Abstract
Background
Glucocorticoids (GCs) are commonly used in many chemotherapeutic protocols and play an important role in the normal regulation of bone remodeling. However, the prolonged use of GCs results in osteoporosis, which is partially due to apoptosis of osteoblasts and osteocytes. In this study, effects of Korean Red Ginseng (KRG) on GC-treated murine osteoblastic MC3T3-E1 cells and a GC-induced osteoporosis mouse model were investigated.
Methods
MC3T3-E1 cells were exposed to dexamethasone (Dex) with or without KRG and cell viability was measured by the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Real-time polymerase chain reaction was performed to evaluate the apoptotic gene expression; osteogenic gene expression and alkaline phosphatase (ALP) activity were also measured. Western blotting was performed to evaluate the mitogen-activated protein kinase (MAPK) proteins. A GC-induced osteoporosis animal model was used for in vivo study.
Results and conclusion
The MTT assay revealed that Korean Red Ginseng (KRG) prevents loss of cell viability caused by Dex-induced apoptosis in MC3T3E1 cells. Real-time polymerase chain reaction data showed that groups treated with both Dex and KRG exhibited lower mRNA levels of caspase-3 and -9, whereas the mRNA levels of Bcl2, IAPs, and XIAP increased. Moreover, groups treated with both Dex and KRG demonstrated increased mRNA levels of ALP, RUNX2, and bone morphogenic proteins as well as increased ALP activity in MC3T3-E1 cells, compared to cells treated with Dex only. In addition, KRG increased protein kinase B (AKT) phosphorylation and decreased c-Jun N-terminal kinase (JNK) phosphorylation. Moreover, microcomputed tomography analysis of the femurs showed that GC implantation caused trabecular bone loss. However, a significant reduction of bone loss was observed in the KRG-treated group. These results suggest that the molecular mechanism of KRG in the GC-induced apoptosis may lead to the development of therapeutic strategies to prevent and/or delay osteoporosis.
Keywords: dexamethasone, Korean Red Ginseng, osteoblast, osteoporosis, Panax ginseng
1. Introduction
Glucocorticoids (GCs) are used most extensively as anti-inflammatory and immunosuppressive drugs to treat a variety of diseases such as inflammation, cancer, and autoimmune disorders. However, protracted usage or a large dose of GC may be the main reason of osteoporosis. GCs have been reported to exhibit detrimental effects on the proliferation and function of osteoblasts. For example, dexamethasone (Dex), a synthetic GC hormone, has been described to inhibit the synthesis of both fibronectin and collagen, as well as stimulating collagenase synthesis [1,2]. Evidence has shown that GCs induce apoptosis in both bone and cartilage, causing excessive or premature loss of osteoblast precursors, osteocytes, and articular and growth plate chondrocytes [3]. The mechanism of GC-induced apoptotic cell death is not elucidated. Weinstein et al [4] demonstrated that prednisone increases the rate of apoptosis in both osteoblasts and osteocytes in adult mice. Gohel et al [5] also reported that corticosterone induces apoptosis in rat and mouse osteoblasts by decreasing the Bcl2/Bax ratio. In addition, Chua et al [6] showed that Dex-induced apoptosis is involved in the activation of several types of caspase genes. All these effects lead to decreased bone formation, ultimately causing bone disease and osteoporosis [7].
For over 2,000 years, ginseng (Panax ginseng Meyer) has been regarded as the most important herbal medicine traditionally in East Asia. Currently, ginseng is one of the extensively used botanical products in the world [8]. It is associated with intrinsic attributes such as antioxidant, anticancer, antidiabetic, and antiadipogenic activities [9,10]. Few studies have investigated the antiosteoporotic activity of ginseng [11]. Korean Red Ginseng (KRG) is essentially Panax ginseng that has been heat processed to enhance its biological and pharmacological activities. KRG and its extracts have been shown to possess multiple pharmacological activities that are useful for treating various human diseases, such as cardiovascular diseases, hypertension, wounds, cerebral ischemia, diabetes mellitus, liver regeneration, antiangiogenesis, and rheumatoid arthritis [12–18]. In recent days, the use of whole ginseng products such as steamed ginseng (KRG), ginseng powder, and ginseng extracts has seen a resurgence in use as alternative medicines in Europe as well as in Asian countries. However, the protective activity of KRG against Dex-induced osteoporosis in vitro and in vivo has not yet been comprehensively explained.
In this study, we determined the protective effects of KRG against Dex-induced apoptosis, as well as the molecular mechanism regulated by KRG in MC3T3-E1 cells in vitro and the alteration of trabecular bone loss in a GC-induced osteoporosis mouse model in vivo.
2. Materials and methods
2.1. Reagents
All the cell culture media and supplements were Gibco products (Life Technologies, Waltham, MA, USA). RNAisol and all polymerase chain reaction (PCR) reagents were obtained from Takara Bio Inc. (Shiga, Japan). Dex, ascorbic acid, β-glycerophosphate, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) were obtained from Sigma-Aldrich (St Louis, MO, USA). Antiphospho-p38 mitogen-activated protein kinase (Thr180/Tyr182), antiphospho-c-Jun N-terminal kinase (p-JNK; Thr183/Tyr185), antiphospho-AKT (p-AKT; ser 473), and anti-β actin antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). KRG extracts were provided by the Korea Ginseng Corporation (Daejeon, Korea) from the roots of a 6-year-old red ginseng (Panax ginseng Meyer) plant harvested in the Republic of Korea. KRG was prepared by steaming fresh ginseng at 90–100°C for 3 h and then drying at 50–80°C. KRG extract was prepared from red ginseng water extract, which was extracted at 85–90°C using three 8-h cycles of circulating hot water. Water content of the pooled extract was 36% of the total weight. KRG was analyzed by high-performance liquid chromatography. The major ginsenosides present in KRG extract were as follows: Rb1, 7.53 mg/g; Rb2, 2.86 mg/g; Rc, 2.86 mg/g; Rd, 0.89 mg/g; Re, 1.90 mg/g; Rf, 1.12 mg/g; Rg1, 1.78 mg/g; Rg2s, 1.12 mg/g; Rg3r, 0.72 mg/g; and Rg3s, 1.37 mg/g; minor ginsenosides were also present.
2.2. Cell culture conditions
Osteoblastic MC3T3-E1 cells (CRL-2593; ATCC, VA, USA) were cultured in a growth medium consisting of minimal essential medium (α-MEM) with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. Cells were incubated in a humid incubator at 37°C (95% O2 and 5% CO2) and maintained in a subconfluent state unless otherwise indicated. Cells were subcultured every 72 h using 0.2% trypsin and 0.02% ethylenediamine tetra-acetic acid. For experiments, cells were cultured for 24 h to obtain monolayers containing α-MEM with 10% FBS. For osteogenic differentiation, when cells reached the confluence, they were cultured in a differentiation medium (α-MEM with 50 μg/mL ascorbic acid and 10mM β-glycerophosphate) in the absence or presence of KRG and/or Dex. The medium was changed every 3 d.
2.3. Cell viability after Dex and KRG treatments
Cell viability was assessed by the MTT assay. Briefly, MC3T3-E1 cells were incubated in 96-well plates and maintained in the growth media for 24 h at 37°C. At 80% confluence, cells were treated with different concentrations of KRG and Dex for 48 h. Then, 10 μL of MTT solution (5 mg/mL) was added to each well, and the cells were incubated for another 4 h at 37°C. After the formation of formazan crystals, the MTT medium was aspirated and replaced with 150 μL of dimethyl sulfoxide (DMSO) for dissolving the formazan crystals. Then, the plates were shaken for 5 min. The absorbance of each well was recorded at 570 nm with a microplate spectrophotometer (Molecular Devices, Sunnyvale, CA, USA). Relative cellular growth was determined by calculating the ratio of the average absorbance in treatment cells to that in control cells. Cell viability was expressed as the ratio of optical densities.
2.4. Determination of alkaline phosphatase activity
To measure alkaline phosphatase (ALP) activity, cells were washed with phosphate-buffered saline twice and sonicated in lysis buffer consisting of 10mM Tris-HCl (pH 7.5), 0.5mM MgCl2, and 0.1% Triton X-100. After centrifugation at 10,000 × g for 20 min at 4°C, ALP activity in the supernatant was indicated in triplicate with the LabAssay ALP kit (Wako Pure Chemicals Industries, Chuo-ku, Osaka, Japan). Protein concentration was analyzed with a bicinchoninic acid protein assay kit (Thermo Pierce, Rockford, IL).
2.5. Isolation of total RNA and quantitative reverse transcription-PCR
Total RNA was isolated with the RNAisol PLUS reagent (Takara Bio Inc.), according to the manufacturer's protocol. The concentration of total RNA was calculated from its absorbance at 260 nm and 280 nm, each with an ND1000 spectrophotometer (Thermo, USA). First-strand cDNA was synthesized with 1 μg of total RNA according to the manufacturer's protocol (Takara Bio Inc.). SYBR-Green-based quantitative real-time PCR was performed using SYBR Primix Ex Taq (Takara Bio Inc.) with the appropriate sense and antisense primers. The primer sets used in this study are shown in Table 1. All reactions were carried out in triplicate and data were analyzed by the 2–ΔΔCT method. Beta-actin was used as an internal standard gene.
Table 1.
List of primer sequence for real-time
| Gene | Forward (5′–3′) | Reverse (5′–3′) |
|---|---|---|
| ALP | GCTGATCATTCCCACGTTTT | CTGGGCCTGGTAGTTGTTGT |
| OCN | AAGCAGGAGGGCAATAAGGT | TTTGTAGGCGGTCTTCAAGC |
| OPN | CGATGATGATGACGATGGAG | TGGCATCAGGATACTGTTCATC |
| RunX2 | GCCGGGAATGATGAGAACTA | GGACCGTCCACTGTCACTTT |
| BMP-2 | GGGACCCGCTGTCTTCTAGT | TCAACTCAAATTCGCTGAGGA |
| BMP-4 | CCTGGTAACCGAATGCTGAT | AGCCGGTAAAGATCCCTCAT |
| BMP-6 | CAACGCCCTGTCCAATGAC | ACTCTTGCGGTTCAAGGAGTG |
| BMP-7 | CGATACCACCATCGGGAGTTC | AAGGTCTCGTTGTCAAATCGC |
| BMP-9 | CAGGTGAGAGCCAAGAGGAG | CCTTTGTGGGGAACTTGAGA |
| Bcl2 | ACTTCTCTCGTCGCTACCGT | CTGTTGACGCTCTCCACACA |
| Bcl-Xl | CGTAGACAAGGAGATGCAGG | TCAGGAACCAGCGGTTGAAG |
| IAP-1 | AAGTGTGTATGGACCGAGAG | GGACCATTAGTCTTGTTCAG |
| IAP-2 | TGTGTATGGACAGAGAGGTT | CAGCTTCTGATGTCCAACAA |
| XIAP | ATACGGAGGATGAGTCAAGT | GGTTGAACGTAATGACGGTG |
| caspase-3 | AGCAGCTTTGTGTGTGTGATTCTAA | AGTTTCGGCTTTCCAGTCAGAC |
| caspase-6 | AGACAAGCTGGACAACGTGACC | CCAGGAGCCATTCACAGTTTCT |
| caspase-7 | GGAGGACTATGGTGATTGGAGC | TAAGGCGCTGGTGGATATGG |
| caspase-9 | GGATCCATGCCCAGACCAGTGGACATTGG | GGTCTAGATTATGATGTTTTAAAGAAAAG |
| β-actin | TCACCCACACTCTGCCCAT | TCCTTAATGTCACGCACCATTT |
PCR, polymerase chain reaction
2.6. Western blot analysis
Treated cells were washed twice with ice-cold phosphate-buffered saline and then solubilized in 100 μL of lysis buffer [20mM Tris-HCl (pH 7.5), 150mM NaCl, 1mM ethylenediamine tetra-acetic acid, 1mM Ethylene glycol tetraacetic acid (EGTA), 1% Triton X-100, 2.5mM sodium pyrophosphate, 1mM β-glycerophosphate, 1mM Na3VO4, 50mM NaF, and 1 μg/mL leupeptin). After a freeze–thaw cycle and vortexing for 1 h at 4°C, the lysate was clarified by centrifugation at 12,000 × g at 4°C for 5 min. The extracts were separated by sodium dodecyl sulfate–polyacrylamide gel electrophoresis and then electroblotted onto a nitrocellulose membrane. The electroblotted membrane was soaked in a blocking solution (5% nonfat dry milk in Tris-buffered saline with 0.1% Tween-20) for 1 h at room temperature. The membrane was then incubated with antibodies overnight at 4°C. The membrane was washed and incubated with horseradish peroxidase-conjugated secondary antibody for 1 h. The blots were finally detected by enhanced chemiluminescence (Amersham Biosciences, Pittsburgh, PA, USA).
2.7. GC-induced osteoporosis animal model
Six-wk-old male Imprinting Control Region (ICR) mice were obtained from Orientbio (Seongnam, Korea). The slow release pellets (Innovative Research of America, Sarasota, FL, USA) of GC (2.1 mg/kg/d prednisolone pellet) were subcutaneously implanted for 5 wks. The GC-implanted mice were divided into four groups: (1) negative control; (2) GC pellet implantation control; (3) GC treated with 100 mg/kg/d of KRG; and (4) GC treated with 500 mg/kg/d of KRG. After 1 wk of GC implantation, mice were orally administered with 100 mg/kg/d or 500 mg/kg/d KRG or saline. After 4 wks of treatment, the mice were euthanized for bone analysis. Radiographic images were taken with a SkyScan1173 microcomputed tomography system (SkyScan, Kontich, Belgium). All animal experimental procedures were approved by the Experimental Animal Ethics Committee at Gachon University, Seongnam, Korea.
2.8. Statistical analysis
All experiments were performed in triplicate. Each value was presented as the mean ± standard deviation. Significant differences were determined using the Sigmaplot program (version 6.0).
3. Results
3.1. Effects of KRG on MC3T3-E1 cell viability
Optimal KRG concentrations for MC3T3-E1 cell viability were determined by the MTT assay. MC3T3-E1 cells (1 × 104 cells/well) were seeded in a plate and treated with various concentrations of KRG for 48 h. The MTT assay indicated that KRG did not affect the cell viability of MC3T3-E1 at concentrations of 1 mg/mL or lower (Fig. 1).
Fig. 1.

Effects of KRG on the viability of MC3T3-E1 cells. Cells were treated with KRG for 48 h. Cell viability was determined using the MTT assay. The experiment was run in triplicate. KRG, Korean Red Ginseng; MTT, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide.
3.2. Inhibitory effects of Dex on the viability of MC3T3-E1 cells
To elucidate whether Dex, an active GC analog, would promote the apoptosis of MC3T3-E1 cells or not, the absorbance of cells was measured by MTT assay. MC3T3-E1 cells were seeded in a 24-well plate for 24 h and then treated with various concentrations of Dex (0μM, 50μM, 125μM, and 250μM) for 48 h. No significant morphological changes occurred at 50μM Dex that could be observed under a light microscope. However, cells treated with 125–250μM Dex underwent apoptosis (data not shown). The MTT assay verified that Dex inhibited cell growth in a dose-dependent manner (Fig. 2). The absorbance of Dex at 125μM in the MTT assay was significantly lower than that of the control group, indicating that the concentration of Dex required to induce half of the MC3T3-E1 cells to go through apoptosis was approximately 125μM.
Fig. 2.
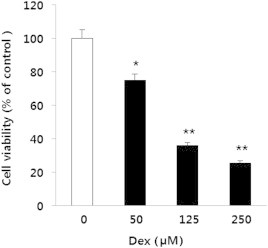
Effect of Dex on the viability of MC3T3-E1 cells. Cells were treated with Dex (0μM, 50μM, 125μM, and 250μM) for 48 h. Treatment with Dex reduced cell viability, which was determined using the MTT assay. *p < 0.05 versus control. **p < 0.01 versus control. Dex, dexamethasone; MTT, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide.
3.3. Effects of KRG on the apoptosis of Dex-exposed MC3T3-E1 cells
To determine whether KRG has protective effects on MC3T3-E1 cells against Dex-induced apoptosis or not, cells were exposed to 100μM Dex and KRG for 48 h. Cell viability was estimated by the MTT assay. A significant decrease in the cell viability of MC3T3-E1 treated with 100μM Dex was observed compared to that of Dex- and KRG-free cells. By contrast, cell viability increased in cells exposed to both Dex and KRG compared to the group treated with only Dex (Fig. 3). These results suggest that KRG prevents Dex-induced apoptosis in MC3T3-E1 cells in a dose-dependent manner.
Fig. 3.

Effect of KRG on the viability of Dex-exposed MC3T3-E1 cells. Cells were treated with KRG at various concentrations and/or cotreated with Dex (100μM) for 48 h. Cell viability was determined using the MTT assay. The experiment was run in triplicate. *p < 0.05 versus Dex control without KRG. **p < 0.01 versus Dex control without KRG. Dex, dexamethasone; KRG, Korean Red Ginseng; MTT, 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide.
3.4. Effects of KRG on the mRNA expression levels of apoptosis-related genes in Dex-exposed MC3T3-E1 cells
Apoptosis is a regulated cellular suicide mechanism that was characterized by nuclear condensation, cell shrinkage, and DNA fragmentation. The increase in MC3T3-E1 cell viability upon treatment with both KRG and Dex suggests that KRG modulates the expression of cell death-related genes. Caspases, a family of cysteine proteases, are the central regulators of apoptosis. To examine the possibility that the expression of these proteins may be modulated, expression levels of both proapoptotic genes (caspase-3, -6, -7, and -9) and antiapoptotic genes (BCL-2, IAPs, and XIPA) were confirmed by quantitative real-time PCR. The treatment of MC3T3-E1 cells with 100μM Dex for 48 h increased the mRNA levels of caspases, whereas cells exposed to Dex and KRG decreased the mRNA levels of caspase-3 and caspase-9 (Fig. 4). However, Dex failed to repress the expression of antiapoptotic genes (BCL-2, IAPs, and XIPA). In fact, Dex significantly upregulated the expression of Bcl-XL, IAP-2, and XIAP (Fig. 5). Therefore, Dex may induce apoptosis by upregulating proapoptotic gene expression.
Fig. 4.
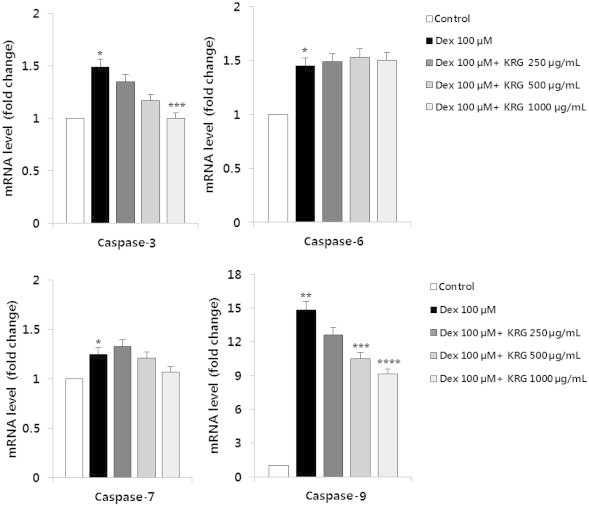
Effect of KRG on mRNA expression level of caspase-3, -6, -7, and -9 in Dex-exposed MC3T3-E1 cells. RNA was extracted from MC3T3-E1 cells treated with various doses of KRG and cotreated with Dex (100μM) for 48 h. The mRNA levels were analyzed using quantitative real-time PCR, and relative expression levels were calculated by comparison with the internal β-actin control. All experiments were performed in triplicate and values are presented as mean ± SD. *p < 0.5 versus control. **p < 0.01 versus control. ***p < 0.5 versus Dex control without KRG. ****p < 0.01 versus Dex control without KRG. Dex, dexamethasone; KRG, Korean Red Ginseng; PCR, polymerase chain reaction; SD, standard deviation.
Fig. 5.

Effect of KRG on mRNA expression level of antiapoptotic genes in Dex-exposed MC3T3-E1 cells. RNA was extracted from MC3T3-E1 cells treated with various doses of KRG and cotreated with Dex (100μM) for 48 h. mRNA levels were analyzed using quantitative real-time PCR, and relative expression levels were calculated by comparison with the internal β-actin control. All experiments were performed in triplicate, and values are presented as mean ± SD. *p < 0.5 versus Dex control without KRG. Dex, dexamethasone; KRG, Korean Red Ginseng; PCR, polymerase chain reaction; SD, standard deviation.
3.5. Effects of KRG on MAPK/AKT signaling in Dex-exposed MC3T3-E1 cells
To survey the molecular mechanism by which KRG exerts its antiapoptotic effects, activation of the MAPK/AKT signaling pathway was examined. MC3T3-E1 cells were incubated with 100μM Dex in the presence or absence of KRG (1 mg/mL) for 24 h. The JNK, p38, and AKT activation states were reviewed by Western blot analysis. When cells were exposed to 100μM Dex, the JNK phosphorylation level increased significantly compared to that of the control, whereas it decreased significantly when treated with both Dex and KRG. Given that AKT activation protects cells from cell apoptosis and cell death, we also investigated whether KRG could induce AKT phosphorylation in Dex-exposed MC3T3-E1 cells or not. When cells were exposed to 100μM Dex, AKT phosphorylation decreased significantly compared to that of the control, whereas it increased significantly when cells were treated with both Dex and KRG (Fig. 6).
Fig. 6.

Effect of KRG on mitogen-activated protein kinase (MAPK) and protein kinase B (AKT) signaling. MC3T3-E1 cells were treated with Dex (100μM) in the presence or absence of KRG (1 mg/mL) for 24 h. After 24-h treatment, Western blot analysis using p-P38, p-JNK, and p-AKT antibody was performed; β-actin was used as a loading control. Dex, dexamethasone; KRG, Korean Red Ginseng; p-AKT, phospho-AKT; p-P38, phospho-p38 mitogen-activated protein kinase; p-JNK, phospho-c-Jun N-terminal kinase.
3.6. Effects of KRG and Dex on the expression of osteogenic gene markers in MC3T3-E1 cells
To determine the effects of KRG on the expression of osteogenic gene markers and ALP activity, cells were treated with various concentrations of KRG and Dex in osteogenic differentiation conditions for 5 d and 7 d. Osteoblastic differentiation was assessed by using quantitative real-time PCR, by measuring the mRNA expression levels of ALP, bone morphogenic proteins (BMPs), osteopontin (OPN), RUNX2, and osteocalcin (OCN). DEX-treated cells showed decreased ALP activity, but in cells treated with Dex and KRG (30 μg/mL and 60 μg/mL; Fig. 7A) this activity was increased significantly. Based on quantitative real-time PCR, cells treated with 100μM Dex exhibited decreased mRNA expression levels of ALP, OCN, OPN, RUNX2, BMP-2, -6, -7, and -9, whereas these expression levels increased in cells treated with both Dex and KRG (Fig. 7B).
Fig. 7.
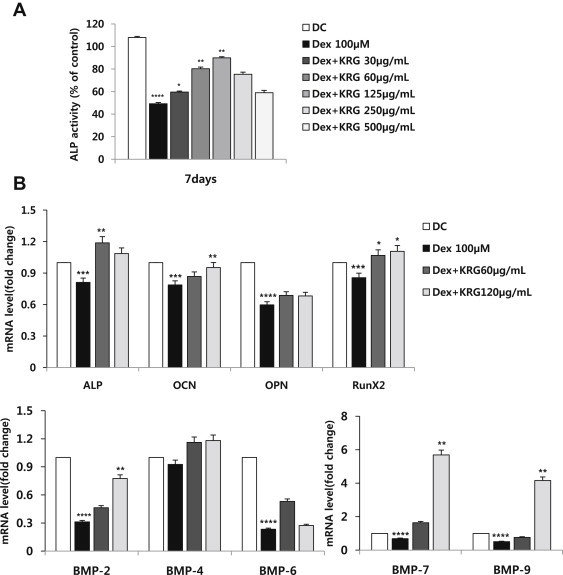
Effect of KRG and 100μM Dex on (A) ALP activity and (B) mRNA expression of MC3T3-E1 cell differentiation. Cells were treated with KRG at various concentrations and/or cotreated with Dex (100μM) for (A) 7 d and (B) 5 d. The mRNA levels were analyzed using quantitative real-time PCR, and relative expression levels were calculated by comparison with the internal β-actin control. *p < 0.05 versus Dex. **p < 0.01 versus Dex. ***p < 0.05 versus DC. ****p < 0.01 versus DC. ALP, alkaline phosphatase; BMP, bone morphogenic protein; DC, differentiation control; Dex, dexamethasone; KRG, Korean Red Ginseng; OCN, osteocalcin; OPN, osteopontin; PCR, polymerase chain reaction.
3.7. Effects of KRG in a GC-induced osteoporosis animal model
To investigate the effects of KRG in a GC-induced osteoporosis model, mice implanted with prednisolone pellets were given KRG (100 mg/kg or 500 mg/kg) orally. In 5 wks, bone loss was measured by microcomputed tomography. Trabecular bone loss in the femur was observed in the GC control group. However, mice in the oral KRG-treated group showed a significant reduction in bone loss (Fig. 8).
Fig. 8.

Effect of KRG on glucocorticoid-induced osteoporosis mice model. Slow release pellets of prednisolone (PDS) (2.1 mg/kg/d) were administrated for 35 d by subcutaneous implantation. One wk after implantation, the mice were orally administrated with KRG extract (100 mg/kg and 500 mg/kg) or saline. After 4 wks, the mice were euthanized for bone analysis. The femurs were imaged with a micro-CT machine. CT, computed tomography; KRG, Korean Red Ginseng; PDS.
4. Discussion
In addition to their use in patients undergoing organ transplantation, GCs have been used in the treatment of autoimmune, pulmonary, and gastrointestinal disorders. A common side effect of long-term GC therapy is reduced bone density, which is the most prevalent form of secondary osteoporosis after menopause. Increased osteoblast apoptosis has been demonstrated in patients with GC-induced osteoporosis [19]. Mice implanted with GCs also have a higher number of apoptotic osteoblasts that inhibit bone formation [20]. In vitro studies have also revealed that GCs can induce the apoptosis of osteoblasts [21]. These findings indicate that increased osteoblast apoptosis is responsible for GC-induced bone loss or osteoporosis.
The apoptotic pathway with multiple interacting components is complicated, and the important steps in this cascade involve caspase enzymes, which are a family of proteins that play a role in the degradation of cells targeted to undergo apoptosis. Caspase-3 is an effector caspase that cleaves nucleases as well as cellular substrates, and caspase-9 is an initiator caspase that is involved in mitochondrial damage [6]. Furthermore, several reports demonstrated that the Extracellular signal-regulated kinase (ERK) activation is essential for cell survival, whereas the activation of JNK and p38 plays an important role in cell death signaling [22,23]. The phosphatidylinositol 3-kinase/AKT pathway is also viewed as a key factor for cell survival in different cell systems [24]. Notably, the inhibition of the phosphatidylinositol 3-kinase pathway and subsequent AKT phosphorylation appear to be important mechanisms of Dex-induced apoptosis. In the present study, the mRNA levels of caspase-3, -6, -7, and -9 in cells treated with both Dex and KRG were observed to decrease compared to those in cells treated with Dex only. This antiapoptotic effect also appeared to be involved in p-AKT activation and p-JNK inhibition.
Bone-forming osteoblasts are derived from mesenchymal precursor cells, and the maturation of preosteoblasts differentiated from mesenchymal precursor cells plays a role in the rebuilding of resorbed bone by elaborating a matrix that becomes mineralized. These preosteoblasts become committed by signals for the activation of osteogenic genes, which are recognizable near the bone surface due to their proximity to surface osteoblasts and the histochemical detection of ALP enzyme activity, one of the earliest markers of the osteoblast phenotype. The active mature osteoblast on the bone surface is distinguished by its morphological properties as well as by the temporal expression of several noncollagenous proteins such as OCN and OPN, which act as markers for mature osteoblasts [25].
BMPs play a major role in the growth and differentiation of osteoblastic cells and have been shown to be potent stimulators of bone formation in various animal models. BMP-2 stimulates the expression of osteogenic genes, such as OCN and ALP [26]. Furthermore, osteogenic BMPs such as BMP-2 and BMP-7 have recently been approved for clinical application in spinal fusion, fracture healing, and dental tissue engineering. Anabolic agents that stimulate BMP expression or its signaling pathway can be used to treat osteoblast-related diseases via bone formation or regeneration [27,28]. Loss of both BMP-2 and -4 has been shown to result in severe impairment of osteogenesis [29]. The network of activities and molecular switches for bone development and osteoblastic differentiation involves BMP-induced transcription factors such as RUNX2. RUNX2 is one of the osteogenic master transcription factors, and its activity is increased by BMP-2 signaling [30,31]. In this study, we observed that the mRNA levels of ALP, OCN, OPN, RUNX2, and BMPs (BMP-2, -6, -7, and -9) in cells treated with both Dex and KRG were slightly higher than those in cells treated with only Dex.
Current research efforts aim to prevent GC-induced osteoporosis and decrease the incidence of fractures. However, few studies have investigated how to improve the repair of fractures that have occurred during GC treatment. A parathyroid hormone-related protein analog has been shown to be effective for impaired bone healing in rabbits receiving corticosteroid therapy. Receptor activator of nuclear factor kappa-B ligand (RANKL), BMP-2, and BMP-7 have been shown to inhibit bone loss in GC-treated animals [32]. In addition, the current study verified that KRG increased bone formation in GC-induced osteoporosis mice model.
In conclusion, GCs have significant pharmacological effects on bone metabolism, including suppression of bone formation and bone resorption. We observed that KRG prevented synthetic GC (Dex)-induced apoptosis of MC3T3-E1 cells by inhibiting the activation of caspase-3 and -9. Gene expression of the osteogenic gene markers (ALP, OCN, OPN, Runx2, and BMPs) was enhanced in cells treated with both Dex and KRG compared to that in cells treated with Dex only. Furthermore, our data indicate that KRG-treated cells not only activated p-AKT, but also inhibited p-JNK. KRG also increased bone formation in GC-induced osteoporosis mice. Thus, KRG can be used as a beneficial therapeutic for the prevention or treatment of GC-induced osteoporosis.
Conflict of interest
All authors have no conflict of interest to declare.
Acknowledgments
This research was sponsored by the grant 2012 from the Korea Ginseng Corporation (GS302-38). The animal experiment in this study was supported by the Experimental Animal Ethics Committee at Gachon University (GC2012-0118).
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Advani S., LaFrancis D., Bogdanovic E., Taxel P., Raisz L.G., Kream B.E. Dexamethasone suppresses in vivo levels of bone collagen synthesis in neonatal mice. Bone. 1997;20:41–46. doi: 10.1016/s8756-3282(96)00314-6. [DOI] [PubMed] [Google Scholar]
- 2.Canalis E., Delany A.M. Mechanisms of glucocorticoid action in bone. Ann N Y Acad Sci. 2002;966:73–81. doi: 10.1111/j.1749-6632.2002.tb04204.x. [DOI] [PubMed] [Google Scholar]
- 3.Zalavras C., Shah S., Birnbaum M.J., Frenkel B. Role of apoptosis in glucocorticoid-induced osteoporosis and osteonecrosis. Crit Rev Eukaryot Gene Expr. 2003;13:221–235. [PubMed] [Google Scholar]
- 4.Weinstein R.S., Jilka R.L., Parfitt A.M., Manolagas S.C. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids. Potential mechanisms of their deleterious effects on bone. J Clin Invest. 1998;102:274–282. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gohel A., McCarthy M.B., Gronowicz G. Estrogen prevents glucocorticoid-induced apoptosis in osteoblasts in vivo and in vitro. Endocrinology. 1999;140:5339–5347. doi: 10.1210/endo.140.11.7135. [DOI] [PubMed] [Google Scholar]
- 6.Chua C.C., Chua B.H.L., Chen Z., Landy C., Hamdy R.C. Dexamethasone induces caspase activation in murine osteoblastic MC3T3-E1 cells. Biochim Biophys Acta Mol Cell Res. 2003;1642:79–85. doi: 10.1016/s0167-4889(03)00100-9. [DOI] [PubMed] [Google Scholar]
- 7.Weinstein R.S., Manolagas S.C. Apoptosis and osteoporosis. Am J Med. 2000;108:153–164. doi: 10.1016/s0002-9343(99)00420-9. [DOI] [PubMed] [Google Scholar]
- 8.Kim D.H. Chemical diversity of Panax ginseng, Panax quinquifolium, and Panax notoginseng. J Ginseng Res. 2012;36:1–15. doi: 10.5142/jgr.2012.36.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim H.G., Yoo S.R., Park H.J., Lee N.H., Shin J.W., Sathyanath R., Cho J.H., Son C.G. Antioxidant effects of Panax ginseng C.A. Meyer in healthy subjects: a randomized, placebo-controlled clinical trial. Food Chem Toxicol. 2011;49:2229–2235. doi: 10.1016/j.fct.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Shin H.R., Kim J.Y., Yun T.K., Morgan G., Vainio H. The cancer-preventive potential of Panax ginseng: a review of human and experimental evidence. Cancer Causes Control. 2000;11:565–576. doi: 10.1023/a:1008980200583. [DOI] [PubMed] [Google Scholar]
- 11.Siddiqi M.H., Siddiqi M.Z., Ahn S., Kang S., Kim Y.J., Sathishkumar N., Yang D.U., Yang D.C. Ginseng saponins and the treatment of osteoporosis: mini literature review. J Ginseng Res. 2013;37:261–268. doi: 10.5142/jgr.2013.37.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konoshima T., Takasaki M., Tokuda H., Nishino H., Duc N.M., Kasai R., Yamasaki K. Anti-tumor-promoting activity of majonoside-R2 from Vietnamese ginseng, Panax vietnamensis Ha et Grushv. (I) Biol Pharm Bull. 1998;21:834–838. doi: 10.1248/bpb.21.834. [DOI] [PubMed] [Google Scholar]
- 13.Morisaki N., Watanabe S., Tezuka M., Zenibayashi M., Shiina R., Koyama N., Kanzaki T., Saito Y. Mechanism of angiogenic effects of saponin from ginseng Radix rubra in human umbilical vein endothelial cells. Br J Pharmacol. 1995;115:1188–1193. doi: 10.1111/j.1476-5381.1995.tb15023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura Y., Sumiyoshi M., Kawahira K., Sakanaka M. Effects of ginseng saponins isolated from Red Ginseng roots on burn wound healing in mice. Br J Pharmacol. 2006;148:860–870. doi: 10.1038/sj.bjp.0706794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon Y.S., Jang K.H. The effect of Korean Red Ginseng on liver regeneration after 70% hepatectomy in rats. J Vet Med Sci. 2004;66:193–195. doi: 10.1292/jvms.66.193. [DOI] [PubMed] [Google Scholar]
- 16.Han K., Shin I.C., Choi K.J., Yun Y.P., Hong J.T., Oh K.W. Korea Red Ginseng water extract increases nitric oxide concentrations in exhaled breath. Nitric Oxide. 2005;12:159–162. doi: 10.1016/j.niox.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Choi K.S., Song H., Kim E.H., Choi J.H., Hong H., Han Y.M., Hahm K.B. Inhibition of Hydrogen sulfide-induced angiogenesis and inflammation in vascular endothelial cells: potential mechanisms of gastric cancer prevention by Korean Red Ginseng. J Ginseng Res. 2012;36:135–145. doi: 10.5142/jgr.2012.36.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shah Z.A., Gilani R.A., Sharma P., Vohora S.B. Cerebroprotective effect of Korean ginseng tea against global and focal models of ischemia in rats. J Ethnopharmacol. 2005;101:299–307. doi: 10.1016/j.jep.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 19.Weinstein R.S. Glucocorticoid-induced osteoporosis. Rev Endocr Metab Disord. 2001;2:65–73. doi: 10.1023/a:1010007108155. [DOI] [PubMed] [Google Scholar]
- 20.O'Brien C.A., Jia D., Plotkin L.I., Bellido T., Powers C.C., Stewart S.A., Manolagas S.C., Weinstein R.S. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145:1835–1841. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y., Porta A., Peng X., Gengaro K., Cunningham E.B., Li H., Dominguez L.A., Bellido T., Christakos S. Prevention of glucocorticoid-induced apoptosis in osteocytes and osteoblasts by calbindin-D28k. J Bone Miner Res. 2004;19:479–490. doi: 10.1359/JBMR.0301242. [DOI] [PubMed] [Google Scholar]
- 22.Tian X.Y., Yung L.H., Wong W.T., Liu J., Leung F.P., Liu L., Chen Y., Kong S.K., Kwan K.M., Ng S.M. Bone morphogenic protein-4 induces endothelial cell apoptosis through oxidative stress-dependent p38MAPK and JNK pathway. J Mol Cell Cardiol. 2012;52:237–244. doi: 10.1016/j.yjmcc.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh W.T., Lin H.Y., Chen J.H., Kuo Y.H., Fan M.J., Wu R.S., Wu K.C., Wood W.G., Chung J.G. Latex of Euphorbia antiquorum induces apoptosis in human cervical cancer cells via c-jun n-terminal kinase activation and reactive oxygen species production. Nutr Cancer. 2011;63:1339–1347. doi: 10.1080/01635581.2011.608481. [DOI] [PubMed] [Google Scholar]
- 24.Zhuang Z., Zhao X., Wu Y., Huang R., Zhu L., Zhang Y., Shi J. The anti-apoptotic effect of PI3K-Akt signaling pathway after subarachnoid hemorrhage in rats. Ann Clin Lab Sci. 2011;41:364–372. [PubMed] [Google Scholar]
- 25.Eijken M., Koedam M., van Driel M., Buurman C.J., Pols H.A., van Leeuwen J.P. The essential role of glucocorticoids for proper human osteoblast differentiation and matrix mineralization. Mol Cell Endocrinol. 2006;248:87–93. doi: 10.1016/j.mce.2005.11.034. [DOI] [PubMed] [Google Scholar]
- 26.Huang W., Rudkin G.H., Carlsen B., Ishida K., Ghasri P., Anvar B., Yamaguchi D.T., Miller T.A. Overexpression of BMP-2 modulates morphology, growth, and gene expression in osteoblastic cells. Exp Cell Res. 2002;274:226–234. doi: 10.1006/excr.2002.5483. [DOI] [PubMed] [Google Scholar]
- 27.Issack P.S., DiCesare P.E. Recent advances toward the clinical application of bone morphogenetic proteins in bone and cartilage repair. Am J Orthop. 2003;32:429–436. [PubMed] [Google Scholar]
- 28.Govender S., Csimma C., Genant H.K., Valentin-Opran A., Amit Y., Arbel R., Aro H., Atar D., Bishay M., Borner M.G. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: a prospective, controlled, randomized study of four hundred and fifty patients. J Bone Joint Surg Am. 2002;84-A:2123–2134. doi: 10.2106/00004623-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 29.Pizette S., Niswander L. Early steps in limb patterning and chondrogenesis. Novartis Found Symp. 2001;232:23–36. doi: 10.1002/0470846658.ch3. discussion 36–46. [DOI] [PubMed] [Google Scholar]
- 30.Lian J.B., Stein G.S., Javed A., van Wijnen A.J., Stein J.L., Montecino M., Hassan M.Q., Gaur T., Lengner C.J., Young D.W. Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord. 2006;7:1–16. doi: 10.1007/s11154-006-9001-5. [DOI] [PubMed] [Google Scholar]
- 31.Phimphilai M., Zhao Z., Boules H., Roca H., Franceschi R.T. BMP signaling is required for RUNX2-dependent induction of the osteoblast phenotype. J Bone Miner Res. 2006;21:637–646. doi: 10.1359/JBMR.060109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hofbauer L.C., Zeitz U., Schoppet M., Skalicky M., Schuler C., Stolina M., Kostenuik P.J., Erben R.G. Prevention of glucocorticoid-induced bone loss in mice by inhibition of RANKL. Arthritis Rheum. 2009;60:1427–1437. doi: 10.1002/art.24445. [DOI] [PubMed] [Google Scholar]


