Abstract
Background
Korean ginseng is a well-known medicinal herb that has been widely used in traditional medicine to treat various diseases, including asthma. Ginseng can be classified as white ginseng (WG) or red ginseng (RG), according to processing conditions. In this study, the authors compared the efficacies of these two ginseng types in a mouse model of acute asthma.
Methods
To produce the acute asthma model, BALB/c mice were sensitized with ovalbumin (OVA) and aluminum hydroxide, and then challenged with OVA. WG and RG extracts were administered to mice orally. The influences of WG and RG on airway hyperresponsiveness (AHR), immune cell distributions in bronchoalveolar lavage fluid (BALF), and OVA-specific immunoglobulin E (IgE), IgG1, and IgG2a in serum were investigated. Cytokine production by lymphocytes isolated from peribronchial lymph nodes and histopathological changes was also examined.
Results
In OVA-sensitized mice, both WG and RG reduced AHR and suppressed immune cell infiltration in bronchoalveolar regions. BALF OVA-specific IgE levels were significantly lower in RG-treated OVA-sensitized mice than in the OVA-sensitized control group. WG and RG also suppressed inflammatory cytokine production by peribronchial lymphocytes. Histopathological findings showed reduced inflammatory cell infiltration and airway remodeling (e.g., epithelial hyperplasia) in WG- and RG-treated OVA mice compared with OVA controls.
Conclusion
In this study, WG and RG showed antiasthmatic effects in an OVA-sensitized mouse model, and the efficacies of RG were found to be better than those of WG.
Keywords: asthma, Korean ginseng, red ginseng, white ginseng
1. Introduction
Korean ginseng, the root of Panax ginseng Meyer, is one of the most popular medicinal herbs in traditional Korean medicine and is also extensively used worldwide to treat various diseases by herbal medicine practitioners [1]. P. ginseng has been used as a general tonic for the promotion of health in Asian countries, and its pharmacological properties have been attributed to its ginsenoside components [2]. Many bioactive constituents are present in ginseng extracts, and ginsenosides, the main constituents of ginseng, are believed to have antiallergic, antioxidant, and immune-stimulatory activities [3].
The two traditional preparations of Korean ginseng, white ginseng (WG) and red ginseng (RG), are presumed to have different bioactivities in traditional medicine. WG is produced by the sun drying of fresh ginseng, whereas RG is manufactured by steaming fresh ginseng and then drying it to a moisture content of < 15% [4]. Many researchers have reported that the steaming process increases the bioactivity of ginseng [4–6]. Few comparative studies have been conducted on the effects of WG and RG on various diseases.
Asthma is a serious health problem and affects people of all ages, and its most common trigger is continuous exposure to allergens [7]. Allergic asthma is characterized by increased mucus production, reversible airway obstruction, eosinophil infiltration, and nonspecific airway hyperresponsiveness (AHR) [8]. The development of asthma is mediated by the overexpression of T helper type 2 (Th2)-mediated or Th1-mediated cytokines, such as interleukin (IL)-4, IL-5, etc. [8,9]. However, currently available therapies cannot completely control the symptoms of asthma, and even intensive treatment shows little effect on healthcare utilization [10]. Consequently, efforts are required to identify new remedies, preferably of natural origin, for mitigating the effects of these immune-related disorders.
P. ginseng is one of most commonly used medicinal herbs to complement the treatment of asthma, allergies, and immunologic conditions [11]. Several researchers have reported that P. ginseng ameliorates asthma in animal models [12,13], but to date, the effects of processing on its medicinal effects have not been studied.
Therefore, in the present study, we compared the effects of WG and RG in a mouse model of acute asthma. In previous studies we reported that herbal remedies offer potential complementary or alternative treatments and showed that the regulation of Th1/Th2 balance could provide a strategy for the treatment of respiratory diseases [14,15].
In this study, we investigated the effects of WG and RG on the infiltration of inflammatory cells, on airway remodeling, and on expressions of inflammation-related cytokines in an ovalbumin (OVA)-sensitized mouse model of acute asthma.
2. Materials and methods
2.1. Animals
Seven-week-old female BALB/c mice (Daehan Biolink, Chungbuk, Korea) were housed in polypropylene cages at 24 ± 4°C under a 12 h light and dark cycle for at least 1 week prior to experiments. Animals were fed with a standard pellet diet and supplied water ad libitum. All experiments were approved by the Institutional Animal Care Committee of Pusan National University and conducted in accordance with the guidelines issued by Pusan National University.
2.2. Preparation of ginseng extracts and reagents
Standardized 5-yr-old Korean WG and RG were purchased from Gwangmyung Natural Pharmaceutical Co. (Busan, Korea); voucher specimens (No. 201KWG and 201KRG) were deposited at the Herbarium of the School of Korean Medicine, Pusan National University. WG and RG (1 kg) were finely ground and extracted with 10 times their volumes of 80% methanol at room temperature for 3 d and then the extraction process was repeated three times. After filtration using filter paper (Advantec, Tokyo, Japan), methanol was removed using a vacuum evaporator (Eyela, Tokyo, Japan) at 45°C, and the resulting extracts (WG and RG) were stored at −20°C until required. OVA (Grade V) was purchased from Sigma-Aldrich (St. Louis, MO, USA). Before use, OVA was detoxified using a DetoxiGel column (Pierce, Rockford, IL, USA).
2.3. High performance thin layer chromatography analysis for WG and RG fingerprinting
For quality assurance purposes, each extract was subjected to high performance thin layer chromatography (HPTLC). Chloroform and methanol (7:3, v/v) were used as the developer solvents, and the bands that developed on HPTLC plates were detected using a Camag visualizer (Camag, Sonnenmattstrasse, Muttenz, Switzerland). It was found that compounds were altered by the steaming process, which suggests this is responsible for their different bioactivities. Fig. 1A lists compounds found in WG and Figs. 1B–1D list compounds found in RG.
Fig. 1.

High performance thin layer chromatography (HPTLC) images of the fingerprints of white ginseng (WG) and of red ginseng (RG). WG (a) and RG (b) extracts were developed on HPTLC plates (silica gel F254; chloroform/methanol, 7:3; under UV or white light) and documented using a visualizer (Camag, Sonnenmattstrasse, Muttenz, Switzerland). (A) Under 254 nm UV light. (B) Under 366 nm UV light. (C) Under 366 nm UV light after spraying with p-anisaldehyde. (D) Under white light after spraying with p-anisaldehyde. Numbers on plates are Rf values. Arrows indicate specific components of WG or RG.
2.4. Induction of asthma and the experimental animal groups
The experimental protocols employed were as described in our previous report [14]. Briefly, 200 μL of phosphate buffer saline (PBS) or emulsion containing 100 μg of OVA and 2 mg of aluminum hydroxide was injected into the mouse intraperitoneally (i.p.) on Day 1 and Day 14. On Day 22, mice were anesthetized with ketamine (100 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.), and on Days 22, 23, and 24 received 30 μL of PBS containing 25 g of OVA by intranasal instillation. WG and RG extracts were administration for 10 consecutive days between 9:00 am and noon from Day 15 to Day 24 (Fig. 2).
Fig. 2.
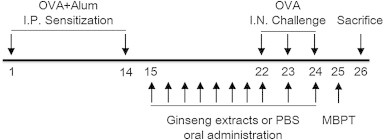
Experimental design and the acute asthma model. Control, white ginseng (WG), and red ginseng (RG) groups were sensitized intraperitoneally on Days 1 and 14, and challenged intranasally on Days 22, 23, and 24. Animals were administered WG or RG from Days 15 to 24. All animals were sacrificed on Day 26. I.N., intranasal; I.P., intraperitoneal; MBPT, methacholine bronchial provocation test; PHS, phosphate buffered saline.
The experimental groups were as follows: (1) naïve: OVA-sensitized but not challenged and administered PBS; (2) control: sensitized and challenged with OVA and administered PBS; and (3) WG or RG: sensitized and challenged with OVA and administered WG or RG, respectively.
2.5. Treatment with WG or RG extracts
Seven-week-old male BALB/c mice were randomly divided into eight groups: 30, 90, and 300 mg/kg WG-treated, 30, 90, and 300 RG-treated, PBS-treated control and the treatment naïve group. Each group consisted of nine mice. In Korean traditional medicine, 30 mg/kg is the recommended daily dose for WG and for RG. Each concentration of WG or RG in PBS was administered by oral intubation for 10 d. Animals in the naïve and PBS-treated control groups were given the same volume of PBS by oral intubation. At the end of treatment, animals were sacrificed and the following samples were collected for analysis; bronchoalveolar lavage fluid (BALF), blood, and lung and bronchial lymph nodes.
2.6. Measurement of AHR
Airway responsiveness to methacholine was measured using a whole body plethysmograph (OCP 3000, Allmedicus, Gyounggi, Korea), which provides a noninvasive measure of airway responsiveness in mice [16]. One day after the last OVA challenge via nasal inhalation (Day 25), mice were exposed to increasing doses of methacholine using an ultrasonic nebulizer (Pari, Starnberg, Germany) for 150 s at each concentration. AHR was calculated in enhanced pause (Penh) as we previously described [14]. The formula used was as follows: Penh = (Te/RT-1) × PEF/PIF, where Te = expiration time (s), RT = relaxation time (s), REF = peak expiratory flow rate (mL/s) and IF = peak inspiratory flow rate (mL/s).
2.7. Analysis of BALF
On Day 26, to obtain BALF, mice were sacrificed with a lethal dose of ketamine and xylazine, and BALF was collected from tracheas; 1.8 mL of PBS was introduced to the lungs, and more than 1.5 mL of buffer was consistently retrieved. BALF cells were analyzed as previously described [17]. Briefly, differential cell counts were performed by counting cytospin preparations stained with Diff-Quick (Dade Behring, Düdingen, Switzerland).
2.8. Determination of serum antibody concentrations
Mice were bled on Day 26, and blood samples were stored at 4°C overnight and then centrifuged at 2,800 × g for 10 min to obtain sera. OVA-specific immunoglobulin (IgE, IgG1 and IgG2a) levels in serum were measured by ELISA, according to the manufacturer's instructions (BD Pharmingen, San Jose, CA, USA).
2.9. Cytokine detection in peribronchial lymphocytes
Levels of cytokine production by peribronchial lymphocytes were measured as previously described [18]. Briefly, on Day 26, peribronchial lymph nodes were isolated and prepared as single cell suspensions. Cells (2 × 105 cells/mL) were then plated on 96-well microplates and cultured for 3 d with OVA (50 mg/mL) in 200 mL RPMI-1640 medium containing 10% fetal bovine serum (FBS). Supernatants were analyzed for IL-4, IL-5, IL-6, transforming growth factor beta (TGF-β) (BD Pharmingen), and IL-13 (R&D Systems, Minneapolis, MN, USA) by ELISA, according to the manufacturer's instructions. OVA-specific cytokine levels were then calculated.
2.10. Lung histology
On Day 26, after obtaining BALF, the lungs and tracheas were resected and fixed overnight in 4% formalin. Specimens were then dehydrated in an alcohol series, embedded in paraffin wax, and sectioned at 5 μm. Sections were placed on glass slides, stained with hematoxylin and eosin, and examined under a light microscope.
2.11. Statistical analysis
Results are expressed as mean ± standard deviation (SD), and all statistical comparisons were performed by one-way analysis of variance followed by Duncan's multiple comparison test. SPSS version 18 (SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Statistical significance was accepted for p values < 0.05.
3. Results
3.1. Effects of WG or RG on numbers of inflammatory cells in BALF
Inflammatory cells were significantly increased in BALF in the PBS-treated control group (OVA + Alum), but treatment with WG or RG significantly decreased total cells including macrophages and other inflammation-related immune cells (Fig. 3). Eosinophilic inflammation is a hallmark of asthma, and RG treatment remarkably decreased BALF eosinophils in dose-dependent manner in the RG-treated group as compared with the PBS-treated control group.
Fig. 3.

Effects of white ginseng (WG) and red ginseng (RG) on total and differential cell counts in bronchoalveolar fluid (BALF). Results are presented as mean ± SD. #p < 0.05 vs. the naïve group; *p < 0.05 vs. the phosphate buffer saline (PBS)-treated control group. Eos, eosinophils; Lym, lymphocytes; Mac, macrophages; Neu, neutrophils.
3.2. Effects of WG or RG on airway responsiveness to methacholine
We examined AHR by methacholine inhalation. AHR resistances were measured as Penh values on Day 25 after methacholine inhalation. AHR in the PBS-treated control group was significantly increased as compared with that of the naïve group (Fig. 4). After exposure to 50 mg/mL of methacholine, Penh in the control group was increased by 443% versus the naïve group (10.05 ± 3.35 vs. 2.27 ± 0.72). In the WG- or RG-treated groups, Penh values were decreased by 21.59% and 35.92%, respectively, versus the control group (2.17 ± 0.76 vs. 10.05 ± 3.35 and 3.61 ± 1.13 vs. 10.05 ± 3.35, respectively) (Figs. 4A and 4B).
Fig. 4.
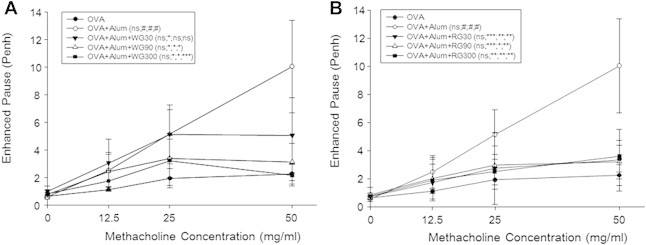
Effects of white ginseng (WG) and red ginseng (RG) on airway hyperresponsiveness. Results are presented as mean ± SD. #p < 0.05 vs. the naïve group; *p < 0.05 vs. the phosphate buffer saline (PBS)-treated control group; **p < 0.01 vs. the PBS-treated control group; ***p < 0.001 vs. the PBS-treated control group. Alum, aluminum hydroxide; ns, not specific; OVA, ovalbumin; Penh, enhanced pause.
3.3. Effects of WG and RG on OVA-specific IgE, IgG1, and IgG2a in serum
Marked increases in the levels of OVA-specific IgE were observed in the control group (Fig. 5). The WG and RG groups showed lower levels of IgE, and RG was more effective than WG.
Fig. 5.
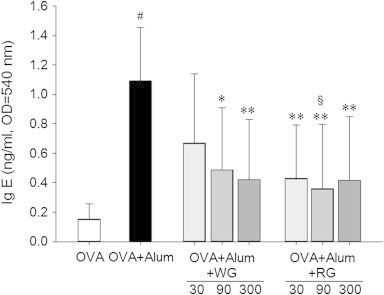
Effect of white ginseng (WG) and red ginseng (RG) on serum immunoglobulin E (IgE). Samples of serum were collected on Day 26, and levels of ovalbumin (OVA)-specific serum IgE were measured. Results are presented as means ± SD. #p < 0.05 vs. the naïve group; *p < 0.05 vs. the phosphate buffer saline (PBS)-treated control group; **p < 0.01 vs. the PBS-treated control group; §p < 0.05 vs. the WG group.
Marked increases in OVA-specific IgG1 and IgG2a levels were observed in the control group as compared with the naïve group. However, treatment with WG or RG did not affect OVA-specific IgG1 and IgG2a production in serum (Figs. 6A–6D).
Fig. 6.
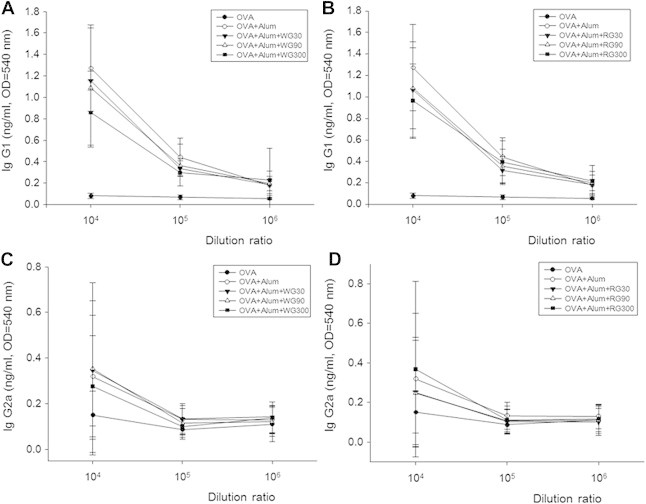
Effects of white ginseng (WG) and red ginseng (RG) on serum IgG1 and IgG2. Samples of serum were collected on Day 26, and levels of ovalbumin (OVA)-specific serum IgG1 and IgG2a were measured.
3.4. Effects of WG and RG on lung histopathology
In the naïve group, few inflammatory cells appeared around respiratory tracts, blood vessels, or alveolar spaces, and no histopathological changes such as mucosal thickening were observed (Fig. 7A). However, in the PBS-treated control group, obvious infiltrations of inflammatory cells were observed in connective tissues (Fig. 7B). Such changes appeared even though alveolar spaces had been washed once with PBS to obtain BAL fluid. Furthermore, marked mucosal thickening was also observed. In the WG- and RG-treated groups, inflammatory cell infiltration and mucosal thickening were less severe than in the PBS-treated control group (Figs. 7C–7H). In the RG group, inflammatory cell infiltration and mucosal thickening were less severe than in the WG group.
Fig. 7.
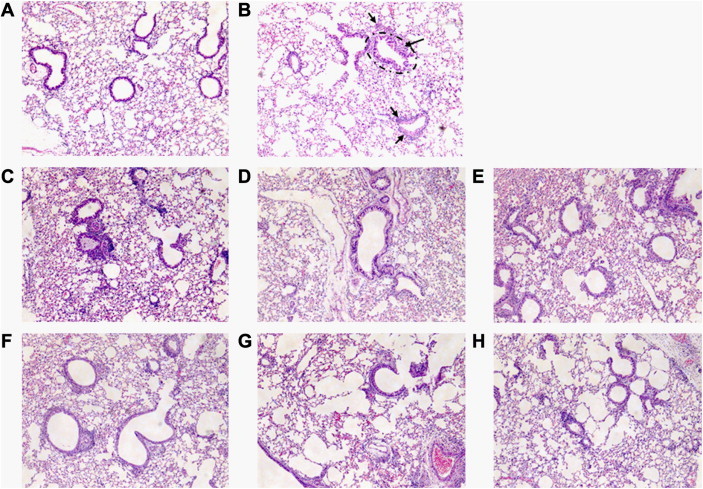
Effect of white ginseng (WG) and red ginseng (RG) on bronchoalveolar inflammation. Light microscopy revealed abnormal respiratory epithelium (ellipsoid area), and high numbers of infiltrated inflammatory cells (arrows) in the phosphate buffer saline (PBS)-treated control group as compared with the naïve group. (A) Treatment naïve group. (B) PBS- treated control group. (C–E) WG-treated groups (treated with 30, 90, and 300 mg/kg/d, respectively). (F–H) RG-treated groups (treated with 30, 90, and 300 mg/kg/d, respectively). Photomicrographs of axial sections of mouse lungs (× 100, H&E stain).
3.5. Effects of WG or RG on cytokine production by peribronchial lymphocytes
The cytokine profiles of peribronchial lymph node cells were analyzed via in vitro OVA stimulation. High levels of IL-4, IL-5, IL-6, and IL-13 production confirmed the Th2 nature of the inflammatory response in OVA-induced asthma (Fig. 8), although TGF-β production was not changed (data not shown). The WG and RG groups of mice showed low levels of cytokine production, and RG was more effective than WG at regulating cytokine production in peribronchial lymphocytes based on statistical analysis between same dosage WG and RG groups (Fig. 8).
Fig. 8.
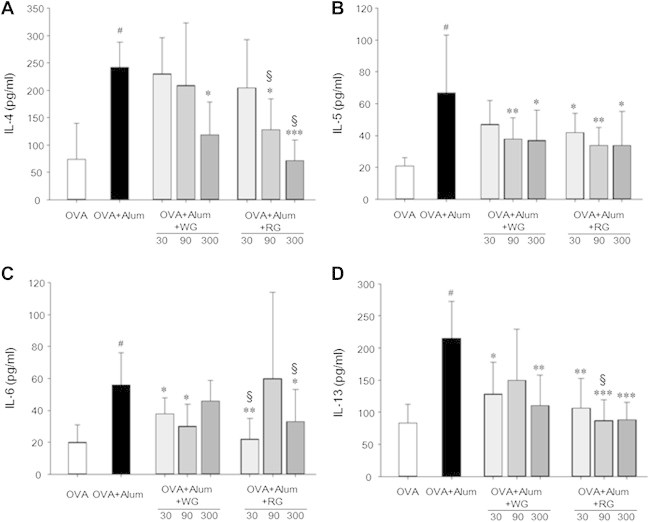
Comparisons of ovalbumin (OVA)-specific cytokine production of peribronchial lymph node cells. Lymph nodes of mice were collected on Day 26, and after 3 d of incubation with OVA, OVA-specific pro-inflammatory cytokine levels in cell culture media were measured. Results are presented as mean ± SD. #p < 0.05 vs. the naïve group; *p < 0.05 vs. the PBS-treated control group; **p < 0.01 vs. the PBS-treated control group; §p < 0.05 vs. the WG group.
4. Discussion
P. ginseng, also called Korean ginseng, is one of the most widely used functional health foods for revitalization and eliminating chronic fatigue, and has been used as a dietary supplement in Asia for > 2000 yr [19]. P. ginseng, both red and white preparations, is most commonly used in traditional Korean medicine, but there are some differences between them, such as in their ginsenoside contents and pharmacological effects. RG is manufactured when WG is produced by additional steaming and sun drying [4], and during this process, ginsenosides undergo chemical changes that could increase physiologic activities [19]. Currently, > 30 different ginsenosides have been isolated and characterized from P. ginseng, and these ginsenosides are known to have different pharmacologic effects [19]. However, the comparative studies of WG and RG on various diseases have not been sufficiently investigated.
Asthma is a serious, worldwide public health problem that affects all ages. It is an inflammatory disease of the airways that can be exacerbated by numerous extrinsic factors, such as continuous exposure to allergens [7]. However, the pathophysiological mechanism of asthma is unclear despite the increasing prevalence of this disease. Furthermore, current therapies fail to provide an adequate therapeutic solution.
Currently, corticosteroids are the drugs most commonly used to control airway inflammation, however, corticosteroid therapy has important adverse effects, and some patients are completely corticoid resistant or fail to show clinical improvement after high dose glucocorticoids treatment [20]. Therefore, the development of safer, more effective antiasthmatic drugs is required, and evaluation of the potential bioactivities of new compounds with unique mechanisms of action remains an important topic of research [20]. Consequently, efforts should be made to identify new antiasthmatic remedies, preferably of natural origin, to mitigate the effects of asthma.
Kim and Yang [12] reported that P. ginseng treatment restores the expression of several genes including EMBP, Muc5ac, and CD40, and the mRNA and protein levels of IL-1β, IL-4, IL-5, and tumor necrosis factor (TNF)-α, but no description was provided of inflammatory cell counts and IgE production in asthmatic mice, which probably underlie the mechanism of asthma. Furthermore, the effects of ginseng on asthma have received little attention. For this reason, we examined and compared the effects of WG and RG in an asthmatic mouse model.
Eosinophils are important immune cells and contribute to the development of allergic and asthmatic inflammation, to the infiltration of eosinophils into airways, and the release of their contents has been linked to symptom severity in asthma [21]. In the present study, eosinophils were absent in the BALF of the naïve group of mice and markedly increased in the PBS-treated control group (Fig. 3). Other inflammatory cells were also significantly up-regulated when asthma was induced. WG or RG administration effectively suppressed eosinophil infiltration into lung bronchioles. Fig. 7 shows the marked infiltrations of inflammatory cells, including eosinophils, neutrophils, and lymphocytes, observed in connective tissues not only around large vessels and airways but also around small vessels and airways in the control group. Although alveolar spaces were washed once with PBS to obtain BALF, many infiltrated inflammatory cells remained. However, in the WG and RG groups, inflammatory cell infiltrations were much reduced as compared with the control group. In addition, mucosal thickening was frequently observed in the control group (Fig. 7B), but in the WG and RG groups, mucosal thickening was moderately reduced, and RG seemed to be more effective than WG.
AHR is a particular feature of asthma and leads to recurrent episodes of shortness of breath, wheezing, and coughing [22]. In the present study, we observed AHR changes by methacholine challenge testing. WG and RG inhibited AHR as evidence by reductions in Penh values to levels similar to those observed in the naïve group (Fig. 4).
Asthma is associated with IgE production, for example, recent studies on the effects of anti-IgE therapy have confirmed that IgE plays an important role in asthma [23,24]. Balza et al [24] reported a positive relationship between airway IgE expression and serum IgE levels. In the present study, the expression of IgE in serum was markedly higher in the PBS-treated control group than in the naïve group, but WG or RG administration significantly decreased IgE serum level (Fig. 5), and RG was more effective than WG. However, neither WG nor RG influenced IgG1 and IgG2a serum levels in asthmatic mice (Fig. 6).
Th2 cell-associated inflammation is considered as an important mediator of asthma, and Th2-type cytokines, such as IL-4, IL-5, and IL-13, are thought to drive the disease pathology [25]. Moreover, there is strong evidence that the Th2-cytokine pattern plays an important role in the pathogenesis of asthma via the release of IL-4, IL-5, and IL-13 [26]. Accordingly, we analyzed the cytokine profiles of bronchial lymph node cells after in vitro OVA stimulation. High levels of IL-4, IL-5, IL-6, and IL-13 production confirmed the Th2 nature of the inflammatory response in the PBS treated control group (Fig. 8). Furthermore, cytokine production by lymphocytes in the WG and RG groups was significantly lower than in the control group, and RG was again more effective than WG.
5. Conclusions
WG and RG effectively suppressed inflammatory cell infiltration into bronchoalveolar regions. AHR and airway remodeling in OVA-induced asthma were also ameliorated by WG and RG. The study shows that WG and RG regulate serum IgE levels, which is an important biomarker of asthma. In addition, WG and RG significantly suppressed pro-inflammatory cytokine production by peribronchial lymphocytes. Furthermore, RG was more potent than WG in all respects. However, as most medicinal herbs have multiple components, it is unclear which component plays key roles in the above-mentioned activities. Therefore, further studies are needed to identify the key molecules underlying these effects and efficacies.
Conflicts of interest
The authors have no potential conflict of interest to declare. The authors alone are responsible for the writing of this paper.
Acknowledgments
This work was supported by the 2011 Specialzation Project Research Grant funded by the Pusan National University.
Footnotes
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/3.0) which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
References
- 1.Jiao L., Li B., Wang M., Liu Z., Zhang X., Liu S. Antioxidant activities of the oligosaccharides from the roots, flowers and leaves of Panax ginseng C.A. Meyer. Carbohydr Polym. 2014;106:293–298. doi: 10.1016/j.carbpol.2014.02.035. [DOI] [PubMed] [Google Scholar]
- 2.Song M., Kim B., Kim H. Influence of Panax ginseng on obesity and gut microbiota in obese middle-aged Korean women. J Ginseng Res. 2014;38:106–115. doi: 10.1016/j.jgr.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choi J.H., Jin S.W., Park B.H., Kim H.G., Khanal T., Han H.J., Hwang Y.P., Choi J.M., Chung Y.C., Hwang S.K. Cultivated ginseng inhibits 2,4-dinitrochlorobenzene-induced atopic dermatitis-like skin lesions in NC/Nga mice and TNF-alpha/IFN-gamma-induced TARC activation in HaCaT cells. Food Chem Toxicol. 2013;56:195–203. doi: 10.1016/j.fct.2013.02.037. [DOI] [PubMed] [Google Scholar]
- 4.Gui Y., Ryu G. Effects of extrusion cooking on physicochemical properties of white and red ginseng (powder) J Ginseng Res. 2014;38:146–153. doi: 10.1016/j.jgr.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H., Li S., Zhang H., Wang Y., Zhao Z., Chen S., Xu H. Holistic quality evaluation of commercial white and red ginseng using a UPLC-QTOF-MS/MS-based metabolomics approach. J Pharm Biomed Anal. 2012;62:258–273. doi: 10.1016/j.jpba.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Jung M.Y., Jeon B.S., Bock J.Y. Free, esterified, and insoluble-bound phenolic acids in white and red Korean ginsengs (Panax ginseng C.A. Meyer) Food Chem. 2002;79:105–111. [Google Scholar]
- 7.Agarwal R., Gupta D. Severe asthma and fungi: current evidence. Med Mycol. 2011;49:S150–S157. doi: 10.3109/13693786.2010.504752. [DOI] [PubMed] [Google Scholar]
- 8.Corrigan C.J., Kay A.B. The roles of inflammatory cells in the pathogenesis of asthma and of chronic obstructive pulmonary disease. Am J Resp Crit Care Med. 1991;143:1165–1168. doi: 10.1164/ajrccm/143.5_Pt_1.1165. [DOI] [PubMed] [Google Scholar]
- 9.Corry D.B. Emerging immune targets for the therapy of allergic asthma. Nat Rev Drug Discov. 2002;1:55–64. doi: 10.1038/nrd702. [DOI] [PubMed] [Google Scholar]
- 10.Moore W.C., Bleecker E.R., Curran-Everett D., Erzurum S.C., Ameredes B.T., Bacharier L., Calhoun W.J., Castro M., Chung K.F., Clark M.P. Characterization of the severe asthma phenotype by the National Heart, Lung, and Blood Institute's Severe Asthma Research Program. J Allergy Clin Immunol. 2007;119:405–413. doi: 10.1016/j.jaci.2006.11.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bielory L. Complementary and alternative interventions in asthma, allergy, and immunology. Ann Allergy Asthma Immunol. 2004;93:S45–S54. doi: 10.1016/s1081-1206(10)61486-x. [DOI] [PubMed] [Google Scholar]
- 12.Kim D.Y., Yang W.M. Panax ginseng ameliorates airway inflammation in an ovalbumin-sensitized mouse allergic asthma model. J Ethnopharmacol. 2011;136:230–235. doi: 10.1016/j.jep.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 13.Mohi El-Din M.M., Mostafa A.M., Abd-Elkader A. Experimental studies on the effect of (Lambda-Cyhalothrin) insecticide on lungs and the ameliorating effect of plant extracts (Ginseng (Panax Ginseng) and garlic (Allium sativum L.) on asthma development in albino rats. BMC Res Notes. 2014;7:243. doi: 10.1186/1756-0500-7-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jung S., Cho S.J., Moon K.I., Kim H.W., Kim B.Y., Cho S.I. Effects of Socheongryong-Tang on immunoglobulin production in asthmatic mice. Kor J Herbol. 2008;23:23–28. [Google Scholar]
- 15.Cho S.J., Kim H.W., Kim B.Y., Cho S.I. Sam So Eum, a herb extract, as the remedy for allergen-induced asthma in mice. Pulm Pharmacol Ther. 2008;21:578–583. doi: 10.1016/j.pupt.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 16.Hamelmann E., Schwarze J., Takeda K., Oshiba A., Larsen G.L., Irvin C.G., Gelfand E.W. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Resp Crit Care. 1997;156:766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- 17.Chung Y., Cho J., Chang Y.S., Cho S.H., Kang C.Y. Preventive and therapeutic effects of oral tolerance in a murine model of asthma. Immunobiology. 2002;206:408–423. doi: 10.1078/0171-2985-00190. [DOI] [PubMed] [Google Scholar]
- 18.Kim J.O., Kim D.H., Chang W.S., Hong C., Park S.H., Kim S., Kang C.Y. Asthma is induced by intranasal coadministration of allergen and natural killer T-cell ligand in a mouse model. J Allergy Clin Immunol. 2004;114:1332–1338. doi: 10.1016/j.jaci.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Ramesh T., Kim S., Hwang S., Sohn S., Yoo S., Kim S. Panax ginseng reduces oxidative stress and restores antioxidant capacity in aged rats. Nutr Res. 2012;32:718–726. doi: 10.1016/j.nutres.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 20.Ribeiro-Filho J., Calheiros A.S., Vieira-de-Abreu A., de Carvalho K.I.M., da Silva Mendes D., Melo C.B., Martins M.A., da Silva Dias C., Piuvezam M.R., Bozza P.T. Curine inhibits eosinophil activation and airway hyper-responsiveness in a mouse model of allergic asthma. Toxicol Appl Pharmacol. 2013;273:19–26. doi: 10.1016/j.taap.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 21.Thomas L.H., Warner J.A. The eosinophil and its role in asthma. Gen Pharmacol Vasc S. 1996;27:593–597. doi: 10.1016/0306-3623(95)02045-4. [DOI] [PubMed] [Google Scholar]
- 22.Lauzon A.M., Bates J.H., Donovan G., Tawhai M., Sneyd J., Sanderson M.J. A multi-scale approach to airway hyperresponsiveness: from molecule to organ. Front Physiol. 2012;3:191. doi: 10.3389/fphys.2012.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ong Y.E., Menzies-Gow A., Barkans J., Benyahia F., Ou T.T., Ying S., Kay A.B. Anti-IgE (omalizumab) inhibits late-phase reactions and inflammatory cells after repeat skin allergen challenge. J Allergy Clin Immunol. 2005;116:558–564. doi: 10.1016/j.jaci.2005.05.035. [DOI] [PubMed] [Google Scholar]
- 24.Balzar S., Strand M., Rhodes D., Wenzel S.E. IgE expression pattern in lung: Relation to systemic IgE and asthma phenotypes. J Allergy Clin Immunol. 2007;119:855–862. doi: 10.1016/j.jaci.2006.12.642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lloyd C.M., Hessel E.M. Functions of T cells in asthma: more than just Th2 cells. Nat Rev Immunol. 2010;10:838–848. doi: 10.1038/nri2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazzarella G., Bianco A., Catena E., De Palma R., Abbate G.F. Th1/Th2 lymphocyte polarization in asthma. Allergy. 2000;55:6–9. doi: 10.1034/j.1398-9995.2000.00511.x. [DOI] [PubMed] [Google Scholar]


