Abstract
Background: Hirudotherapy is often used successfully in modern medicine, especially in plastic and reconstructive surgery. However, Aeromonas infections are the most common complications of post-operative leech application. Hence, prophylactic antibiotic administration is recommended before and during leech therapy. It has been confirmed that patient safety and achieving the desired therapeutic effect depend mainly on the microbiologic purity of the animals used. The aims of this study were to find a safe and practical way to eradicate symbiotic Aeromonas spp. occuring in the intestine of Hirudo verbana.
Methods: Leeches were fed artificially with 1.5 mL of sterile defibrinated sheep blood supplemented with ciprofloxacin (CIP) or cefotaxime (CTX), at bacteriostatic concentrations of 0.2 mcg/mL or 1.5 mcg/mL, and bactericidal concentrations of 20 mcg/mL or 50 mcg/mL, respectively. Bacteria were isolated from the leech intestines before and after feeding at different time intervals: 1, 7, 14, 21, and 28 d.
Results: Biochemical identification of bacterial isolates from water samples and intestines of H. verbana using the API-NE20 test showed that A. veronii biovar sobria was predominant. Bacteria belonging to the genus Aeromonas were detected in all control leeches. The results showed that optimum eradication of bacteria from leech intestines was obtained using 20 mcg/mL of CIP and 50 mcg/mL of CTX, which decreased the number of Aeromonas spp. to undetectable levels for two weeks after feeding in all treated leeches. A statistically significant reduction in the number of bacterial colonies (p<0.0001) was observed in leeches treated with bacteriostatic concentrations of CIP or CTX; no bacterial growth was found on the plates after only seven days of feeding with antibiotics. All water samples in which the leeches were kept before treatment were contaminated with Aeromonas spp., whereas these samples were negative after antibiotic feeding of animals.
Conclusions: All leeches were ready to take a blood meal after treatment, suggesting the possibility of using ciprofloxacin-treated or cefotaxime-treated leeches instead of chemoprophylaxis in patients undergoing hirudotherapy.
Leech therapy has been used in various human diseases since ancient Egyptian times. The leeches were originally used mainly for blood-letting, which was regarded as the panacea for all ailments. The discovery in 1884 of hirudin, an anti-coagulant substance in leech saliva, led to increased popularity of hirudotherapy for the treatment of venous insufficiency, varicose veins, hemorrhoids, and other venous diseases [1]. The subsequent recognition of a number of antithrombotic substances, vasodilators, and anti-inflammatory agents in the saliva of Hirudo medicinalis generated renewed interest in the use of leech therapy in various medical specialties, especially in replantation, reconstructive, and plastic surgery [2–5]. However, the application of leeches is associated with a risk of microbial infection, especially symbiotic Aeromonas spp., which may vary from minor wound complications to serious illnesses such as meningitis, bacteremia, and sepsis, especially in immunocompromised patients [6–9]. The transmission of the bacterial flora of leeches to patients can occur in several ways, the most common being inoculation of pathogens into the feeding site via the saliva, and by close contact between the body of the leech and the wound. Additionally, the ingested blood in the leech digestive tract could be re-injected into the host, along with endosymbiotic bacteria, by regurgitation during the manipulation of leech removal [10].
It has been confirmed that the blood-sucking leech is a potential source of many human and animal pathogens including viruses, bacteria, protozoa, and fungi [11–13]. However, the symbiotic bacterial species Aeromonas veronii biovar sobria and Aeromonas hydrophila living in the leech digestive tract are the most common pathogens responsible for the infection of patients [14]. A high incidence of Aeromonas transmission to human beings (from 2.4% to 36%) has been noted after application of medicinal leeches [8,15]. Aeromonas colonization of necrotic tissue has been reported, lasting between 24 h to more than 10 d after leech application [16]. Nosocomial infections with other bacteria such as Morganella morganii, Klebsiella pneumoniae, Serratia marcesens, Vibrio fluvialis, and Pseudomonas spp., associated with complications after hirudotherapy, are also described, although less frequently [3,17,18]. To avoid serious bacterial complications, prophylactic antibiotics have been recommended before and during hirudotherapy. Patients with open wounds should continue oral antibiotic therapy until wound closure. Effective antibiotic therapy against Aeromonas spp. comprises third-generation cephalosporins, ciprofloxacin, aminoglycosides, tetracycline, and trimethoprim-sulfamethoxazole. However, the use of antibacterial drugs is often associated with side effects leading to worsening of the condition [4].
To ensure the safety of patients undergoing hirudotherapy, and to reduce the cost of treating infectious complications, there is a need to find effective methods to reduce the risk of bacterial infection during contact with leeches. Only a few experimental attempts at external sterilization have been done to control Aeromonas populations on the body surface and in the digestive tract of medicinal leeches, in order to avoid chemoprophylaxis [4,15,19]. Unfortunately, external chemical decontamination proved unsuccessful and resulted in impaired leech sucking function [20–22]. According to Mumcuoglu et al. [23], the elimination of endosymbiotic bacteria by feeding leeches artificially with antibiotics appears to be a more effective method than immersing them in sterilizing solutions.
The aim of this study was the elimination of symbiotic Aeromonas spp. from the digestive tract of Hirudo verbana by feeding with ciprofloxacin and cefotaxime. A practical method of decontamination of leeches while preserving the sucking ability will prevent the transmission of pathogenic bacteria to patients without the use of chemoprophylaxis.
Materials and Methods
Animals
The farm-raised Hirudo verbana used in this study were obtained from the Hirudinea Natural Medicine Center (Lodz, Poland). Before the experiment, 260 animals were maintained without feeding in leech tanks containing boiled mineral water at a temperature of around 7°C. Twenty-five leeches were kept in each 5L container for three weeks prior to the experiment. The water was changed weekly.
Antibiotics used for elimination of intestinal bacteria
The selection of antibiotics used in the present study was based on the results of our previous research, in which antimicrobial susceptibility of isolated bacteria from the surface, jaws, and intestine of Hirudo verbana was examined. Of the tested drugs, two antibiotics, ciprofloxacin and cefotaxime, were selected for intestinal decontamination based on their susceptibility, solubility, and therapeutic effectiveness against Aeromonas infection. All strains of isolated Aeromonas spp. were sensitive to CIP and CTX. The concentrations of the above antibiotics used in the present study were estimated based on the minimum inhibitory concentration (MIC) values earlier determined by E-test (bioMerieux, Marcy a'Etoile, France) (unpublished data). Sterile sheep blood was supplemented with bacteriostatic or bactericidal concentrations of these drugs, which amounted to 0.2 mcg/mL or 20 mcg/mL for CIP and 1.5 mcg/mL or 50 mcg/mL for CTX, respectively.
Artificial feeding
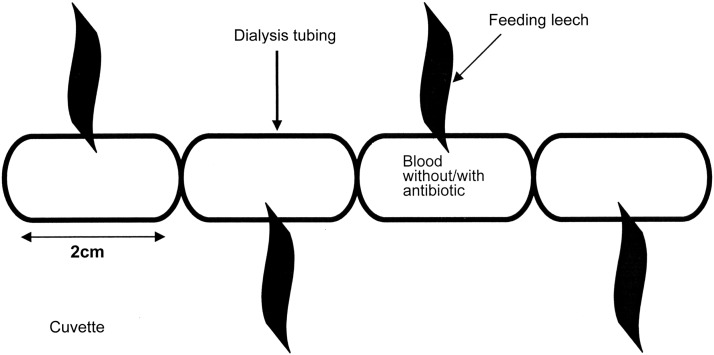
The leeches were divided into five groups: One control, fed without antibiotic, and four experimental groups, fed with antibiotic. All leeches were fed on 1.5 mL of sterile defibrinated sheep blood (GRASO Biotech, Poland). Each experimental group of 50 leeches received blood-meal with either 2 mcg/mL CIP, 20 mcg/mL CIP, 1.5 mcg/mL CTX or 50 mcg/mL CTX, depending on the group. The blood was warmed to 37°C and placed in 21-mm diameter SERVAPOR® sterile dialysis tubing membrane (Serva Electrophoresis GmbH, Heidelberg, Germany), which was tied with a thread every two cm to form 1.5 mL containers for the blood. This tubing was then moistened with 4% arginine solution in 0.85% NaCl, used as a phagostimulant [24], and then placed in a sterile cuvette. Next, one leech was attached to each container (Fig. 1). The amount of blood consumed by each individual leech was about 1.5 mL. The sign that the leech had fed was the empty container. After feeding, the leeches were maintained separately in water containing either 20 mg/L CIP or 50 mg/L CTX and examined periodically for the presence of intestinal bacteria.
FIG 1.
Schematic representation of the artificial feeding of leeches using dialysis tubing.
Isolation of digestive tract flora
Bacteria were isolated from the leech intestines before and after feeding. The intestines were dissected at different time intervals: 1, 7, 14, 21 and 28 d after consuming blood. All leeches were held with sterile gloves and prepared in sterile conditions. Before dissection, the anterior and posterior ends of the animal were tied off with string and the animal was submerged for three minutes in 70% ethanol for euthanasia and to disinfect the body surface [25].
The leech intestines were prepared under a stereomicroscope, as 20x magnification, according to Worthen et al. [25]. A longitudinal incision of intestine was made and each organ was placed in a sterile tube with 1 mL of 0.85% NaCl. The contents of the tube were shaken for 10 min and 0.5 mL of fluid was transferred to 5% sheep blood agar (bioMerieux). Bacterial growth was observed after 24–48 h of incubation at 37°C, when a colony count was carried out for every cultured plate. Bacterial macrocultures were subcultured to obtain pure isolates. Oxidase-positive organisms that grew on MacConkey agar (bioMerieux) and fermented glucose were identified using the API 20NE test (bioMerieux).
Bacteriologic examination of water samples
The microbiologic purity of the water in which the leeches were kept before and after being fed with blood was examined as described previously [13]. Samples (20 mL) were concentrated by centrifugation at 5,000×g for 20 min at 20°C in sterile test tubes, and 1 mL volumes of the suspensions were transferred onto 5% sheep blood agar. The cultures were then incubated at 37°C and examined after 48 h. As the water in all leech containers was changed every week, water samples were collected after seven days of leech incubation.
Sucking tests
To assess the readiness of leeches to take a blood meal after antibiotic treatment, three leeches from each experimental group were selected randomly for sucking tests on rabbits. Two leeches were attached to each rabbit ear. They sucked blood for approximately 10 min. Blood feeding was stopped when the leeches were observed to excrete plasma from ingested blood meals. Blood sucking was stopped by touching the leech with a tampon moistened with 70% ethanol.
Statistical analysis
The Fisher exact test was used to compare differences between proportions. The Mann-Whitney U test was used to compare the average numbers of bacterial colonies counted. Values of p<0.05 were taken as significant. All calculations were performed using STATISTICA v. 10 software (StatSoft, Tulsa, OK).
Results
All leeches from control and experimental groups willingly sucked the blood from dialysis tubing, and all survived feeding on blood supplemented with antibiotics. Moreover, all examined leeches for the sucking test sucked rabbit blood successfully, independent of antibiotic concentrations.
Aeromonas spp. was detected in all control leeches both before and after feeding on blood without antibiotics (Table 1). All cultures from the intestines of these leeches were positive for four weeks after feeding, i.e., to the end of the experiment, and the number of colonies growing on plates peaked after 24 h of feeding on sterile blood (Table 1). The differences between the mean numbers of colonies before and after blood consumption were found to be significant (p<0.0001). The optimum eradication of bacteria from intestine leeches was obtained by using 20 mcg/mL for CIP, and 50 mcg/mL for CTX (Tables 2 and 3), which reduced the counts of Aeromonas spp. to undetectable levels for two weeks after feeding in all treated leeches. Blood containing bacteriostatic concentrations of CIP or CTX significantly (p<0.0001) decreased the numbers of bacterial colonies compared with controls. In these cases, from two to four weeks after consumption, only single colonies (1–3) were observed on plates, and the mean number of colonies did not exceed 1.6 per leech; no bacterial growth was observed on the plates after seven days of feeding (Tables 2 and 3). Both CIP and CTX had similar effectiveness in eradicating intestinal bacteria (Table 4) (p>0.05).
Table 1.
Bacterial Growth of Intestine Cultures on the Plates from Control Hirudo verbana before and after Feeding on Blood without Antibiotic
| No. of bacterial colonies from individual leeches N=10 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | Mean±SD | |
| Before feeding | 24 | 31 | 21 | 43 | 39 | 45 | 54 | 19 | 27 | 33 | 33.6±11.44 |
| After feeding: | |||||||||||
| 1 d | 270 | 188 | 213 | 169 | 254 | 124 | 111 | 147 | 92 | 176 | 174.4±58.96* |
| 7 d | 256 | 214 | 192 | 177 | 163 | 143 | 134 | 117 | 130 | 112 | 163.8±46.34* |
| 14 d | 120 | 112 | 187 | 134 | 149 | 118 | 128 | 97 | 111 | 115 | 127.1±25.36* |
| 21 d | 114 | 97 | 111 | 133 | 145 | 113 | 99 | 86 | 90 | 86 | 107.4±19.87* |
| 28 d | 94 | 137 | 87 | 123 | 91 | 69 | 76 | 98 | 82 | 81 | 93.8±21.15* |
P<0.0001-significant differences between mean number of colonies before and after feeding.
Table 2.
Comparison of Bacterial Growth from Intestine Cultures on Plates from CIP–Treated Leeches
| Leeches feeding with CIP | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.2 mcg/mL | 20 mcg/mL | |||||||||||||||
| Day after feeding | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Mean no. of colonies per leech±SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Mean no. of colonies per leech±SD |
| 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 7 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 14 | - | - | - | - | +(1) | - | - | <0.3 | - | - | - | - | - | - | - | - |
| 21 | - | +(1) | +(1) | - | +(1) | +(1) | +(1) | 0.7±0.48 | - | - | - | - | +(1) | +(1) | - | <0.3 |
| 28 | - | +(1) | +(1) | - | +(2) | +(1) | +(2) | 1.00±0.81 | +(1) | - | +(1) | - | +(1) | +(1) | +(1) | 0.7±0.48 |
- no growth
+presence of at least one colony
in parenthesis - No. of colonies on plates
Table 3.
Comparison of Bacterial Growth from Intestine Cultures on Plates from CTX-Treated Leeches
| Leeches feeding with CTX | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1.5 mcg/mL | 50 mcg/mL | |||||||||||||||
| Day after feeding | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Mean no. of colonies per leech±SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Mean no. of colonies per leech±SD |
| 1 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 7 | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - | - |
| 14 | +(1) | - | +(1) | - | - | - | - | < 0.3 | - | - | - | - | - | - | - | - |
| 21 | +(2) | +(1) | +(1) | - | +(2) | +(1) | +(1) | 1.10±0.69 | - | - | - | - | - | +(1) | +(1) | <0.3 |
| 28 | +(2) | +(1) | +(1) | +(1) | +(3) | +(2) | +(1) | 1.60±0.79 | - | +(1) | +(1) | - | - | +(1) | +(1) | 0.60±0.53 |
- no growth
+presence of at least one colony
in parenthesis - No. of colonies on plates
Table 4.
Presence of Aeromonas spp. in the Intestines of Hirudo verbana Treated with Ciprofloxacin (CIP) and Cefotaxime (CTX)
| No. of leeches with Aeromonas spp. (%) | ||||
|---|---|---|---|---|
| CIPN=7 | CTXN=7 | |||
| Day of examinationafter feeding | 0.2 mcg/mL | 20 mcg/mL | 1.5 mcg/mL | 50 mcg/mL |
| 1 | 0 | 0 | 0 | 0 |
| 7 | 0 | 0 | 0 | 0 |
| 14 | 1 (14.3) | 0 | 2 (28.6) | 0 |
| 21 | 5 (71.4) | 2 (28.6) | 6 (85.7) | 2 (28.6) |
| 28 | 5 (71.4) | 5 (71.4) | 7 (100) | 4 (57.1) |
Whereas all water samples in which H. verbana were kept before treatment were contaminated with Aeromonas spp., no such contamination was detected during the experimental period in the water containing 20 mg/L CIP or 50 mg/L CTX.
The dominant species was identified by the API 20NE kit as A. veronii biovar sobria. Two different phenotypes within this taxon were designated as 7176755 (77.4% of all detected isolates) and 7176744 (19.2%). The second species was identified as Aeromonas hydrophila, profile 3577754 (3.4%). In this experiment, the isolated bacterial strains were identified by biochemical assay. Molecular analysis of the bacterial flora of leeches has been documented extensively in earlier studies [25,26], and the purpose of present study was to determine the effectiveness of elimination of bacteria from the intestine.
Discussion
Recently, hirudotherapy has been used successfully in plastic and reconstruction surgery to decrease the venous congestion of skin flaps and to improve micro-revascularization of flaps, grafts and replants. It was reported that the use of leech therapy is associated with 60%–83% greater success of flap, graft, and replantation procedures [15,16]. However, the application of leeches carries the risk of microbial infection. The presence of Aeromonas infections as a complication of the medicinal use of leeches reduces the chances of successful re-implantations or flaps by 30% [16,19]. Retrospective studies of bacterial complications after using leech therapy revealed Aeromonas spp. to be one of the major pathogens [6]. It is worth noting that most isolated bacteria causing nosocomial infection after hirudotherapy were described previously as A. hydrophila, but were later found to be A. veronii biovar sobria by the use of biochemical tests and 16S rRNA gene sequences [25,26].
In the present study, of the two species isolated from the intestines of H. verbana, A. veronii biovar sobria was clearly dominant. No isolates belonging to any other genera were detected, suggesting that the genus Aeromonas is the dominant symbiont. It was confirmed previously by molecular assays that two species dominate in the digestive tract flora of medicinal leeches: A. veronii biovar sobria and a Rikenella-like bacterium [25–28]. However, the anaerobic, non-pathogenic Rikenella are difficult to culture, and only detection of their specific rRNA indicates their presence in the leech environment. Aeromonas veronii biovar sobria, as well as other aeromonads such as A. hydrophila, are gram-negative, facultative anaerobic bacteria which are potentially pathogenic to human beings [6–9,15].
Clinical observations demonstrate that the endosymbiotic flora of the leech is capable of transmission into the patient during attachment and feeding [6]. Thus, the crucial technical problem of hirudotherapy is the sterility of the leeches. The new method of eradicating Aeromonas spp. from the intestine of H. verbana presented in this study is effective and also safe for the leeches. Moreover, the leeches still showed a readiness to consume rabbit blood after the antibiotics entered the digestive tract, which guarantees their use in treatment. It was decided to apply a lower concentration of antibiotics than used by Mumcuoglu et al. [23] as leeches are sensitive to chemicals, and they may refuse to feed on blood supplemented with drugs. In addition, a different mode of delivery was chosen, as the use of arginine to carry the drug into the digestive tract of leeches described by this team proved ineffective. Mumcuoglu et al. [23] note that leeches fed artificially with a 2 g/L arginine solution with CIP (100 mg/L) did not suck blood meals willingly afterwards: Almost 60% of leeches refused to feed in the first two weeks after being treated with antibiotic. Teh et al. [24] also confirmed poor consumption when only L-arginine was added to saline solution. Additionally, mortality rates as high as 40% were observed among arginine-feeding leeches. Hence, in the present study, antibiotics against Aeromonas spp. (CIP and CTX) were introduced to the leeches with a small portion of sterile ovine blood, which all leeches sucked willingly. Our experiment is the first attempt at internal sterilization of medicinal leeches after feeding on sterile blood supplemented with antibiotic. The results indicate that the greatest bacterial growth from intestinal cultures of control leeches was achieved after one and seven days after blood feeding, whereas complete elimination of Aeromonas bacteria from antibiotic treated-leeches was noted in this time.
As the external decontamination of the leeches with chlorhexidine used commonly in clinics is not sufficient to eradicate Aeromonas spp. from medicinal leeches, prophylactic antibiotics are applied [3,4]. Lineaweaver et al. [19] noted a significant reduction in the bacterial population occurring in the digestive tracts of leeches applied to patients receiving an antibiotic effective against Aeromonas spp. Earlier studies have shown that attempts to eliminate the bacterial population inside and outside leeches by the exterior application of antiseptics and antibiotics only reduces the number of bacteria on the surface of the animals; a complete lack of bacterial growth is needed to confirm that sterilization has been successful. Although Mackay et al. [20] observed a significant colony count reduction after incubating leeches for 12 h in a solution of 4 mcg/mL tetracycline, no significant differences were noted with 12 mcg/mL of tetracycline. According to Golden et al. [29], pre-treating maintenance leeches with a solution of CIP for 24 h before application minimizes the complication of infection. Hokelek et al. [21] noted that the optimum eradication of bacteria from leeches was obtained by immersion of animals for 18 h in antibiotic solutions (500 mcg/mL CIP or 1 mcg/mL CTX), but complete elimination of bacteria from the body and intestine was not observed.
It should be noted that preserving the sucking ability of the leeches is important in these eradication studies. After decontamination, the leeches should be healthy and willing to feed. However, most antiseptics are toxic to leeches or decrease their suction activity. For example, povidone-iodine or ethanol-water solutions, are highly toxic to leeches [18]. Attempts have been made to disinfect medical leeches before attachment to patients by immersion in 0.02% chlorhexidine for 15 s, or hypochloric acid solutions for 10 min, but with unsuccessful results [20–22]. The oral mode of administration of antibiotics to the digestive tract used in the present study eliminates bacteria effectively from the intestine of medicinal leeches and preserves the ability to suck blood. Aeromonas spp. were not detected in cultures from the intestines of leeches treated with antibiotics for two weeks after blood feeding, nor in the water in which they were kept during the experimental period.
Prophylaxis with antimicrobial agents active against Aeromonas spp. has been recommended, but it is controversial, because this therapy may promote the undesirable emergence of drug-resistant bacteria in patients. The first case of surgical site infection with CIP-resistant A. hydrophila following leech therapy was reported recently [30]. However, decontamination of the bacterial flora of leeches by antibiotic treatment can lead also to the acquisition of resistance in symbiotic bacteria. Nevertheless, it appears safer to eradicate Aeromonas from the intestinal tract of leeches used for therapy than subject the patient to antibiotic prophylaxis.
The increasing use of medicinal leeches has been accompanied by increasing numbers of reports of Aeromonas infections after leech application. The procedure described in the present study used for the effective eradication of digestive tract bacteria without affecting the readiness of the leech to suck blood, allows leeches treated with CIP or CTX to used instead of chemoprophylaxis in patients undergoing hirudotherapy. Suppression of leech enteric bacteria may be an effective tactic to prevent invasive infection by Aeromonas spp. as well as bacterial colonization of devitalized tissue, which could be a source of late infection.
Acknowledgments
This work was carried out in the framework of Research Grant for Doctoral Candidates and Young Scientists (No. 502-03/1-013-01/502-14-012) awarded and financial supported by the Medical University of Lodz (Poland).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Elliott JM, Kutschera U. Medicinal leeches: Historical use, ecology, genetics and conservation. Freshwater Rev 2011;4:21–41 [Google Scholar]
- 2.Riede F, Koenen W, Goerdt S, et al. . Medicinal leeches for the treatment of venous congestion and hematoma after plastic reconstructive surgery. J Dtsch Dermatol Ges 2010;8:881–888 [DOI] [PubMed] [Google Scholar]
- 3.Porshinsky BS, Saha S, Grossman MD, et al. . Clinical uses of the medicinal leech: A practical review. J Postgrad Med 2011;57:65–71 [DOI] [PubMed] [Google Scholar]
- 4.Whitaker IS, Oboumarzouk O, Rozen WM, et al. . The efficacy of medicinal leeches in plastic and reconstructive surgery: A systematic review of 277 reported clinical cases. Microsurgery 2012;32:240–250 [DOI] [PubMed] [Google Scholar]
- 5.Mumcuoglu KY. Recommendations for the use of leeches in reconstructive plastic surgery. Evid base. Compl Alternative Med 2014:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bauters TG, Buyle FM, Verschraegen G, et al. . Infection risk related to the use of medicinal leeches. Pharm World Sci 2007;29:122–125 [DOI] [PubMed] [Google Scholar]
- 7.Bourdais L, Heusse JL, Aillet S, et al. . Leech-borne infection on a TRAM flap: A case report. Ann Chir Plast Esthet 2010;55:71–73 [DOI] [PubMed] [Google Scholar]
- 8.Schnabl SM, Kunz C, Unglaub F, et al. . Acute postoperative infection with Aeromonas hydrophila after using medical leeches for treatment of venous congestion. Arch Orthop Trauma Surg 2010;130:1323–1328 [DOI] [PubMed] [Google Scholar]
- 9.Levine SM, Frangos SG, Hanna B, et al. . Aeromonas septicemia after medicinal leech use following replantation of severed digits. Am J Crit Care 2012;19:469–471 [DOI] [PubMed] [Google Scholar]
- 10.Yantis MA, O'Toole KN, Ring P. Leech therapy. Am J Nurs 2009;109:36–42 [DOI] [PubMed] [Google Scholar]
- 11.Nehili M, Ilk C, Mehlhorn H, et al. . Experiments on the possible role of leeches as vectors of animal and human pathogens: a light and electron microscopy study. Parasitol Res 1994;80:277–290 [DOI] [PubMed] [Google Scholar]
- 12.Al-Khleif A, Roth M, Menge C, et al. . Tenacity of mammalian viruses in the gut of leeches fed with porcine blood. J Med Microbiol 2011;6:787–92 [DOI] [PubMed] [Google Scholar]
- 13.Litwinowicz A, Blaszkowska J. Hirudo verbana is a source of fungal isolates potentially pathogenic to humans. Afr J Microbiol Res 2013;7:5358–5363 [Google Scholar]
- 14.Graf J, Kikuchi J, Rio RVM. Leeches and their microbiota: naturally simple symbiosis models. Trends Microbiol 2006;14:365–371 [DOI] [PubMed] [Google Scholar]
- 15.Maetz B, Abbou R, Andreoletti JB, Bruant-Rodier C. Infections following the application of leeches: Two case reports and review of the literature. J Med Case Rep 2012;25:1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sartor C, Limouzin-Perotti F, Legré R, et al. . Nosocomial infections with Aeromonas hydrophila from leeches. Clin Infect Dis 2002;35:1–5 [DOI] [PubMed] [Google Scholar]
- 17.Varghese MR, Farr RW, Wax MK, et al. . Vibrio fluvialis wound infection associated with medicinal leech therapy. Clin Infect Dis 1996;22:709–710 [DOI] [PubMed] [Google Scholar]
- 18.Pereira JA, Greig JR, Liddy H, et al. . Leech-borne Serratia marcescens infection following complex hand injury. Br J Plast Surg 1998;51:640–641 [DOI] [PubMed] [Google Scholar]
- 19.Lineaweaver WC, Hill MK, Buncke GM, et al. . Aeromonas hydrophila infections following use of medicinal leeches in replantation and flap surgery. Ann Plast Surg 1992;29:238–44 [DOI] [PubMed] [Google Scholar]
- 20.Mackay DR, Manders EK, Saggers GC, et al. . Aeromonas species isolated from medicinal leeches. Ann Plast Surg 1999;42:275–279 [DOI] [PubMed] [Google Scholar]
- 21.Hokelek M, Guneren E, Eroglu C. An experimental study to sterilize medical leeches. Eur J Plast Surg 2002; 25:81–85 [Google Scholar]
- 22.Aydin A, Nazik H, Kuvat SV, et al. . External decontamination of wild leeches with hypochloric acid. BMC Infect Dis 2004;4:1–8 http://www.biomedcentral.com/1471-2334/4/28 [DOI] [PMC free article] [PubMed]
- 23.Mumcuoglu KY, Huberman L, Cohen R, et al. . Elimination of symbiotic Aeromonas spp. from the intestinal tract of the medicinal leech, Hirudo medicinalis, using ciprofloxacin feeding. Clin Microbiol Infect 2010;16:563–567 [DOI] [PubMed] [Google Scholar]
- 24.Teh JC, Kamarudin MS, Rahim A, Saad CR. Performance of selected chemical compounds in eliciting feeding of Asian buffalo leech, Hirudinaria manillensis. J Fish Aquat Sci 2011;6:846–851 [Google Scholar]
- 25.Worthen PL, Gode CJ, Graf J. Culture-Independent characterization of the digestive-tract microbiota of the medicinal leech reveals a tripartite symbiosis. Appl Environ Microbiol 2006;72:4775–4781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graf J. Symbiosis of Aeromonas veronii biovar sobria and Hirudo medicinalis, the medicinal leech: A novel model for digestive tract associations. Infect Immun 1999;67:1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silver AC, Rabinowitz NM, Küffer S, Graf J. Identification of Aeromonas veronii genes required for colonization of the medicinal leech, Hirudo verbana. J Bacteriol 2007;189:6763–6772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelson MC, Graf J. Bacterial symbioses of the medicinal leech Hirudo verbana. Gut Microbes 2012;3:322–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Golden MA, Quinn JJ, Partington MT. Leech therapy in digital replantation. AORN J 1995;62:364–375 [DOI] [PubMed] [Google Scholar]
- 30.Giltner CL, Bobenchik AM, Uslan DZ, et al. . Ciprofloxacin-resistant Aeromonas hydrophila cellulitis following leech therapy 2013;51:1324–1326 [DOI] [PMC free article] [PubMed]