Abstract
Background
Korean Red Ginseng (KRG) is a representative traditional herbal medicine with many different pharmacological properties including anticancer, anti-atherosclerosis, anti-diabetes, and anti-inflammatory activities. Only a few studies have explored the molecular mechanism of KRG-mediated anti-inflammatory activity.
Methods
We investigated the anti-inflammatory mechanisms of the protopanaxadiol saponin fraction (PPD-SF) of KRG using in vitro and in vivo inflammatory models.
Results
PPD-SF dose-dependently diminished the release of inflammatory mediators [nitric oxide (NO), tumor necrosis factor-α, and prostaglandin E2], and downregulated the mRNA expression of their corresponding genes (inducible NO synthase, tumor necrosis factor-α, and cyclooxygenase-2), without altering cell viability. The PPD-SF-mediated suppression of these events appeared to be regulated by a blockade of p38, c-Jun N-terminal kinase (JNK), and TANK (TRAF family member-associated NF-kappa-B activator)-binding kinase 1 (TBK1), which are linked to the activation of activating transcription factor 2 (ATF2) and interferon regulatory transcription factor 3 (IRF3). Moreover, this fraction also ameliorated HCl/ethanol/-induced gastritis via suppression of phospho-JNK2 levels.
Conclusion
These results strongly suggest that the anti-inflammatory action of PPD-SF could be mediated by a reduction in the activation of p38-, JNK2-, and TANK-binding-kinase-1-linked pathways and their corresponding transcription factors (ATF2 and IRF3).
Keywords: activating transcription factor 2, anti-inflammatory activity, interferon regulatory transcription factor 3, Korean red ginseng, protopanaxadiol saponin fraction
1. Introduction
Inflammation is an important process because it is one of the natural defense mechanisms caused by the release of inflammatory mediators [e.g., (nitric oxide) NO and prostaglandin (PG)E2], cytokines [e.g., tumor necrosis factor (TNF)-α], and chemokines [1,2]. This event requires the activation of inflammatory cells such as macrophages via the ligation of their surface receptors (e.g., Toll-like receptors) [3]. The activation of Toll-like receptors in macrophages by ligands derived from pathogens triggers various cellular signaling cascades to activate transcription factors including nuclear factor (NF)-κB (p50 and p65), activator protein (AP)-1 [c-Fos, c-Jun, and activating transcription factor (ATF)-2], and interferon regulatory transcription factor (IRF)-3 to trigger the new expression of inflammatory genes [4–6]. Although inflammation is a normal response, acutely, excessive induced, or chronically sustained inflammatory responses are known to cause serious diseases including cancer, stroke, and diabetes. Therefore, it must be stressed that normalization of upregulated inflammation is crucial in prevention of such diseases [7–9].
Korean Red Ginseng (KRG, steamed root of Panax ginseng Meyer, Araliaceae) is a well-known herbal medicine traditionally used in Korea [10]. It has been used for a long time without displaying any toxic properties, thus, developing some anti-inflammatory preparation with KRG could be considered beneficial. Unlike acid polysaccharides that are known as major components contributing to upregulation of the body's immune responses [11], red ginseng saponin fractions enriched with protopanaxadiol (PPD)-type ginsenosides have been reported as strong anti-inflammatory preparations [12]. Some PPD-type ginsenosides such as ginsenoside (G)-Rb1, G-Rb2, and G-Rd display strong anti-inflammatory properties under various conditions [13]. This notion led us to establish a hypothesis that PPD-type saponins could be used as an anti-inflammatory remedy. In this study, therefore, we investigated the anti-inflammatory activity and molecular mechanism of the protopanaxadiol saponin fraction (PPD-SF).
2. Materials and methods
2.1. Materials
PPD-SFs, prepared by previously established methods [14], from KRG with higher amounts of protopanaxadiol-type ginsenosides (G-Rb1, G-Rc, G-Re, and G-Rb2) were kindly supplied by the Korea Ginseng Cooperation (Daejeon, Korea). Nω-Nitro-l-arginine methyl ester hydrochloride (l-NAME), (3-4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), phorbol 12-myristate 13-acetate (PMA), and lipopolysaccharide (LPS, Escherichia coli 0111:B4) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). BX795 and SP600125 were obtained from Calbiochem (La Jolla, CA, USA). Luciferase constructs containing promoters with binding sites for NF-κB, AP-1, and IRF-3 were used, as reported previously [15]. RAW264.7 cells, a BALB/c-derived murine macrophage cell line (ATCC No. TIB-71), and HEK293 cells, a human embryonic kidney cell line (ATCC No. CRL-1573), were obtained from American Tissue Culture Collection (Rockville, MD, USA). TANK (TRAF family member-associated NF-kappa-B activator)-binding kinase (TBK)1 and adaptor molecule [TIR-domain-containing adapter-inducing interferon-β (TRIF) or myeloid differentiation primary response gene 88 (MyD88)] were used as reported previously [16]. Fetal bovine serum and RPMI 1640 were purchased from Gibco (Grand Island, NY, USA), and phospho-specific or total antibodies to c-Jun, c-Fos, ATF-2, IRF-3, extracellular signal-regulated kinase (ERK), p38, C-Jun N-terminal kinase (JNK), mitogen-activated protein kinase kinase 4 (MKK4), MKK3/6, transforming growth factor-β-activated kinase 1 (TAK1), TBK1, lamin A/C, and β-actin were purchased from Cell Signaling (Beverly, MA, USA). All other chemicals were purchased from Sigma Chemical Co.
2.2. Treatment of PPD-SF
A stock solution (8 mg/mL) of PPD-SF was prepared with culture medium and diluted to 0–400 μg/mL: with media for in vitro, cellular assays, or suspended in 1% sodium carboxymethylcellulose for in vivo experiments.
2.3. Animal experiments
Male imprinting control region (ICR) mice (6–8 weeks old, 17–21 g) were obtained from Daehan Biolink (Chungbuk, Korea) and maintained in plastic cages under standard conditions. Water and pelleted food (Samyang, Daejeon, Korea) were supplied ad libitum. Studies (approval ID: SKKUBBI 13-6-2) were performed in accordance with guidelines established by the Institutional Animal Care and Use Committee at Sungkyunkwan University, Suwon, Korea.
2.4. Cell culture
RAW 264.7 and HEK293 cells were cultured in RPMI 1640 medium supplemented with 10% heat-inactivated fetal bovine serum, glutamine, and antibiotics (penicillin and streptomycin) at 37°C under 5% CO2. For experiments, cells were detached with a cell scraper. Under our experimental cell density (2 × 106 cells/mL), the proportion of dead cells was < 1% according to Trypan blue dye exclusion tests.
2.5. NO, PGE2, and TNF-α production
After preincubation for 18 hours, RAW264.7 cells (1 × 106 cells/mL) were pretreated with PPD-SF (0–400 μg/mL) or the standard compounds (l-NAME, SP600125, or BX795), and incubated with LPS (1 μg/mL) for 24 hours. The inhibitory effects of PPD-SF or standard compounds on NO, TNF-α, or PGE2 production were determined by analyzing the NO, PGE2, or TNF-α levels quantified with Griess reagent, enzyme immunoassay, or enzyme-linked immunosorbent assay, respectively, as described previously [17,18].
2.6. Cell viability test
After preincubation for 18 hours, PPD-SF (0–400 μg/mL) was added to RAW264.7 cells (1 × 106 cells/mL) followed by incubation for 24 hours. The cytotoxic effects of PPD-SF were evaluated by MTT assay, as reported previously [19,20].
2.7. HPLC of PPD-SF
Phytochemical characteristics of PPD-SF with standard ginsenosides were identified by high performance liquid chromatography (HPLC) as reported previously [21,22]. The HPLC system was equipped with a Knauer (Wellchrom, Berlin, Germany) HPLC-pump K-1001, a Wellchrom fast scanning spectrophotometer K-2600, a WellChrom UV Detector K-2600, and a four-channel degasser K-500. Elution solvent (acetonitrile), step gradients (0, 20%, 32%, 50%, 65%, or 90% for 0 minutes, 10 minutes, 40 minutes, 55 minutes, 70 minutes, or 80 minutes, 1.6 mL/minute, 203 nm), and a phenomenex gemini C18 ODS (250 mm × 4.6 mm, 5 μm) column were used. Based on these conditions, the contents of ginsenosides from PPD-SF were calculated with the peak area curve of standard ginsenosides.
2.8. mRNA analysis by quantitative reverse transcriptase-polymerase chain reaction
To evaluate cytokine mRNA expression levels, RAW264.7 cells pretreated with PPD-SF (0–400 μg/mL) for 30 minutes were incubated with LPS (1 μg/mL) for 6 hours. Total RNA was isolated with TRIzol Reagent (Gibco BRL) according to the manufacturer's instructions and stored at −70°C until use. The mRNA was quantified by real-time reverse transcriptase polymerase chain reaction (RT-PCR) with SYBR Premix Ex Taq, according to the manufacturer's instructions (Takara, Shiga, Japan), using a real-time thermal cycler (Bio-Rad, Hercules, CA, USA), as reported previously [23,24]. Results were expressed as the ratio of the optical density relative to glyceraldehyde 3-phosphate dehydrogenase. The primers used (Bioneer, Seoul, Korea) are described in Table 1.
Table 1.
Primers used for real-time polymerase chain reaction
| Gene name | Sequence (5′–3′) | |
|---|---|---|
| iNOS | F | CCCTTCCGAAGTTTCTGGCAGCAG |
| R | GGCTGTCAGAGCCTCGTGGCTTTGG | |
| COX-2 | F | CACTACATCCTGACCCACTT |
| R | ATGCTCCTGCTTGAGTATGT | |
| TNF-α | F | TGCCTATGTCTCAGCCTCTTC |
| R | GAGGCCATTTGGGAACTTCT | |
| GAPDH | F | CACTCACGGCAAATTCAACGGCA |
| R | GACTCCACGACATACTCAGCAC |
COX = cyclo-oxygenase; GAPDH = glyceraldehyde 3-phosphate dehydrogenase; iNOS = inducible NO synthase; TNF = tumor necrosis factor
2.9. Plasmid transfection and luciferase reporter gene activity assay
HEK293 cells (1 × 106 cells/mL) were transfected with 1 μg of plasmid containing β-galactosidase and NF-κB-Luc, AP-1-Luc, or IRF-3-Luc in the presence or absence of PMA, or overexpressed adaptor molecules (TRIF or MyD88) using the polyethylenimine (PEI) method in 12-well plates. The cells were treated with PPD-SF for 12 hours prior to termination. Luciferase assays were performed using the Luciferase Assay System (Promega, Madison, WI, USA), as previously reported [24,25].
2.10. Preparation of total lysates and nuclear extracts, and immunoblotting analysis
Stomach tissues or RAW264.7 cells (5 × 106 cells/mL) were washed three times in cold phosphate-buffered saline with 1mM sodium orthovanadate, and then lysed using a sonicator (Thermo Fisher Scientific, Waltham, MA, USA) or a Tissuemizer (Qiagen, Germantown, MD, USA) in lysis buffer [26] for 30 minutes with rotation at 4°C. Lysates were clarified by centrifugation at 16,000 × g for 10 minutes at 4°C and stored at −20°C until use. Nuclear fractions were prepared with RAW264.7 cell-derived lysates in a three-step procedure [27]. After treatment, cells were collected with a rubber policeman, washed with 1 × phosphate-buffered saline, and lysed in 500 μL lysis buffer [28] on ice for 4 minutes. Lysates were centrifuged at 19,326 × g for 1 minute in a microcentrifuge. The pellet (nuclear fraction) was washed once in washing buffer (lysis buffer without Nonidet P-40) and then treated with extraction buffer (lysis buffer containing 500mM KCl and 10% glycerol). The nuclei/extraction buffer mixture was frozen at −80°C, thawed on ice, and centrifuged at 19,326 × g for 5 minutes. The supernatant was collected as a nuclear extract. Soluble cell lysates (30 μg/lane) were immunoblotted. Either the phosphorylated or total levels of c-Jun, c-Fos, ATF2, FRA (Fos-related antigen), IRF3, ERK, p38, JNK, MKK4, MKK3/6, TAK1, TBK1, lamin A/C, and β-actin were visualized as previously described [29].
2.11. Enzyme assay
To evaluate the inhibition of MKK4, MKK6, and MKK7 kinase activities using purified enzymes, we used the kinase profiler service from Millipore (Billerica, MA, USA).
2.12. Ethanol/HCl-induced gastritis
Stomach inflammation was induced in mice using HCl/ethanol according to a published method [30,31]. Fasted ICR mice (7 mice/group) were orally treated with PPD-SF (200 mg/kg) or ranitidine (40 mg/kg) twice daily for 3 days. At 30 minutes after the final injection, 400 μL of 60% ethanol in 150mM HCl was administered orally. Animals were anaesthetized and sacrificed with urethane 1 hour after the administration of necrotizing agents. Stomachs were excised and gently rinsed under running tap water. After opening the stomachs along the greater curvature and spreading them out on a board, the area (mm2) of mucosal erosive lesions was measured using a pixel counter by a technician blinded to the treatment conditions. Experimental groups included a normal group (sham-operated/treated with vehicle), control group (HCl/ethanol injected/treated with vehicle), and drug-treated groups [HCl/ethanol injected/treated with PPD-SF (200 mg/kg) or ranitidine (40 mg/kg)]. Immunoblotting analysis was used to detect the phosphorylated and total levels of JNK from stomach tissue lysates.
2.13. Statistical analysis
The data in this paper are presented as the mean ± standard error of the mean of three different experiments performed using four samples for the in vitro experiments, or as the mean ± standard deviation for the six mice used in the in vivo tests and the kinase assay for three samples. For statistical comparisons, these results were analyzed using analysis of variance/Scheffe's post hoc and Kruskal–Wallis/Mann–Whitney tests. A p value < 0.05 was considered statistically significant. All statistical tests were performed using the SPSS 16.0 computer program (SPSS Inc., Chicago, IL, USA).
3. Results and discussion
To test the anti-inflammatory activity of PPD-SF, we first used in vitro inflammatory models established with LPS-treated RAW264.7 cells. Under these conditions, we could achieve optimal levels of NO, PGE2, and TNF- after 24 hours incubation with LPS, as reported previously [15]. The levels of these inflammatory mediators during LPS exposure were 45μM (NO), 21.6 ng/mL (PGE2), and 6.8 ng/mL (TNF-α), whereas normal levels of these mediators were below 0.6μM (NO), 0.01 ng/mL (PGE2), and 0.3 ng/mL (TNF-α). As we expected, PPD-SF (0–400 μg/mL) dose-dependently suppressed the production of these molecules (Fig. 1A left panel), which shows a higher activity than those of KRG water extract [32]. In particular, this fraction more strongly inhibited the release of PGE2, indicating that this fraction is able to ameliorate more effectively PGE2-derived pain and inflammatory responses. The fact that l-NAME, a standard inducible NO synthase inhibitor, diminished NO production (Fig. 1A right panel) validates our in vitro inflammatory models. There was no cytotoxicity seen at up to 400 μg/mL PPD-SF (Fig. 1B), therefore, the inhibitory activity of PPD-SF in in vitro models could not have been due to its nonspecific cytotoxicity. Meanwhile, HPLC analysis showed that this fraction (PPD-SF) mostly contained G-Rb1 (33.2%), G-Rc (29.4%), G-Rb2 (31.7%), and G-Rb3 (5.4%) (Fig. 1C), implying that these specific ginsenosides could contribute to the mediation of the anti-inflammatory activity of PPD-SF.
Fig. 1.
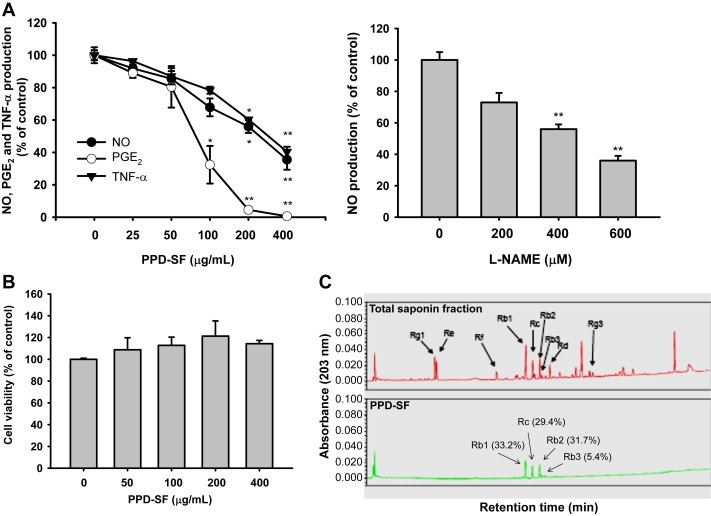
In vitro anti-inflammatory activity of PPD-SF in RAW264.7 cells and HPLC analysis of PPD-SF. (A) Levels of NO, PGE2, and TNF-α were determined from culture supernatants of RAW264.7 cells treated with LPS (1 μg/mL) in the presence or absence of PPD-SF (left panel) or l-NAME (right panel) for 24 hours. (B) Viability of RAW264.7 cells under PPD-SF exposure in the absence of LPS was determined by MTT assay. (C) Phytochemical characteristics of ginsenosides in PPD-SF were analyzed using high performance liquid chromatography. *p < 0.05 compared to the control. **p < 0.01 compared to the control. l-NAME = Nω-Nitro-l-arginine methyl ester hydrochloride; LPS = lipopolysaccharide; MTT = (3-4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; PGE2 = prostaglandin E2; PPD-SF = protopanaxadiol saponin fraction; TNF = tumor necrosis factor.
To understand the molecular mechanism of PPD-SF-induced anti-inflammatory activity, we next examined whether this fraction inhibited the secretion of inflammatory mediators at the transcriptional level. We measured the mRNA levels of iNOS, TNF-α, and cyclo-oxygenase-2 by real-time PCR. Like the upregulation of inflammatory mediators, the mRNA levels of their corresponding genes were also markedly upregulated by LPS, up to 200–1,400-fold (Fig. 2A), similar to findings that have been reported previously [15]. Similarly, PPD-SF strongly decreased the mRNA levels of the genes in a dose-dependent manner (Fig. 2A). Moreover, the promoter-binding activities of AP-1 and IRF3, but not NF-κB, triggered by PMA (Fig. 2B, 2E) and adaptor molecules (TRIF and MyD88) (Fig. 2C, 2D, 2F) were also dose-dependently inhibited by PPD-SF, indicating that this red ginseng fraction could modulate the transcriptional activation of AP-1 and IRF-3. In agreement with these results, this fraction suppressed the nuclear translocation of c-Jun and the phosphorylation of ATF-2 and IRF-3 (Fig. 2G), implying that the nuclear translocation and phosphorylation events of these transcription factors could be targeted by PPD-SF. Considering that red ginseng marc oil was able to block the expression of inflammatory genes in LPS-treated RAW264.7 cells by suppression of NF-κB [33], and that Panax notoginseng saponins were also found to block the NF-κB pathway [34], the pharmacological features of PPD-SF from KRG seem to be distinctive from those of marc oil and P. notoginseng saponins. However, because there is still a possibility that PPD-SF can suppress the activation of NF-κB, we will further evaluate its potential inhibitory activity under LPS-stimulated conditions.
Fig. 2.
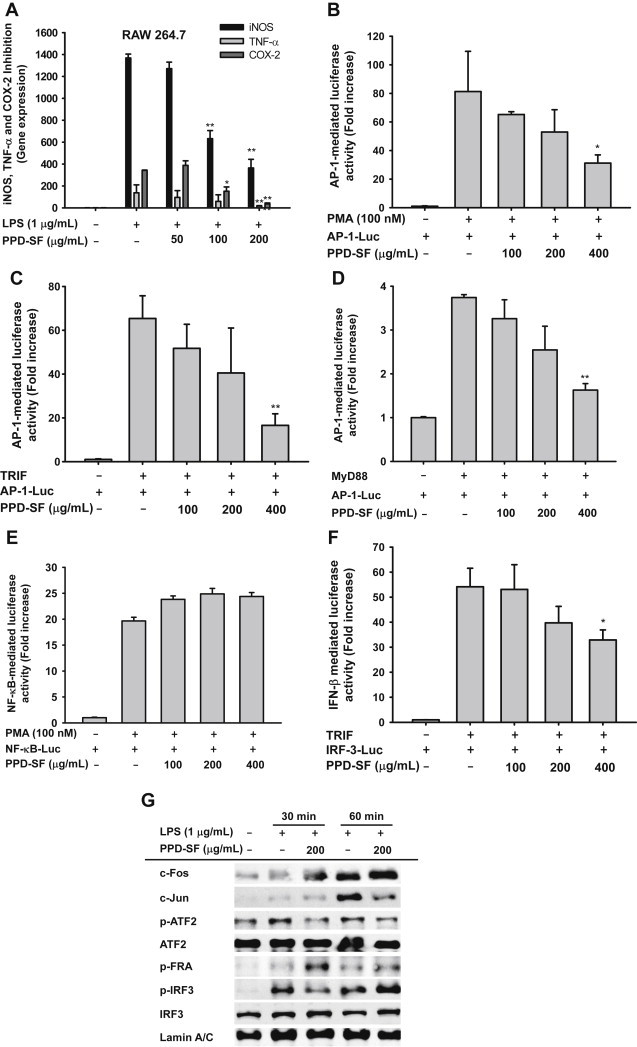
Effect of PPD-SF on the transcriptional regulation of inflammatory genes in LPS-treated RAW264.7 cells. (A) RAW264.7 cells (5×106 cells/mL) were incubated with LPS (1 μg/mL) in the presence or absence of PPD-SF for 6 hours. The mRNA levels of iNOS, TNF-α, and COX-2 were determined by real-time PCR. (B–F) HEK293 cells transfected with plasmid constructs (NF-κB-Luc, AP-1-Luc, and IRF-3-Luc) and β-gal (as a transfection control) were treated with PMA (100nM) or cotransfected with adaptor molecules (MyD88 or TRIF). Then, the cells were further incubated with PPD-SF for 12 hours. Luciferase activity was measured using a luminometer. (G) The phospho- or total protein levels of transcription factors were identified by immunoblotting analysis of the lysates of LPS-treated RAW264.7 cells. *p < 0.05 compared to the control. **p < 0.01 compared to the control. AP = activator protein; COX = cyclo-oxygenase; iNOS = inducible NO synthase; IRF = interferon regulatory factor; LPS = lipopolysaccharide; MyD88 = myeloid differentiation primary response gene 88; NF = nuclear factor; PCR = polymerase chain reaction; PMA = phorbol 12-myristate 13-acetate; PPD-SF = protopanaxadiol saponin fraction; TNF = tumor necrosis factor; TRIF = TIR-domain-containing adapter-inducing interferon-β.
Therefore, we further investigated PPD-SF-targeted molecular events regulating the activation and translocation of AP-1 and IRF-3 in LPS-treated RAW264.7 cells. Previously, it has been reported that ERK, p38, and JNK are major proteins involved in the regulation of AP-1 family activation [35]. TBK1 is also regarded as an important upstream enzyme regulating IRF-3 phosphorylation [4]. PPD-SF clearly suppressed the phosphorylation of p38 from 5 minutes to 30 minutes after treatment, and the phosphorylation of JNK at 15–30 minutes after treatment (Fig. 3A), suggesting that these two enzymes could be directly or indirectly inhibited by PPD-SF. However, the upstream enzymes for p38 and JNK phosphorylation were not inhibited by PPD-SF between 2 minutes and 10 minutes after treatment (Fig. 3B), whereas the phosphorylation of TBK1, the phosphorylating enzyme of IRF-3 [4], was suppressed (Fig. 3C). These results seem to imply that MKK4, MKK6, MKK7, and TBK1 upstream kinase could be directly targeted by this fraction. However, we did not observe any inhibitory effect of PPD-SF in a direct enzyme assay performed with purified MKK4, MKK7, and MKK6, indicating that these enzymes are not targets of PPD-SF. Moreover, we could not test the upstream TBK1-phosphorylating enzyme, because the TBK1-phosphorylating enzymes have not yet been identified [36]. Therefore, we will continue to identify targets specifically inhibited by PPD-SF for the suppression of AP-1 and IRF-3 pathways. Meanwhile, the inhibitory activities of SP600125, a JNK inhibitor, and BX795, a TBK1 inhibitor, on the production of PGE2 (Fig. 3E) strongly suggested the critical involvement of these enzymes in the inflammatory process. Other research groups have also found that the enzymes, JNK and TBK1, play important pathological roles in many different inflammatory responses and symptoms, such as colitis [37–39].
Fig. 3.
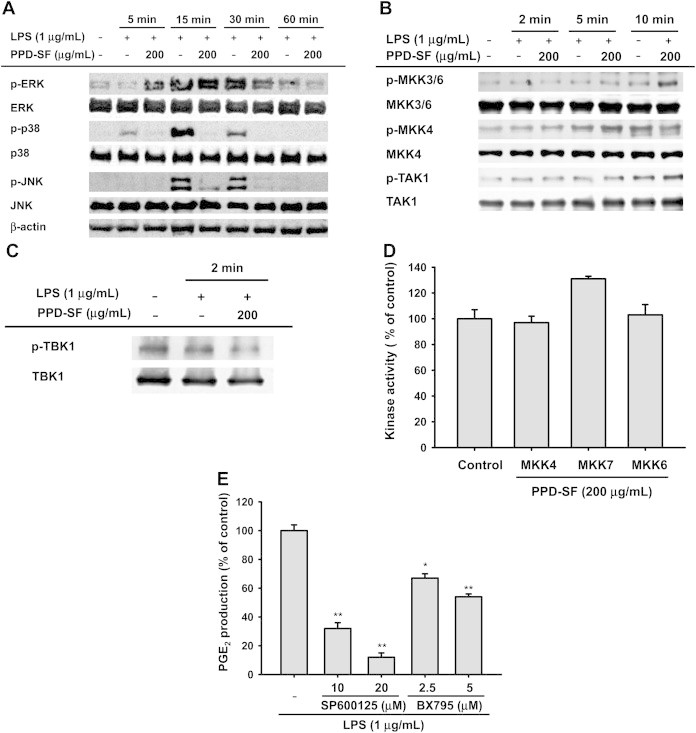
Effect of PPD-SF on the upstream signaling activation of AP-1 and IRF-3 pathways. (A–C) RAW264.7 cells were incubated with LPS (1μg/mL) in the presence or absence of PPD-SF for the indicated times. Total or phospho-levels of ERK, p38, JNK, MKK4, MKK3/6, TAK1, and TBK1 in whole lysates were determined by immunoblotting analysis. (D) Effects of PPD-SF on the kinase activities of MKK4, MKK7, and MKK6 were determined by direct enzyme assays. (E) PGE2 inhibitory activities of SP600125 and BX795 were determined by Griess assay and EIA. *p < 0.05 compared to the control. **p < 0.01 compared to the control. AP = activator protein; COX = cyclo-oxygenase; ERK = extracellular signal-regulated kinase; iNOS = inducible NO synthase; IRF = interferon regulatory factor; JNK = C-Jun N-terminal kinase; LPS = lipopolysaccharide; MKK = mitogen-activated protein kinase kinase; PGE2 = prostaglandin E2; PPD-SF = protopanaxadiol saponin fraction; TAK = transforming growth factor-β-activated kinase 1; TBK = TNAK-binding kinase.
To develop a strong and safe anti-inflammatory remedy, determining whether the preparation is orally active in an in vivo model is critical. Although orally administered KRG-water extract is reported to have anti-inflammatory activity in a mouse inflammation model with allergic rhinitis [40], whether PPD-SF is able to ameliorate in vivo inflammatory symptoms was examined using a HCl/ethanol-induced mouse gastritis model. As Fig. 4A shows, PPD-SF strongly suppressed the formation of gastric ulcer triggered by HCl/ethanol. In particular, it was also revealed that the level of phospho-JNK2 was markedly decreased by PPD-SF, according to immunoblotting analysis with stomach lysates (Fig. 4B). Therefore, these results also strongly suggest that PPD-SF can be an orally effective anti-inflammatory preparation with JNK inhibitory properties.
Fig. 4.
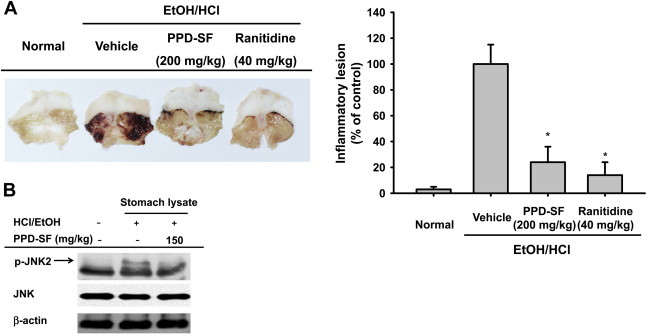
In vivo anti-inflammatory activity of PPD-SF. (A) Mice were orally administered PPD-SF (200 mg/kg) or ranitidine (40 mg/kg) for 3 days and were then orally treated with HCl/ethanol. After 1 hour, gastric stomach lesions were measured with a ruler (right panel), and photographs were taken (left panel). Gastric lesions formed after treatment with inducer alone were set as 100%. (B) Stomachs prepared from HCl/ethanol-treated mice preadministered with PPD-SF were lysed with lysis buffer. Total or phospho-levels of JNK and β-actin were determined by immunoblotting analysis. *p < 0.05 compared to the control. JNK = C-Jun N-terminal kinase; PPD-SF = protopanaxadiol saponin fraction.
In summary, we found that PPD-SF is capable of diminishing in vitro inflammatory responses mediated by macrophage-like RAW264.7 cells treated with LPS and suppressing in vivo gastritis symptoms induced by HCl/ethanol in mice. Through the analysis of transcription factors and their upstream signaling enzymes, it was demonstrated that c-Jun, ATF-2, and IRF-3 and their upstream activation pathways including p38, JNK, and TBK1 could be targeted by PPD-SF, as summarized in Fig. 5. Therefore, our results strongly suggest that PPD-SF can be developed as a KRG-derived anti-inflammatory remedy.
Fig. 5.
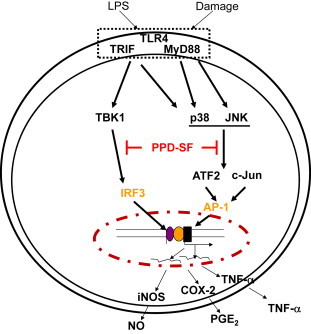
The putative inhibitory pathway of PPD-SF-mediated anti-inflammatory responses. PPD-SF = protopanaxadiol saponin fraction.
Conflicts of interest
The authors declare that there is no conflict of interests regarding the publication of this paper.
Acknowledgments
This work was supported by a grant (2012-2013) from the Korean Society of Ginseng.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Contributor Information
Ji Hye Kim, Email: jhkim1@chonbuk.ac.kr.
Jae Youl Cho, Email: jaecho@skku.edu.
References
- 1.Sodenkamp J., Behrends J., Forster I., Muller W., Ehlers S., Holscher C. gp130 on macrophages/granulocytes modulates inflammation during experimental tuberculosis. Eur J Cell Biol. 2011;90:505–514. doi: 10.1016/j.ejcb.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 2.Roberts-Thomson I.C., Fon J., Uylaki W., Cummins A.G., Barry S. Cells, cytokines and inflammatory bowel disease: a clinical perspective. Expert Rev Gastroenterol Hepatol. 2011;5:703–716. doi: 10.1586/egh.11.74. [DOI] [PubMed] [Google Scholar]
- 3.Ribeiro-Gomes F.L., Silva M.T., Dosreis G.A. Neutrophils, apoptosis and phagocytic clearance: an innate sequence of cellular responses regulating intramacrophagic parasite infections. Parasitology. 2006;132(Suppl.):S61–S68. doi: 10.1017/S0031182006000862. [DOI] [PubMed] [Google Scholar]
- 4.Yu T., Yi Y.S., Yang Y., Oh J., Jeong D., Cho J.Y. The pivotal role of TBK1 in inflammatory responses mediated by macrophages. Mediators Inflamm. 2012;2012:979105. doi: 10.1155/2012/979105. http://dx.doi.org/10.1155/2012/979105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Byeon S.E., Yi Y.S., Oh J., Yoo B.C., Hong S., Cho J.Y. The role of Src kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2012;2012:512926. doi: 10.1155/2012/512926. http://dx.doi.org/10.1155/2012/512926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeon J.W., Park B.C., Jung J.G., Jang Y.S., Shin E.C., Park Y.W. The soluble form of the cellular prion protein enhances phagocytic activity and cytokine production by human monocytes via activation of ERK and NF-kappaB. Immune Netw. 2013;13:148–156. doi: 10.4110/in.2013.13.4.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiurchiu V., Maccarrone M. Chronic inflammatory disorders and their redox control: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2011;15:2605–2641. doi: 10.1089/ars.2010.3547. [DOI] [PubMed] [Google Scholar]
- 8.Przemyslaw L., Boguslaw H.A., Elzbieta S., Malgorzata S.M. ADAM and ADAMTS family proteins and their role in the colorectal cancer etiopathogenesis. BMB Rep. 2013;46:139–150. doi: 10.5483/BMBRep.2013.46.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kang D.H., Kang S.W. Targeting cellular antioxidant enzymes for treating atherosclerotic vascular disease. Biomol Ther (Seoul) 2013;21:89–96. doi: 10.4062/biomolther.2013.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baeg I.H., So S.H. The world ginseng market and the ginseng (Korea) J Ginseng Res. 2013;37:1–7. doi: 10.5142/jgr.2013.37.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Byeon S.E., Lee J., Kim J.H., Yang W.S., Kwak Y.-S., Kim S.Y., Choung E.S., Rhee M.H., Cho J.Y. Molecular mechanism of macrophage activation by red ginseng acidic polysaccharide from Korean red ginseng. Mediators Inflamm. 2012;2012:732860. doi: 10.1155/2012/732860. http://dx.doi.org/10.1155/2012/732860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yayeh T., Jung K.H., Jeong H.Y., Park J.H., Song Y.B., Kwak Y.S., Kang H.S., Cho J.Y., Oh J.W., Kim S.K. Korean Red Ginseng saponin fraction downregulates proinflammatory mediators in LPS stimulated RAW264.7 cells and protects mice against endotoxic shock. J Ginseng Res. 2012;36:263–269. doi: 10.5142/jgr.2012.36.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim H.A., Kim S., Chang S.H., Hwang H.J., Choi Y.N. Anti-arthritic effect of ginsenoside Rb1 on collagen induced arthritis in mice. Int Immunopharmacol. 2007;7:1286–1291. doi: 10.1016/j.intimp.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Kim J.H., Kang S.A., Han S.M., Shim I. Comparison of the antiobesity effects of the protopanaxadiol- and protopanaxatriol-type saponins of red ginseng. Phytother Res. 2009;23:78–85. doi: 10.1002/ptr.2561. [DOI] [PubMed] [Google Scholar]
- 15.Kim M.H., Son Y.J., Lee S.Y., Yang W.S., Yi Y.S., Yoon D.H., Yang Y., Kim S.H., Lee D., Rhee M.H. JAK2-targeted anti-inflammatory effect of a resveratrol derivative 2,4-dihydroxy-N-(4-hydroxyphenyl)benzamide. Biochem Pharmacol. 2013;86:1747–1761. doi: 10.1016/j.bcp.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 16.Kim M.H., Yoo D.S., Lee S.Y., Byeon S.E., Lee Y.G., Min T., Rho H.S., Rhee M.H., Lee J., Cho J.Y. The TRIF/TBK1/IRF-3 activation pathway is the primary inhibitory target of resveratrol, contributing to its broad-spectrum anti-inflammatory effects. Pharmazie. 2011;66:293–300. [PubMed] [Google Scholar]
- 17.Cho J.Y., Baik K.U., Jung J.H., Park M.H. In vitro anti-inflammatory effects of cynaropicrin, a sesquiterpene lactone, from Saussurea lappa. Eur J Pharmacol. 2000;398:399–407. doi: 10.1016/s0014-2999(00)00337-x. [DOI] [PubMed] [Google Scholar]
- 18.Green L.C., Wagner D.A., Glogowski J., Skipper P.L., Wishnok J.S., Tannenbaum S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 19.Gerlier D., Thomasset N. Use of MTT colorimetric assay to measure cell activation. J Immunol Methods. 1986;94:57–63. doi: 10.1016/0022-1759(86)90215-2. [DOI] [PubMed] [Google Scholar]
- 20.Hong Y.J., Yang K.S. Anti-inflammatory activities of crocetin derivatives from processed Gardenia jasminoides. Arch Pharm Res. 2013;36:933–940. doi: 10.1007/s12272-013-0128-0. [DOI] [PubMed] [Google Scholar]
- 21.Kang T.H., Park H.M., Kim Y.B., Kim H., Kim N., Do J.H., Kang C., Cho Y., Kim S.Y. Effects of red ginseng extract on UVB irradiation-induced skin aging in hairless mice. J Ethnopharmacol. 2009;123:446–451. doi: 10.1016/j.jep.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 22.Kim I.W., Sun W.S., Yun B.S., Kim N.R., Min D., Kim S.K. Characterizing a full spectrum of physico-chemical properties of (20S)- and (20R)-ginsenoside Rg3 to be proposed as standard reference materials. J Ginseng Res. 2013;37:124–134. doi: 10.5142/jgr.2013.37.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang R., Zhu J., Cao H.-Z., Xie X.-L., Huang J.-J., Chen X.-H., Luo Z.-Y. Isolation and characterization of LHT-type plant amino acid transporter gene from Panax ginseng Meyer. J Ginseng Res. 2013;37:361–370. doi: 10.5142/jgr.2013.37.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park Y.J., Kim M.J., Kim H.R., Yi M.S., Chung K.H., Oh S.M. Chemopreventive effects of Ginkgo biloba extract in estrogen-negative human breast cancer cells. Arch Pharm Res. 2013;36:102–108. doi: 10.1007/s12272-013-0002-0. [DOI] [PubMed] [Google Scholar]
- 25.Back S.S., Kim J., Choi D., Lee E.S., Choi S.Y., Han K. Cooperative transcriptional activation of ATP-binding cassette sterol transporters ABCG5 and ABCG8 genes by nuclear receptors including Liver-X-Receptor. BMB Rep. 2013;46:322–327. doi: 10.5483/BMBRep.2013.46.6.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu T., Ahn H.M., Shen T., Yoon K., Jang H.J., Lee Y.J., Yang H.M., Kim J.H., Kim C., Han M.H. Anti-inflammatory activity of ethanol extract derived from Phaseolus angularis beans. J Ethnopharmacol. 2011;137:1197–1206. doi: 10.1016/j.jep.2011.07.048. [DOI] [PubMed] [Google Scholar]
- 27.Kim E.H., Lee M.J., Kim I.H., Pyo S., Choi K.T., Rhee D.K. Anti-apoptotic effects of red ginseng on oxidative stress induced by hydrogen peroxide in SK-N-SH cells. J Ginseng Res. 2010;34:138–144. [Google Scholar]
- 28.Lee J.Y., Lee Y.G., Lee J., Yang K.J., Kim A.R., Kim J.Y., Won M.H., Park J., Yoo B.C., Kim S. Akt Cys-310-targeted inhibition by hydroxylated benzene derivatives is tightly linked to their immunosuppressive effects. J Biol Chem. 2010;285:9932–9948. doi: 10.1074/jbc.M109.074872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee Y., Kim J., Jang S., Oh S. Administration of phytoceramide enhances memory and upregulates the expression of pCREB and BDNF in hippocampus of mice. Biomol Ther (Seoul) 2013;21:229–233. doi: 10.4062/biomolther.2013.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okabe S., Miyake H., Awane Y. Cytoprotective effects of NC-1300 and omeprazole on Hcl. ethanol-induced gastric lesions in rats. Jpn J Pharmacol. 1986;42:123–133. doi: 10.1254/jjp.42.123. [DOI] [PubMed] [Google Scholar]
- 31.Lee S.-J., Park J.-Y., Choi K.-S., Ock C.-Y., Hong K.-S., Kim Y.-J., Chung J.-W., Hahm K.-B. Efficacy of Korean red ginseng supplementation on eradication rate and gastric volatile sulfur compound levels after Helicobacter pylori eradication therapy. J Ginseng Res. 2010;34:122–131. [Google Scholar]
- 32.Yang Y., Yang W.S., Yu T., Sung G.H., Park K.W., Yoon K., Son Y.J., Hwang H., Kwak Y.S., Lee C.M. ATF-2/CREB/IRF-3-targeted anti-inflammatory activity of Korean red ginseng water extract. J Ethnopharmacol. 2014;154:218–228. doi: 10.1016/j.jep.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 33.Bak M.J., Hong S.G., Lee J.W., Jeong W.S. Red ginseng marc oil inhibits iNOS and COX-2 via NFkappaB and p38 pathways in LPS-stimulated RAW 264.7 macrophages. Molecules. 2012;17:13769–13786. doi: 10.3390/molecules171213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dou L., Lu Y., Shen T., Huang X., Man Y., Wang S., Li J. Panax notogingseng saponins suppress RAGE/MAPK signaling and NF-kappaB activation in apolipoprotein-E-deficient atherosclerosis-prone mice. Cell Physiol Biochem. 2012;29:875–882. doi: 10.1159/000315061. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y., Kim S.C., Yu T., Yi Y.S., Rhee M.H., Sung G.H., Yoo B.C., Cho J.Y. Functional roles of p38 mitogen-activated protein kinase in macrophage-mediated inflammatory responses. Mediators Inflamm. 2014;2014:352371. doi: 10.1155/2014/352371. http://dx.doi.org/10.1155/2014/352371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark K., Plater L., Peggie M., Cohen P. Use of the pharmacological inhibitor BX795 to study the regulation and physiological roles of TBK1 and IkappaB kinase epsilon: a distinct upstream kinase mediates Ser-172 phosphorylation and activation. J Biol Chem. 2009;284:14136–14146. doi: 10.1074/jbc.M109.000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan L., Liu S., Wang C., Wang F., Song Y., Yan N., Xi S., Liu Z., Sun G. JNK and NADPH oxidase involved in fluoride-induced oxidative stress in BV-2 microglia cells. Mediators Inflamm. 2013;2013:895975. doi: 10.1155/2013/895975. http://dx.doi.org/10.1155/2013/895975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kersting S., Behrendt V., Kersting J., Reinecke K., Hilgert C., Stricker I., Herdegen T., Janot M.S., Uhl W., Chromik A.M. The impact of JNK inhibitor D-JNKI-1 in a murine model of chronic colitis induced by dextran sulfate sodium. J Inflamm Res. 2013;6:71–81. doi: 10.2147/JIR.S40092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abe T., Barber G.N. Cytosolic-DNA-mediated, STING-dependent proinflammatory gene induction necessitates canonical NF-kappaB activation through TBK1. J Virol. 2014;88:5328–5341. doi: 10.1128/JVI.00037-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jung J.H., Kang I.G., Kim D.Y., Hwang Y.J., Kim S.T. The effect of Korean red ginseng on allergic inflammation in a murine model of allergic rhinitis. J Ginseng Res. 2013;37:167–175. doi: 10.5142/jgr.2013.37.167. [DOI] [PMC free article] [PubMed] [Google Scholar]


