Abstract
Background
Panax ginseng (i.e., ginseng) root is extensively used in traditional oriental medicine. It is a modern pharmaceutical reagent for preventing various human diseases such as cancer. Ginsenosides—the major active components of ginseng—exhibit immunomodulatory effects. However, the mechanism and function underlying such effects are not fully elucidated, especially in human monocytes and dendritic cells (DCs).
Methods
We investigated the immunomodulatory effect of ginsenosides from Panax ginseng root on CD14+ monocytes purified from human adult peripheral blood mononuclear cells (PBMCs) and on their differentiation into DCs that affect CD4+ T cell activity.
Results
After treatment with ginsenoside fractions, monocyte levels of tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-10 increased through phosphorylation of extracellular signal-regulated kinase (ERK)1/2 and c-Jun N-terminal kinase (JNK), but not p38 mitogen-activated protein kinase (MAPK). After treatment with ginsenoside fractions, TNF-α production and phosphorylation of ERK1/2 and JNK decreased in lipopolysaccharide (LPS)-sensitized monocytes. We confirmed that DCs derived from CD14+ monocytes in the presence of ginsenoside fractions (Gin-DCs) contained decreased levels of the costimulatory molecules CD80 and CD86. The expression of these costimulatory molecules decreased in LPS-treated DCs exposed to ginsenoside fractions, compared to their expression in LPS-treated DCs in the absence of ginsenoside fractions. Furthermore, LPS-treated Gin-DCs could not induce proliferation and interferon gamma (IFN-γ) production by CD4+ T cells with the coculture of Gin-DCs with CD4+ T cells.
Conclusion
These results suggest that ginsenoside fractions from the ginseng root suppress cytokine production and maturation of LPS-treated DCs and downregulate CD4+ T cells.
Keywords: CD14+ monocytes, CD4+ T cells, dendritic cells, ginsenosides, Panax ginseng
1. Introduction
Panax ginseng (i.e., ginseng) is a well-known traditional oriental medicine used to prevent various human diseases such as inflammatory diseases and cancer [1,2]. Ginsenosides are a major component of ginseng and more than 25 ginsenosides reportedly exist [3]. Ginsenosides can activate macrophages to produce reactive nitrogen intermediates and induce a tumoricidal effect [4]. However, they may also attenuate cytokine production [5].
Monocytes comprise approximately 5–10% of blood leukocytes in humans [6] and mice [7]. They have an important role in establishing innate immune responses. Monocytes differentiate into macrophages or dendritic cells (DCs) in the presence of appropriate mediators such as granulocyte macrophage colony-stimulating factor (GM-CSF), macrophage colony-stimulating factor (M-CSF), or interleukin 4 (IL-4) [8]. On stimulation with lipopolysaccharide (LPS), monocytes and macrophages produce proinflammatory cytokines such as tumor necrosis factor (TNF)-α and the chemokines. Dendritic cells have a major role in initiating and inducing innate immunity and, perhaps more importantly, bridging with antigen-specific immune responses elucidated by T cells. Compared to monocytes, immature DCs display higher expression levels of CD80, CD86, CD11c, and major histocompatibility complex (MHC) class II, and have increased antigen uptake [9]. After antigen uptake, immature DCs become mature and sensitize naive T cells, which leads to clonal expansion and differentiation into effector helper T cells and cytotoxic T cells, which produce IFN-γ.
Mouse DCs treated with ginsenosides in a recent study showed a suppressed maturation process [10]. In mouse DCs stimulated with LPS, the ginsenosides inhibit the secretion of IL-12, an important cytokine that induces T cell activation. However, no reports have revealed the effect of ginsenosides on the differentiation of immature DCs from human monocytes. In the present study, we therefore explored the effect of ginsenoside fractions on the differentiation of CD14+ monocytes to DCs, and explored the expression of cell surface markers (e.g., CD80, CD86, CD40, and MHC class II) on the differentiated DCs and interferon gamma (IFN-γ) production in CD4+ T cells when cocultured with DCs that were differentiated in the presence of ginsenoside fractions.
2. Materials and methods
2.1. Reagents and chemicals
Roswell Park Memorial Institute (RPMI) 1640 medium, fetal bovine serum (FBS), and antibiotics (e.g., penicillin and streptomycin) were purchased from Gibco-BRL (Grand Island, NY, USA). Escherichia coli LPS (026:B6), the c-Jun N-terminal kinase (JNK) inhibitor SP600125, and polymyxin B (PMB) were purchased from Sigma–Aldrich (St. Louis, MO, USA). The mitogen-activated protein kinase (MAPK) inhibitor U0126 was purchased from EMD Millipore (San Diego, CA, USA). Human recombinant IL-4, GM-CSF, and anti-Annexin-V-FITC antibody were purchased from R&D Systems (Minneapolis, MN, USA). Rabbit antiphospho-extracellular signal-regulated kinase 1/2 (antiphospho-ERK1/2), anti-ERK1/2, antiphospho-JNK, anti-JNK, antiphospho-p38, anti-p38, and anti-inhibitory kappa B (anti-IκB) antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). Goat antimouse immunoglobulin G-horseradish peroxidase (IgG-HRP), mouse antirabbit IgG-HRP, and mouse monoclonal anti-β-actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The specific antibodies for flow cytometric analysis, which included human anti-CD80-PE, anti-CD86-antigen-presenting cell (APC), anti-CD40-fluorescein isothiocyanate (FITC), anti-CD14-FITC, anti-CD11c-APC, and anti-human leukocyte antigen DR (HLA-DR)-FITC were purchased from BD Biosciences (San Diego, CA, USA). Unless otherwise noted, all other reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Ginsenoside preparation
Ginsenoside fractions were extracted from Panax ginseng, as previously described [11]. In brief, the dried root of Panax ginseng was refluxed twice with 80% methanol and concentrated with a vacuum-evaporator. The concentrate was diluted with water and the solution was extracted with 1 L of diethyl ether. The aqueous phase was briefly evaporated under vacuum to remove the remaining ether. The solution was then extracted with n-butanol. The organic phase was finally collected and evaporated. Endotoxin levels in the ginsenoside preparations were determined using a Limulus amebocyte lysate test kit (Cambrex Bio Science, Walkersville, MD, USA) in accordance with the manufacturer's instructions. For each experiment, 100 mg of the ginsenoside fractions were dissolved in 5 mL sterile double-distilled water and diluted 1:1 with phosphate-buffered saline (PBS, Gibco-BRL) for a final concentration of 10 mg/mL.
2.3. Thin-layer chromatography and high-performance liquid chromatography analyses
For TLC, 8 μL of ginseng extract solution in butanol was spotted onto a TLC plate (silica gel 60) with standard samples and developed to 5.5 cm distance in a chamber containing a mobile phase chloroform-methanol-water mixture (65:35:10, v/v/v; lower phase). The bands on the TLC plates were detected by spraying with 10% sulfuric acid, followed by heating at 110°C for 10 min. High-performance liquid chromatography was performed by using the NS 3000i system (Futecs Co., Ltd, Jinju, Korea), which is equipped with a UV detector and a gradient pump. A 20-μL sample was injected into a C18 column (250 mm × 4.6 mm, 5μm), and the eluent was withdrawn at a flow rate of 1.6 mL/min using a solvent gradient consisting of acetonitrile (A) and water (W). The solvent A/solvent W ratios were 15:85, 21:79, 58:42, 65:35, 90:10, 90:10, and 15:85 with runtimes of 0–5 min, 5–25 min, 25–70 min, 70–85 min, 85–87 min, 87–97 min, and 97–110 min, respectively. Each ginsenoside fraction peak was monitored and compared with the peak corresponding to the standards (i.e., Rb1, Rc, Rd, Rh2, Rg1, Rg3, and compound K) prepared from steamed and dried Panex ginseng root (KT&G, Daejeon, Korea).
2.4. Preparation of CD14+ monocytes and differentiation into DCs
The Institutional Review Board (IRB Number 0705/001-002) of the Seoul National University (Seoul, South Korea) approved all experiments using human blood. Peripheral blood mononuclear cells (PBMCs) were prepared by density gradient centrifugation of blood obtained from healthy donors by using the Ficoll-Paque Plus centrifuge (Amersham Bioscience, Buckinghamshire, UK). Mononuclear cells in the buffy coat were collected and washed three times with PBS. The CD14+ monocytes were isolated from the PBMCs by using an IMag anti-human CD14 antibody kit (BD Biosciences). The CD14+ monocytes were suspended in a complete medium composed of RPMI-1640 glutamax supplemented with 10% FBS and 1% antibiotics/antimycotics. To generate DCs, 1 × 106 CD14+ monocytes were cultured for 3 d or 5 d at 37°C under 5% carbon dioxide in RPMI complete medium containing 500 U/mL IL-4 and 800 U/mL GM-CSF in a 24-well culture plate (Nalgene Nunc International, Rochester, NY, USA). The medium was changed every 3 d.
2.5. Enzyme-linked immunosorbent assay
For 24 h, CD14+ monocytes (1 × 106 cells) were treated with ginsenoside fractions at a concentration of 0 μg/mL, 1 μg/mL, or 10 μg/mL in the presence or absence of LPS (50 ng/mL). The supernatants were then harvested. In some experiments, CD14+ monocytes were pretreated for 1 h with U0126, SP600125, or PMB. The levels of IL-1β, IL-6, IL-10, and TNF-α in the supernatants were analyzed using enzyme-linked immunosorbent assay (ELISA) kits (R&D Systems) in accordance with the manufacturer's instructions.
2.6. Western blot analysis
The CD14+ monocytes (1 × 106 cells) were stimulated with ginsenoside fractions at a concentration of 0 μg/mL, 1 μg/mL, and 10 μg/mL in the presence or absence of LPS (50 ng/mL). The cells were washed with cold PBS and lysed in cold radioimmunoprecipitation assay lysis buffer containing 50mM Tris-HCl, pH 8, 150mM sodium chloride, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), a protease inhibitor cocktail (Roche, Mannheim, Germany), 2mM sodium fluoride, 0.1mM sodium orthovanadate, and 2mM glycerol phosphate. Insoluble material was removed by centrifugation at 22,000 × g for 10 min at 4°C. The protein concentration was determined using the Bio-Rad Protein Assay Kit (Bio-Rad Laboratories, Hercules, CA, USA). The lysates were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and transferred to a polyvinylidene difluoride microporous membrane (Amersham Biosciences, Piscataway, NJ, USA). The membranes were blocked at room temperature for 1 h with 3% bovine serum albumin (BSA) in tris-buffered saline (TBS) containing 0.1% Tween 20 prior to probing with a primary antibody for the nonphosphorylated or phosphorylated forms of MAPKs or mouse anti-β-actin. Primary antibodies were detected using goat antimouse IgG-HRP or mouse antirabbit IgG-HRP antibodies. They were visualized with an enhanced chemiluminescence system (GE Healthcare, Buckinghamshire, UK), after the membrane had been extensively washed with TBS containing 0.1% Tween 20. For the MAPK signaling inhibition test, the cells were pretreated for 1 h with 20μM SP600125 (i.e., JNK inhibitor) and 10μM U0126 (i.e., MAPK inhibitor) prior to being treated with ginsenoside fractions.
2.7. Flow cytometric analysis
The CD14+ monocytes were seeded into a 24-well plate at a density of 1 × 106 cells/mL in RPMI complete media containing GM-CSF and IL-4. The cells were then treated with ginsenoside fractions for 3 d or 5 d. In an additional experiment, immature DCs were stimulated with LPS (50 ng/mL) in the presence or absence of the ginsenoside fractions. The cells were then harvested and stained with an appropriate combination of antihuman-CD80-PE, anti-CD86-APC, anti-CD40-FITC, anti-CD14-FITC, anti-CD11c-APC, and anti-HLA-DR-FITC antibodies. After staining for 25 min at 4°C, the cells were washed three times, and differences in the expression of cell surface molecules were analyzed by a flow cytometer (BD FACScalibur; BD Biosciences) with CellQuest software (BD Biosciences). All flow cytometric data were analyzed by FlowJo software (Tree Star, San Carlos, CA, USA).
2.8. Apoptosis assay
The CD14+ monocytes were seeded onto a 24-well plate at a density of 1 × 106 cells/mL in RPMI complete media containing GM-CSF and IL-4. The cells were treated with ginsenoside fractions for 5 d and then harvested and stained with anti-Annexin V antibody and propidium iodide (PI). After staining for 15 min at 4°C under dark conditions, the cells were washed with cold PBS. Cell death was assessed by using a flow cytometer (BD Biosciences) and FlowJo software (Tree Star).
2.9. Measurement of T cell activity
The CD4+ T cells were isolated and activated, as previously described [12]. In brief, after differentiating DCs with or without ginsenoside fraction treatment, the cells were stimulated for 2 d with ethanol-killed Staphylococcus aureus (107 colony-forming units (CFU)/mL) [12]. After washing with PBS, 2 × 105 cells were cocultured in a 96-well plate with CD4+ T cells (2 × 105 cells) labeled with carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen, Carlsbad, NM, USA). After 5 d, the cells were harvested and washed with PBS. The intensity of CFSE was determined by flow cytometry. After culturing for 3 d, the IFN-γ levels in the supernatants were determined using an ELISA kit (R&D Systems).
2.10. Statistical analysis
Comparative data were analyzed by the Student t test using the SAS statistical software package, version 9.3 (SAS Institute Inc., Cary, NC, USA). Differences were considered statistically significant when p < 0.05.
3. Results
3.1. Characterization of ginsenoside fractions
We initially examined the proportion of each ginsenoside fraction in the sample by using TLC, which is a common technique for the fingerprint analysis of a mixed complex because of its ease of use, low cost, and versatility. As Fig. 1A shows, Rg3, Rd, and Rb1 were the predominant components. We then examined the ginsenoside fraction further by using high performance liquid chromatography. As expected from TLC results, Rb1, Rg3, and Rd were the major components in the ginseng root, and the largest fraction was Rc (Fig. 1B).
Fig. 1.
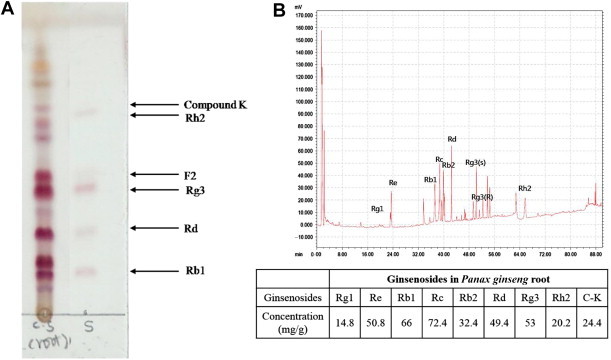
Components of crude root ginsenoside. The qualitative analysis of the ginsenoside fractions are by (A) thin-layer chromatography and (B) high-performance liquid chromatography.
3.2. Induction of cytokines in CD14+ monocytes treated with ginsenoside fractions
First, to examine the cytotoxicity of the ginsenoside fractions on CD14+ monocytes, we analyzed apoptosis of CD14+ monocytes by using Annexin V/PI for the first 5 d of differentiation. The ginsenoside fractions did not show any major signs of inducing apoptosis (Fig. 2A and B). These results suggested that 1 μg/mL or 10 μg/mL of ginsenoside fractions was a valid concentration to use for further experiments during DC differentiation.
Fig. 2.
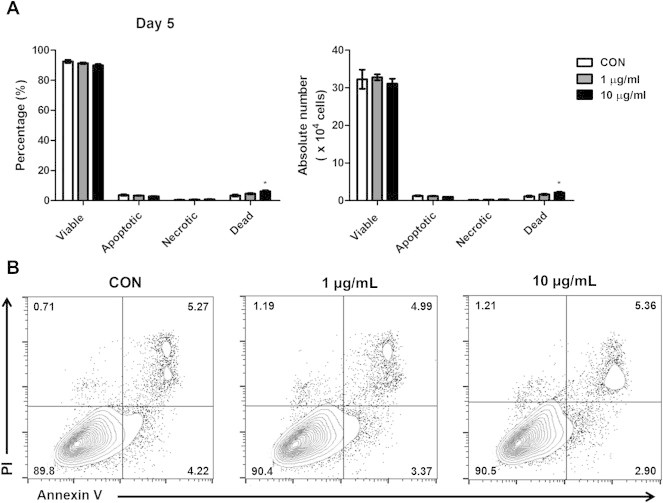
Apoptosis in CD14+ monocytes in the presence or absence of ginsenoside fractions during their differentiation into dendritic cells. (A and B) For 5 d, the CD14+ monocytes (1 × 106 cells) were cultured with interleukin-4 (IL-4; 500 U/mL) and granulocyte macrophage colony-stimulating factor (GM-CSF; 800 U/mL) in the presence or absence of ginsenoside fractions (1 μg/mL or 10 μg/mL). The expression levels of Annexin V and propidium iodide were measured by flow cytometry. *Indicates statistical significance at p < 0.05, compared to the control group. CON, control; PI, Propidium iodide.
Second, to determine the effect of ginsenoside fractions on cytokine responses of CD14+ monocytes, the cells were treated for 24 h with ginsenoside fractions at a concentration of 0 μg/mL, 1 μg/mL, or 10 μg/mL. The supernatant was examined for cytokine production. As Fig. 3A shows, the expression of TNF-α (p < 0.001) and IL-6 (p < 0.01) increased significantly after treatment with ginsenoside fractions (at the concentration of 10 μg/mL), but IL-1β showed minimal changes. As Fig. 3B shows, IL-10 interestingly also increased in a dose-dependent manner. To confirm whether the induction of cytokines was because the ginsenoside fractions were contaminated with LPS, an LPS neutralization assay was performed, after the addition of PMB, which inhibits the LPS response [13]. As expected, the production of TNF-α in LPS-treated cells decreased significantly (p = 0.00082) after PMB treatment, whereas no change occurred with PMB treatment in the cells stimulated with the ginsenoside fractions (Fig. 3C). This suggests that there was no LPS contamination in the ginsenosides. When cotreated with LPS and ginsenosides, TNF-α induction decreased significantly (p = 0.00005), compared to the cells treated with LPS alone. These results indicate that ginsenoside fractions induce cytokine production in CD14+ monocytes and suppress LPS-induced immune responses.
Fig. 3.
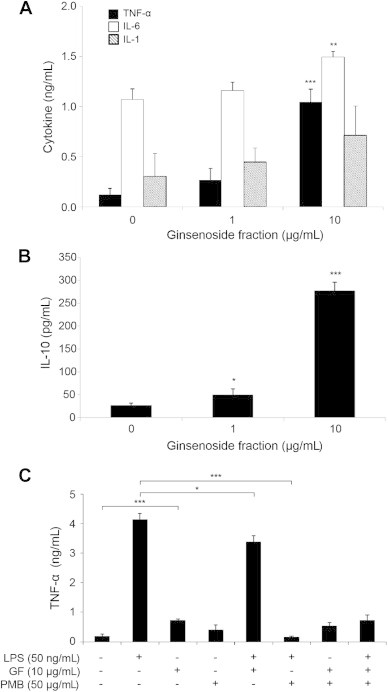
Cytokine production by lipopolysaccharide (LPS)-sensitized CD14+ monocytes in the presence or absence of ginsenoside fractions. For 24 h, CD14+ monocytes were treated with ginsenoside fractions (0 μg/mL, 1 μg/mL, or 10 μg/mL). The production of (A) TNF-α, IL-6, IL-1β, and (B) IL-10 was measured by an enzyme-linked immunosorbent assay kit. (C) For 1 h, the cells were pretreated with PMB (50 μg/mL). This was followed by treatment with ginsenoside fractions (10 μg/mL) and/or lipopolysaccharide (LPS; 50 ng/mL) for an additional 24 h. The culture supernatants were collected and the TNF-α levels were measured. Statistical significance is denoted by *p < 0.05, **p < 0.01, and ***p < 0.001, compared to the control group. GF, ginsenoside fraction; IL, interleukin; PMB, polymyxin B; TNF-α, tumor necrosis factor alpha.
3.3. The ability of ginsenoside fractions to enhance cytokine production is mediated through ERK1/2 and JNK signaling
Most studies on ginseng have focused on a single ginsenoside compound. However, the mechanisms by which total ginsenosides modulate the activity of human monocytes have not yet been reported. Thus, we examined the changes in MAPK (ERK1/2, JNK, and p38) and nuclear factor kappa B (NF-κB) signaling in CD14+ monocytes treated with ginsenoside fractions. The phosphorylation of ERK1/2 and JNK increased in cells treated with ginsenoside fractions in a time-dependent manner (Fig. 4A), whereas the phosphorylation of p38 and IκB did not change (data not shown). To confirm these results, cytokine production was measured after blocking the activities of ERK1/2 and JNK. The production of TNF-α in cells treated with ginsenoside fractions decreased significantly (Fig. 4B and C) after the addition of ERK1/2 or JNK inhibitors (Fig. 4D and E). These data suggest that ginsenosides induce cytokine secretion via the activation of phosphorylated ERK1/2 (pERK1/2) and phosphorylated JNK (pJNK) signaling in CD14+ monocytes.
Fig. 4.
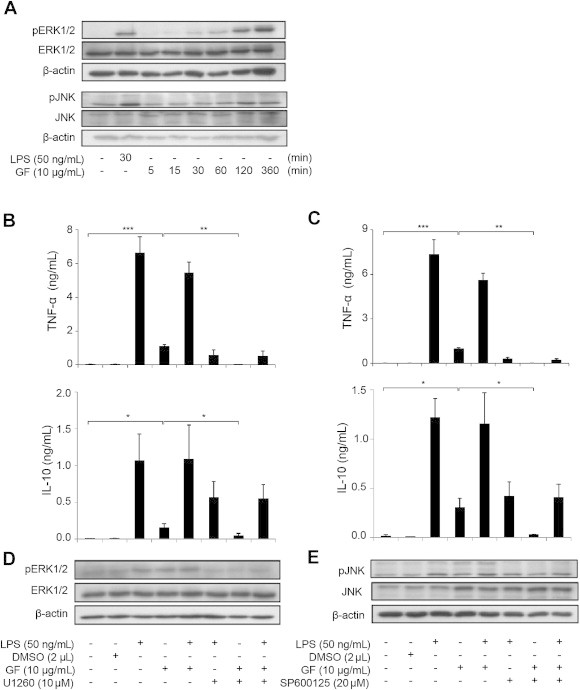
Activation of ERK1/2 and JNK signaling in lipopolysaccharide (LPS)-sensitized CD14+ monocytes with or without ginsenoside fractions. (A) The CD14+ monocytes were stimulated with LPS (50 ng/mL) for 30 min or with ginsenoside fractions (10 μg/mL) for 0 min, 5 min, 15 min, 30 min, 60 min, 120 min, or 360 min. Forty micrograms of protein from each cell lysate were subjected to Western blot analysis for changes in pERK and pJNK activity. The results are representative of three independent experiments. After 1 h pretreatment with (B and D) 10 μM U0126 or (C and E) 20 μM SP600125, the cells were treated with ginsenoside fractions (10 μg/mL) for 24 h in the presence or absence of LPS (50 ng/mL). (B and C) The production of TNF-α was measured by enzyme-linked immunosorbent assay. (D and E) The phosphorylation of ERK1/2 and JNK was evaluated by Western blot. ERK1/2, extracellular signal-regulated kinase 1/2; GF, ginsenoside fraction; IL-10, interleukin 10; JNK, c-Jun N-terminal kinase; pERK, phosphorylated ERK; TNF-α, tumor necrosis factor alpha.
3.4. Ginsenosides attenuate the differentiation of CD14+ monocytes into DCs
Monocytes differentiate into DCs when cultured in the presence of GM-CSF and IL-4 [8]. To test whether ginsenoside fraction is involved in DC differentiation, CD14+ monocytes were incubated with GM-CSF and IL-4 in the presence or absence of ginsenoside fractions for 3 d or 5 d, and the expression of cell surface and maturation markers (i.e., CD80, CD86, CD40, CD11c, CD14, and MHC class II) was measured [9]. Three days after the treatment, little to no change had occurred (Fig. 5A). However, 5 d after the treatment, the ginsenoside fractions suppressed the expression of CD80, CD86, CD40, and CD11c, but not MHC class II and CD14 (Fig. 5B). These results indicate that DCs treated with ginsenoside fractions during the maturation process express low levels of costimulatory molecules.
Fig. 5.
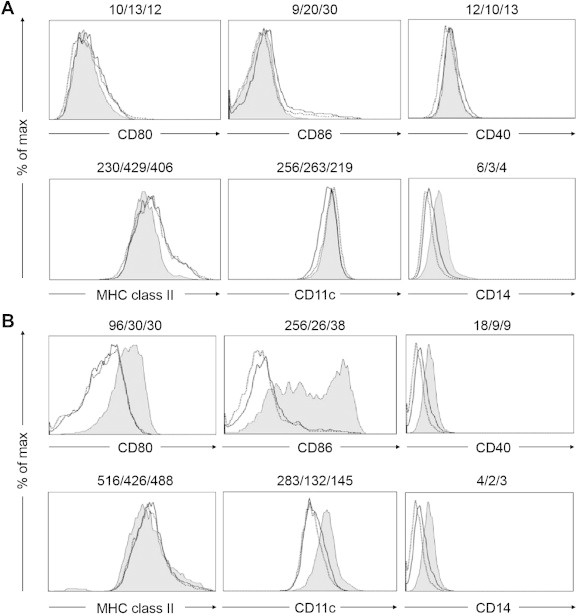
Suppression of the expression of cell surface molecules during dendritic cell differentiation in the presence or absence of ginsenoside fractions. The CD14+ monocytes were treated with interleukin 4 (IL-4; 500 U/mL) and granulocyte macrophage colony-stimulating factor (GM-CSF; 800 U/mL) in the presence or absence of ginsenoside fractions (gray area, 0 μg/mL; dotted line, 1 μg/mL; unbroken line, 10 μg/mL) for (A) 3 d or (B) 5 d. The expression levels of the surface markers CD80, CD86, CD40, MHC class II, CD11c, and CD14 were measured by flow cytometry. The numbers in the panels indicate the mean fluorescence intensity of each ginsenoside molecule at the concentration of 0 μg/mL, 1 μg/mL, or 10 μg/mL, respectively. The data are representative of three independent experiments. MHC, major histocompatibility complex.
3.5. DCs differentiated in the presence of ginsenoside fractions exhibit impaired maturation status and T cell activation
Mature DCs express higher levels of surface markers such as CD80, CD86, CD40, and CD83, compared to immature DCs [14]. Therefore, to further examine the characteristics of DCs differentiated in the presence of ginsenoside fractions (Gin-DCs), the Gin-DCs were treated with LPS. To identify the impact of Gin-DCs on the maturation process, we measured the expression of the surface markers CD80, CD86, CD40, and MHC class II. As Fig. 6A shows, the expression of these markers decreased in a dose-dependent manner, whereas the expression of CD40 remained relatively unchanged.
Fig. 6.
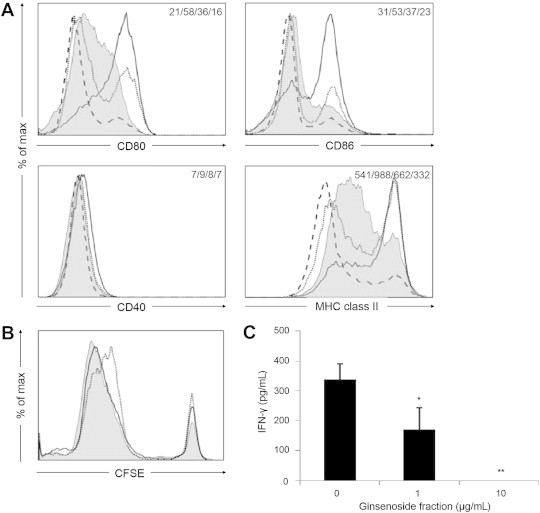
Dendritic cell (DC) maturation in the presence of ginsenoside fractions and the regulation of CD4+ T cells. (A) The DCs treated with ginsenoside fractions (the unbroken, dotted, and broken lines indicate 0 μg/mL, 1 μg/mL, and 10 μg/mL, respectively) during differentiation were stimulated with lipopolysaccharide (LPS) for 24 h. Their expression of CD80, CD86, CD40, and MHC class II was assessed by flow cytometry. The gray area indicates the untreated DCs (i.e., the control). The numbers in the panels indicate the mean fluorescence intensity (MFI) of the gray area, unbroken line, dotted line, and broken line. (B) During differentiation for 48 h, ethanol-killed Staphylococcus aureus (107 CFU/mL) was primed to DCs treated with ginsenoside fractions (the gray area, unbroken line, and dotted line indicate 0 μg/mL, 1 μg/mL, and 10 μg/mL, respectively). The cells were cocultured with CFSE-labeled CD4+ T cells for 3 d or 5 d. Proliferation was then evaluated by flow cytometry. (C) The interferon gamma (IFN-γ) levels in the supernatants at the end of the 3-d culture were measured by enzyme-linked immunosorbent assay. Statistical significance is denoted at *p < 0.05 and **p < 0.01. CFSE, carboxyfluorescein succinimidyl ester; MHC, major histocompatibility complex.
To investigate whether Gin-DCs activate CD4+ T cells, the Gin-DCs were primed for 2 d with ethanol-killed S. aureus [12]. They were then cocultured with CFSE-labeled CD4+ T cells for an additional 3 d or 5 d. The CD4+ T cell proliferation was slightly suppressed when cocultured with S. aureus-primed Gin-DCs for 5 days (Fig. 6B). In addition, IFN-γ production decreased significantly (p < 0.05) under the same conditions (Fig. 6C). These results suggest that ginsenoside fractions reduce the capacity of DCs to activate CD4+ T cells, compared to control DCs.
4. Discussion
The major findings of the current study were the following: (1) ginsenoside fractions increased the production of IL-6, IL-10, and TNF-α by human CD14+ monocytes; (2) treatment with ginsenoside fractions increased the production of TNF-α through ERK1/2 and JNK signaling pathways, but they inhibited LPS-induced cytokine production; (3) ginsenoside fractions suppressed the expression of cell surface molecules during the differentiation of monocytes to DCs; and (4) Gin-DCs exhibited low expression of costimulatory molecules, thereby inhibiting their capacity to activate CD4+ T cells.
The levels IL-6, TNF-α, and IL-10, but not IL-1β, significantly increased in human monocytes after ginsenoside fraction treatment, which suggests that ginsenosides could modulate the action mode of monocytes. The expression of IL-10 increased in monocytes treated with ginsenosides, which interestingly indicated possible anti-inflammatory activity under inflammatory conditions. Ginsenoside showed no effect on IL-1β production. In LPS-stimulated human monocytes, TNF-α and IL-1β are differentially regulated [15]. Therefore, it is reasonable to assume that the various ginsenoside components exert different effects on cytokine induction. These results led us to investigate the mechanism by which ginsenoside fractions induce cytokine production in monocytes.
The Rg1 ginsenoside activates ERK1/2 in MCF-7 human breast cancer cells [16], and compound K activates JNK and p38 phosphorylation in HT-29 human colon cancer cells [17].
The anticancer and immune-regulative effects of ginseng are controversial. The ginsenoside Rg1 suppresses the expression of TNF-α, whereas Rh1 increases TNF-α expression in THP-1 human leukemia cells [18]. In addition, the ginsenoside Rh1 inhibits the activation of MAPK signaling in THP-1 cells [19]. The ginsenosides Rg and Rh2 inhibit the production of proinflammatory cytokines via suppressing activator protein 1 and protein kinase A activity, but they have no effect on NF-κB activity [20]. Our results suggest that the ERK1/2 and JNK pathways, but not the p38 MAPK pathway, are responsible for the ginsenoside-mediated expression of TNF-α.
Ginsenoside fraction-treated LPS-sensitized monocytes showed ERK1/2 and JNK phosphorylation that was superior to that of the cells stimulated with LPS alone. These results indicate that the ginsenosides are forceful activators of these signaling pathways. Our results further suggested that ginsenoside fractions modulate LPS-induced inflammatory effects in human monocytes.
Because of the wide variability in the type and concentration of ginsenosides in ginseng extracts, the definition of the immunomodulatory potential of each ginsenoside component is crucial. The ginsenoside Rg1 indeed inhibited the production of TNF-α and IL-6, whereas Rb1 affected IL-6 production only. The combination of Rg1 and Rb1 unexpectedly diminished such inhibitory effects. These findings are consistent with our results and with reports from other studies that suggest that ginseng extracts differentially affect immune cell function, based on their specific ginsenoside profile [21].
Our results are in agreement with those of previous reports showing that DCs expressing low levels of costimulatory molecules weakly induce T cell proliferation and T cell secretion of IFN-γ [22,23]. Furthermore, LPS-stimulated Gin-DCs expressed low levels of costimulatory molecules. When cocultured with CD4+ T cells, ethanol-killed S. aureus–primed Gin-DCs induced decreased CD4+ T cell proliferation and IFN-γ production, compared to the control DCs [12].
Several studies have recently suggested that tolerogenic DCs that express low levels of costimulatory molecules and produce low levels of proinflammatory cytokines also suppress T cell proliferation and cytokine production [24–26]. The Gin-DCs share some characteristics with tolerogenic DCs such as the low expression levels of costimulatory molecules; however, Gin-DCs continuously produce proinflammatory cytokines (data not shown). As mentioned previously, ginsenosides consist of a number of compounds such as Re, Rh, and Rg. Different combinations of these compounds may cause different responses in DCs. These features of the ginsenosides (not a single compound) may therefore be responsible for the low expression levels of costimulatory molecules by DCs. Because of the immunomodulatory activities reported in this paper, the precise mechanism by which ginsenosides regulate the expression of costimulatory molecules by DCs should be investigated further.
In conclusion, ginsenoside fractions promote the production of inflammatory cytokines in CD14+ monocytes via ERK1/2 and JNK signaling pathways. However, DCs differentiated from monocytes do not fully activate CD4+ T cells in the presence of ginsenoside fractions. This is likely because they express low levels of costimulatory molecules. These results suggest that ginsenosides may alleviate inflammatory symptoms.
Conflicts of interest
The authors have no financial conflicts of interest.
Acknowledgments
This work was supported by National Research Foundation grants (2010-0003291, 2010-0029116) and the World Class University Program (R31-10056, funded through the Ministry of Education, Science, and Technology, Korea). This work was also partially supported by a grant from the Next-Generation BioGreen 21 Program (PJ008127012011), Rural Development Administration, Korea.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Kiefer D., Pantuso T. Panax ginseng. Am Fam Physician. 2003;68:1539–1542. [PubMed] [Google Scholar]
- 2.Lee T.K., Johnke R.M., Allison R.R., O'Brien K.F., Dobbs L.J., Jr. Radioprotective potential of ginseng. Mutagenesis. 2005;20:237–243. doi: 10.1093/mutage/gei041. [DOI] [PubMed] [Google Scholar]
- 3.Kang K.A., Kang J.H., Yang M.P. Ginseng total saponin enhances the phagocytic capacity of canine peripheral blood phagocytes in vitro. Am J Chin Med. 2008;36:329–341. doi: 10.1142/S0192415X08005801. [DOI] [PubMed] [Google Scholar]
- 4.Fan Z.H., Isobe K., Kiuchi K., Nakashima I. Enhancement of nitric oxide production from activated macrophages by a purified form of ginsenoside (Rg1) Am J Chin Med. 1995;23:279–287. doi: 10.1142/S0192415X9500033X. [DOI] [PubMed] [Google Scholar]
- 5.Park E.K., Choo M.K., Han M.J., Kim D.H. Ginsenoside Rh1 possesses antiallergic and anti-inflammatory activities. Int Arch Allergy Immunol. 2004;133:113–120. doi: 10.1159/000076383. [DOI] [PubMed] [Google Scholar]
- 6.Whitelaw D.M. Observations on human monocyte kinetics after pulse labeling. Cell Tissue Kinet. 1972;5:311–317. doi: 10.1111/j.1365-2184.1972.tb00369.x. [DOI] [PubMed] [Google Scholar]
- 7.van Furth R., Cohn Z.A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968;128:415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu H., Kramer M., Spengler H.P., Peters J.H. Dendritic cells differentiated from human monocytes through a combination of IL-4, GM-CSF and IFN-gamma exhibit phenotype and function of blood dendritic cells. Adv Exp Med Biol. 1995;378:75–78. doi: 10.1007/978-1-4615-1971-3_15. [DOI] [PubMed] [Google Scholar]
- 9.Pickl W.F., Majdic O., Kohl P., Stockl J., Riedl E., Scheinecker C., Bello-Fernandez C., Knapp W. Molecular and functional characteristics of dendritic cells generated from highly purified CD14+ peripheral blood monocytes. J Immunol. 1996;157:3850–3859. [PubMed] [Google Scholar]
- 10.Tung N.H., Quang T.H., Son J.H., Koo J.E., Hong H.J., Koh Y.S., Song G.Y., Kim Y.H. Inhibitory effect of ginsenosides from steamed ginseng-leaves and flowers on the LPS-stimulated IL-12 production in bone marrow-derived dendritic cells. Arch Pharm Res. 2011;34:681–685. doi: 10.1007/s12272-011-0419-2. [DOI] [PubMed] [Google Scholar]
- 11.Cheng L.Q., Na J.R., Bang M.H., Kim M.K., Yang D.C. Conversion of major ginsenoside Rb1 to 20(S)-ginsenoside Rg3 by Microbacterium sp. GS514. Phytochemistry. 2008;69:218–224. doi: 10.1016/j.phytochem.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 12.Son Y.M., Ahn S.M., Jang M.S., Moon Y.S., Kim S.H., Cho K.K., Han S.H., Yun C.H. Immunomodulatory effect of resistin in human dendritic cells stimulated with lipoteichoic acid from Staphylococcus aureus. Biochem Biophys Res Commun. 2008;376:599–604. doi: 10.1016/j.bbrc.2008.09.037. [DOI] [PubMed] [Google Scholar]
- 13.Jacobs D.M., Morrison D.C. Inhibition of the mitogenic response to lipopolysaccharide (LPS) in mouse spleen cells by polymyxin B. J Immunol. 1977;118:21–27. [PubMed] [Google Scholar]
- 14.de Saint-Vis B., Vincent J., Vandenabeele S., Vanbervliet B., Pin J.J., Ait-Yahia S., Patel S., Mattei M.G., Banchereau J., Zurawski S. A novel lysosome-associated membrane glycoprotein, DC-LAMP, induced upon DC maturation, is transiently expressed in MHC class II compartment. Immunity. 1998;9:325–336. doi: 10.1016/s1074-7613(00)80615-9. [DOI] [PubMed] [Google Scholar]
- 15.Molnar-Kimber K.L., Yonno L., Heaslip R.J., Weichman B.M. Differential regulation of TNF-alpha and IL-1beta production from endotoxin stimulated human monocytes by phosphodiesterase inhibitors. Mediators Inflamm. 1992;1:411–417. doi: 10.1155/S0962935192000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lau W.S., Chen W.F., Chan R.Y., Guo D.A., Wong M.S. Mitogen-activated protein kinase (MAPK) pathway mediates the oestrogen-like activities of ginsenoside Rg1 in human breast cancer (MCF-7) cells. Br J Pharmacol. 2009;156:1136–1146. doi: 10.1111/j.1476-5381.2009.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee I.K., Kang K.A., Lim C.M., Kim K.C., Kim H.S., Kim D.H., Kim B.J., Chang W.Y., Choi J.H., Hyun J.W. Compound K, a metabolite of ginseng aaponin, induces mitochondria-dependent and caspase-dependent apoptosis via the generation of reactive oxygen species in human colon cancer cells. Int J Mol Sci. 2010;11:4916–4931. doi: 10.3390/ijms11124916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y., Wang B.X., Liu T.H., Minami M., Nagata T., Ikejima T. Metabolism of ginsenoside Rg1 by intestinal bacteria. II. Immunological activity of ginsenoside Rg1 and Rh1. Acta Pharmacol Sin. 2000;21:792–796. [PubMed] [Google Scholar]
- 19.Choi Y.J., Yoon J.H., Cha S.W., Lee S.G. Ginsenoside Rh1 inhibits the invasion and migration of THP-1 acute monocytic leukemia cells via inactivation of the MAPK signaling pathway. Fitoterapia. 2011;82:911–919. doi: 10.1016/j.fitote.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Bae E.A., Kim E.J., Park J.S., Kim H.S., Ryu J.H., Kim D.H. Ginsenosides Rg3 and Rh2 inhibit the activation of AP-1 and protein kinase A pathway in lipopolysaccharide/interferon-gamma-stimulated BV-2 microglial cells. Planta Med. 2006;72:627–633. doi: 10.1055/s-2006-931563. [DOI] [PubMed] [Google Scholar]
- 21.Attele A.S., Wu J.A., Yuan C.S. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693. doi: 10.1016/s0006-2952(99)00212-9. [DOI] [PubMed] [Google Scholar]
- 22.Wheat W.H., Pauken K.E., Morris R.V., Titus R.G. Lutzomyia longipalpis salivary peptide maxadilan alters murine dendritic cell expression of CD80/86, CCR7, and cytokine secretion and reprograms dendritic cell-mediated cytokine release from cultures containing allogeneic T cells. J Immunol. 2008;180:8286–8298. doi: 10.4049/jimmunol.180.12.8286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavassani K.A., Aliberti J.C., Dias A.R., Silva J.S., Ferreira B.R. Tick saliva inhibits differentiation, maturation and function of murine bone-marrow-derived dendritic cells. Immunology. 2005;114:235–245. doi: 10.1111/j.1365-2567.2004.02079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steinman R.M., Hawiger D., Nussenzweig M.C. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- 25.Boks M.A., Kager-Groenland J.R., Haasjes M.S., Zwaginga J.J., van Ham S.M., ten Brinke A. IL-10-generated tolerogenic dendritic cells are optimal for functional regulatory T cell induction–a comparative study of human clinical-applicable DC. Clin Immunol. 2012;142:332–342. doi: 10.1016/j.clim.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 26.Wolfle S.J., Strebovsky J., Bartz H., Sahr A., Arnold C., Kaiser C., Dalpke A.H., Heeg K. PD-L1 expression on tolerogenic APCs is controlled by STAT-3. Eur J Immunol. 2011;41:413–424. doi: 10.1002/eji.201040979. [DOI] [PubMed] [Google Scholar]


