Abstract
Dynamic network analysis based on resting-state magnetic resonance imaging (rsMRI) is a fairly new and potentially powerful tool for neuroscience and clinical research. Dynamic analysis can be sensitive to changes that occur in psychiatric or neurologic disorders and can detect variations related to performance on individual trials in healthy subjects. However, the appearance of time-varying connectivity can also arise in signals that share no temporal information, complicating the interpretation of dynamic functional connectivity studies. Researchers have begun utilizing simultaneous imaging and electrophysiological recording to elucidate the neural basis of the networks and their variability in animals and in humans. In this article, we review findings that link changes in electrically recorded brain states to changes in the networks obtained with rsMRI and discuss some of the challenges inherent in interpretation of these studies. The literature suggests that multiple brain processes may contribute to the dynamics observed, and we speculate that it may be possible to separate particular aspects of the rsMRI signal to enhance sensitivity to certain types of neural activity, providing new tools for basic neuroscience and clinical research.
Key words: : dynamic analysis, EEG, functional connectivity, neural activity, resting-state fMRI
Introduction
Functional connectivity mapping with resting-state magnetic resonance imaging (rsMRI) has grown rapidly in popularity, as its introduction in Biswal and associates (1995) as the power of the technique to demonstrate clinically and cognitively relevant differences in brain networks has become apparent. Until recently, analysis methods assumed that the functional networks were stationary, maintaining the same structure over the course of the scan. Since improved imaging and analysis methods reduced contributions from physiological noise and increased sensitivity, interest began to grow in examining variability in the networks over time, creating a need for new twists on the classic analysis techniques (Hutchison et al., 2013a). Most of these methods involve using sliding windows or temporal segmentation to create network maps that vary over time (Allen et al., 2014; Chang and Glover, 2010; Hutchison et al., 2013b; Keilholz et al., 2013; Kiviniemi et al., 2011). Others identify spatiotemporal patterns of activity that repeat over time (Handwerker et al., 2012; Liu and Duyn, 2013; Majeed et al., 2011) or activity related to single events (Liu and Duyn, 2013; Petridou et al., 2013). These dynamic analysis methods are sensitive to changes that occur in psychiatric or neurologic disorders (Leonardi et al., 2013; Li et al., 2014b; Sakoglu et al., 2010) and are also related to variations in performance on individual trials in healthy subjects (Thompson et al., 2012; Yang et al., 2014). However, the appearance of time-varying connectivity can also arise in signals that share no temporal information, complicating the interpretation of dynamic functional connectivity studies (Handwerker et al., 2012; Keilholz et al., 2013). A few labs have begun utilizing simultaneous imaging and electrophysiological recording to elucidate the neural basis of the networks and their variability in animals (Magri et al., 2012; Pan et al., 2011, 2013; Scholvinck et al., 2010; Shmuel and Leopold, 2008; Thompson et al., 2013a, 2013b) and in humans (Chang et al., 2013; Tagliazucchi et al., 2012; Wu et al., 2010). In this article, we review findings that link changes in electrically recorded brain states to changes in the networks obtained with rsMRI and discuss some of the challenges inherent in the interpretation of these studies. The literature suggests that multiple brain processes may contribute to the dynamics observed, and we speculate that it may be possible to separate particular aspects of the rsMRI signal to enhance sensitivity to certain types of neural activity, providing new tools for basic neuroscience and clinical research.
Neural Correlates of Traditional Steady-State (Stationary) Functional Connectivity
The existence of a neural basis for traditional functional connectivity measurements is a necessary prerequisite if dynamic rsMRI network analysis is to reflect changes in coordinated neural activity. The evidence for neural correlates of blood-oxygenation-level-dependent (BOLD)-based functional connectivity comes from three sources: noninvasive electroencephalography (EEG) and magnetoencephalography (MEG) studies in healthy subjects, intracranial recordings in patient populations, and animal experiments.
Pioneering studies that combined EEG and MRI provided the first insight into the neural basis of BOLD-based functional connectivity (Laufs et al., 2003; Mantini et al., 2007). The broadband EEG signal is traditionally separated into five bands: δ (1–4 Hz), θ (4–8 Hz), α (8–12 Hz), β (12–30 Hz), and γ (30–100 Hz), with the divisions between frequency bands varying slightly across studies. A large body of research has linked different frequencies to different activities of the subject. For example, the alpha band is strong in a subject at rest with eyes closed, while gamma activity has been implicated in high-level processing of information. Not unexpectedly, combined EEG and MRI studies found that individual resting-state networks were linked to multiple frequency bands of the EEG, although different networks had distinctive spectral “fingerprints” (Mantini et al., 2007). The link between EEG and BOLD varied across subjects, suggesting that the relationship between EEG and BOLD was state dependent (Gonçalves et al., 2006). While all of these studies found a link between electrical recordings and rsMRI, the findings were difficult to interpret in the context of a direct link between BOLD and neural activity due to the limited spatial localization and depth penetration available with noninvasive recording methods.
To obtain more localized electrical measurements, researchers turned to invasive studies in neurosurgery patients. Using intracranial electrocorticography in epilepsy patients undergoing monitoring before surgical resection, Nir and associates (2008) found significant interhemispheric correlations in the sensory cortex in low-frequency (<0.1 Hz) modulations of neuronal firing rates and gamma local field potential (LFP) power. In a similar study, He and associates observed a correlation in the <0.5 and 1–4 Hz bands in the sensorimotor cortex whether subjects were awake or sleeping, which closely corresponded to BOLD correlation maps obtained from the same subjects. Gamma band-limited power (BLP) was also correlated during wakefulness or rapid eye movement (REM) sleep but not during slow wave sleep (He et al., 2008). Keller and associates (2013) examined the relationship between functional connectivity based on BOLD and electrocorticography and showed that both positive and negative BOLD correlation appear to be tied to positive and negative correlation, respectively, in high gamma power.
The studies in patient populations are uniquely valuable but have their limitations. Only sites of clinical interest can be examined; neural activity may be somewhat altered by pathology; and simultaneous imaging and recording is usually not feasible. Animal studies provide complementary information that fills some of these gaps. An early study by Lu and associates (2007) using epidural electrocorticography over sensory cortex and rsMRI in separate groups of animals showed that power correlation in the delta band (1–4 Hz) was modulated by increasing levels of anesthetic in the same way as BOLD correlation. Shmuel and Leopold (2008) used simultaneous MRI and intracortical recording in the anesthetized monkey to show that neural features such as gamma band power, multiunit spiking activity (MUA), and spiking were correlated with the BOLD signal from the same site in the visual cortex. Pan and associates (2011) found a correlation between broadband LFP power and the BOLD signal in isoflurane-anesthetized rats, with correlation between BLPs in the lower frequencies (δ, θ) that is most predictive of correlation in the BOLD signal, though correlation at a single site was higher in the gamma band. Similarly, in the monkey, Wang and associates (2012) found that oscillations below 20 Hz best corresponded to BOLD functional connectivity, and that these lower frequencies modulated local gamma activity. Studies that combined imaging of hemodynamics and electrophysiology showed that the hemodynamic response to spontaneous neural “events” was similar to the response to a stimulus and strongly correlated across hemispheres (Bruyns-Haylett et al., 2013; Liu et al., 2012). Using laser Doppler flowmetry and microelectrode arrays, Huang and associates (2014) showed that low-frequency oscillations (<0.26 Hz) in spiking rate and LFP power were causally related to low-frequency hemodynamic oscillations.
Most of the studies in animals have focused on the traditional EEG frequency bands of activity above 1 Hz, but the BOLD fluctuations used to map functional connectivity are much slower. Pan and associates (2013) recorded electrical activity in the same frequency range as the BOLD response and showed that the infraslow activity (<1 Hz) from a single electrode in primary somatosensory cortex (SI) was directly correlated with the BOLD signal from SI in both hemispheres.
Taken together, these studies strongly indicate that the BOLD signal reflects contributions from multiple frequency bands and possibly from multiple brain processes. Gamma band activity and infraslow activity, in particular, have been linked to the BOLD signal in multiple species under multiple anesthetic conditions (Table 1). At least two studies have shown that delta band power correlation is predictive of BOLD correlation (Lu et al., 2007; Pan et al., 2011), but since both used varying levels of anesthetic to manipulate correlation, the relationship may be due to common effects of anesthesia on both electrical activity and functional connectivity without proving a causal relationship. The state dependence of both animal and human studies suggests a complex relationship between neural activity and the functional connectivity measured with rsMRI, while simultaneously hinting at the intriguing possibility of teasing out more information about neural activity from the BOLD signal.
Table 1.
Summary of Selected Studies Linking Functional Connectivity to Electrical Activity
| Study | Species | Condition/state | Recording method | BOLD analysis method | Finding |
|---|---|---|---|---|---|
| Laufs et al. (2003) | Human | Awake, eyes closed | EEG | Seed-based correlation | Negative correlation between alpha power and BOLD in lateral frontal and parietal areas; positive correlation with beta power in restrosplenial, temporoparietal, and dorsomedial prefrontal areas |
| Mantini et al. (2007) | Human | Awake, eyes closed | EEG | ICA | Each BOLD network associated with a specific fingerprint of multiple EEG frequency bands |
| Goncalves et al. (2006) | Human | Awake, eyes closed | EEG | Voxel by voxel correlation with EEG | Pattern of BOLD correlation with alpha power varies across and within subjects |
| He et al. (2008) | Human | Awake, slow wave sleep, REM sleep | ECoG | Seed-based correlation | Slow cortical potentials exhibit a correlation structure similar to BOLD across all states; gamma band power correlation similar to BOLD in wakefulness or REM |
| Keller et al. (2013) | Human | Awake | ECoG | Seed-based correlation | Positive and negative correlations were present in both BOLD and high gamma band power |
| Lu et al. (2007) | Rat | Alpha-chloralose anesthesia | ECoG | Seed-based correlation | Delta band power coherence was the most predictive of BOLD correlation as the level of anesthesia deepened |
| Shmuel and Leopold (2008) | Nonhuman primate | Isoflurane and fentanyl anesthesia, eyes open or closed | LFP, MUA | Voxel-by-voxel correlation with LFP | Slow fluctuations in BLP (particularly gamma band) and MUA correlate with spontaneous BOLD fluctuations |
| Pan et al. (2011) | Rat | Isoflurane anesthesia | LFP | Voxel-by-voxel correlation with LFP, seed-based correlation | Broadband LFP power correlation with local BOLD (particularly gamma power); BOLD correlation closest to delta, theta power correlation as function of anesthesia depth |
| Pan et al. (2013) | Rat | Isoflurane and dexmedetomidine anesthesia | LFP | Voxel-by-voxel correlation with LFP | Infraslow LFP activity is correlated with BOLD near electrode and in opposite hemisphere; time-lagged correlation gives pattern of alternating positive and negative correlation along cortex |
| Wang et al. (2012) | Nonhuman primate | Tiletamine/zolazepam anesthesia, eyes closed; awake, eyes open | LFP | Seed-based correlation | Frequencies below 20 Hz contribute most to BOLD correlation and modulate localized gamma activity |
| Thompson et al. (2013a) | Rat | Isoflurane anesthesia | LFP | Sliding window correlation | Sliding window power correlation in theta, beta, and gamma frequencies was linked to sliding window BOLD correlation |
| Chang et al. (2013) | Human | Awake, eyes closed | EEG | Sliding window correlation | Alpha power inversely related to correlation between DMN and TPN |
| Tagliazucchi et al. (2012) | Human | Awake, asleep | EEG | Sliding window correlation | Increased alpha and beta power linked to decreased BOLD connectivity; increased gamma linked to increased BOLD connectivity; patterns altered in subjects changing from wakefulness to sleep |
| Britz et al. (2010) | Human | Awake, eyes closed | EEG | ICA; voxel-by-voxel correlation with microstate time course | EEG microstate time courses were correlated with BOLD network time courses |
| Musso et al. (2010) | Human | Awake, eyes closed | EEG | ICA; voxel-by-voxel correlation with microstate time course | EEG microstates correlated with BOLD in patterns that resemble BOLD resting-state networks |
| Thompson et al. (2013b) | Rat | Isoflurane or dexmedetomidine anesthesia | LFP | Spatiotemporal pattern finding; voxel-by-voxel correlation | Time-lagged patterns of infraslow LFP-BOLD correlation match quasi-periodic patterns observed in BOLD alone |
| Magri et al. (2012) | Nonhuman primate | Remifentanil anesthesia | LFP, MUA | Mutual information | Gamma band power was the most informative about local BOLD signal; alpha and beta bands carry complementary information |
| Thompson et al. (2014) | Rat | Isoflurane or dexmedetomidine anesthesia | LFP | Phase-amplitude coupling; partial correlation | No phase-amplitude coupling was observed between BOLD and LFP or between infraslow LFP and higher frequencies except under isoflurane; partial correlation suggests infraslow and high frequencies carry complementary information |
BLP, band-limited power; BOLD, blood-oxygenation-level-dependent; DMN, default mode network; ECoG, electrocorticography; EEG, electroencephalography; ICA, independent component analysis; LFP, local field potential; MUA, multiunit spiking activity; REM, rapid eye movement; TPN, task positive network.
Detecting Significant Variability in Correlation Over Time
Along with the excitement of obtaining new information about network dynamics with rsMRI comes a host of questions about how to best measure and interpret those dynamics. One of the problems with dynamic analysis is determining which changes in connectivity are significant. The paper by Chang and Glover (2010) found that only some areas exhibited dynamics significantly different from random. Handwerker and associates (2012) similarly showed that connectivity exhibited periodicity but so did phase-scrambled data. Using data that were mismatched across scans and/or rats, Keilholz and associates (2013) demonstrated that periods of high and low correlation could be observed in time courses which shared no temporal information, and that the variation in correlation from real data was mostly indistinguishable from the randomly matched data. Simply because the amount of variability cannot be distinguished from that of randomized data does not mean that the variability does not carry useful information, but it certainly highlights the difficulties involved in the interpretation of dynamic analysis. What portion of the variation in connectivity is meaningful, and how can we identify it?
Ideally, of course, we would like to link network dynamics from rsMRI studies to a more direct indicator of neural activity to ensure that we are detecting meaningful changes. In healthy humans, this usually means EEG. In some ways, though, it is not clear what sort of relationship should be expected between a poorly localized, cortically dominated EEG signal and the functional connectivity matrices that map changes in BOLD correlation throughout the brain. Changes in EEG power reflect large-scale alterations in the state of the brain due to varying levels of wakefulness, for example, and one would certainly expect corresponding changes in the functional connectivity matrices. However, it is conceivable that more subtle cognitive changes during free thought in the scanner (e.g., remembering a past vacation as compared with planning to write a manuscript) might be reflected in changing connectivity but have no effect on the EEG signal. Source localization methods may provide a closer examination of the relationship between the electrical signal and BOLD correlation, with coherence or synchrony in the EEG signal from certain areas linked to varying BOLD correlation between the same areas. EEG microstates are also promising candidates to link to changes in functional connectivity. However, the limited spatial localization of EEG makes a direct comparison far from simple for most areas of the brain.
Even for more invasive recording techniques that allow localized measurements, the key parameters are unclear. What do we measure? The relationship between the BOLD signal and the neural activity at a single site is not necessarily the same as the relationship between BOLD correlation and coordinated neural activity across sites (Pan et al., 2011). For example, gamma band power could be most correlated with fluctuations in the local BOLD signal, while delta band power was the strongest mediator between sites. It may then be possible to separate the two contributions to the BOLD signal if some of their properties are known, enabling a separate examination of the two bands. This is particularly interesting in the context of a widespread theory in neuroscience that high-frequency activity organizes relatively small areas of the brain, while low-frequency activity has a larger spatial footprint.
Most studies to date have looked at band-limited power as a potential source for the BOLD fluctuations, and correlation between BLPs as a comparison to correlated BOLD signals from different areas. This choice originally arose as a way to manage the large discrepancy between the high temporal resolution of the electrophysiological recordings and the slow nature of the BOLD fluctuations. It is unlikely that correlations in the BOLD signal reflect millisecond-level coordination of neural activity due to the low-pass filter inherent in the vasculature, and converting the electrical signals to band-limited power can provide a meaningful measure of activity on the same temporal scale as the BOLD signal. Most groups do not record electrical activity in the infraslow frequencies (<1 Hz) that are directly comparable to the slow changes in the BOLD signal, but recent work shows that these signals themselves are directly linked to hemodynamic and BOLD oscillations (Li et al., 2014a; Pan et al., 2013), and in this case, correlation between the raw electrical signals is quite appropriate for a comparison to BOLD correlation. Future studies should also examine the relationship between measures of synchrony and coherence in electrical signals and BOLD correlation. A recent study by Musall and associates (2014) showed that neural synchrony can modulate EEG signals independently of the amplitude of activity, suggesting that synchrony may prove an important parameter in linking neural activity to the measured rsMRI signal.
Time Scales of Stationarity
During undirected cognition, multiple processes occur. For example, a single thought may last a few seconds; a single topic may last tens of seconds; and a slow increase in drowsiness may occur over hundreds of seconds. The spatial scale of these processes should also vary. While no one knows precisely the extent of activity involved in a single “thought,” it could conceivably affect a minimum of only a few small areas, while large-scale changes throughout the brain can be caused by a reduction in wakefulness.
One of the first questions that arose during studies of network dynamics is that of the time scale at which activity converges to a steady state. rsMRI scans typically last 5–10 min. A study using MR encephalography to acquire images with a repetition time (TR) of 100 msec found that for frequencies of 0.5–0.8 Hz, correlation appeared stable even with short 30 sec windows;, while for more typical frequencies of 0.01–0.1 Hz, variability was apparent even in the 120 sec windows (Lee et al., 2013). A study using typical rsMRI parameters in humans found that correlation began to plateau at around 4 min (Van Dijk et al., 2010). In comparison, an intracranial electrophysiology study found that stable, frequency-dependent patterns of correlation emerge after ∼100 sec (Kramer et al., 2011). These findings do not include the effect of ongoing tasks, which can affect the spontaneous oscillations even after the task is completed. Barnes and associates (2009) found that the fractal characteristics of the spontaneous fluctuations did not return to pre-task value for minutes after the task was completed, and that the time required for this return depended on the difficulty of the task.
In contrast to MRI studies, MEG studies performed under the assumption of stationarity over a 5 min scan resulted in mostly unilateral networks, while functional connectivity maps created from shorter time periods corresponding to maximal network correlation were more bilaterally symmetric and similar to the resting-state networks seen in MRI (de Pasquale et al., 2010).
In anesthetized rats, Thompson and associates (2013a) found that the correlation between BOLD correlation and BLP correlation in multiple bands reached a plateau at window lengths of 30–70 sec. However, the error also increased to a plateau on a similar time frame, suggesting that despite the relatively low signal-to-noise ratio (SNR) of short window measurements, they may be sensitive to transient changes in neural activity. It should be noted that this study utilized a shorter TR (500 msec) and a higher field strength (9.4 T) than typical human studies. Window lengths for most dynamic studies that use sliding windows or temporal segmentation currently range from ∼30 sec to 2 min, limiting the scale of the changes which can be detected. The advent of fast imaging sequences that enable whole brain coverage in less than a second will increase the number of samples in a given time window and may improve sensitivity to short-lived changes (Moeller et al., 2010; Posse et al., 2012).
EEG or MEG and MRI Studies of Network Dynamics
EEG signals reflect fairly widespread cortical activity in the brain and are particularly well suited for examining large-scale changes in brain state, such as those that distinguish sleep from waking. EEG signals, particularly those acquired without a specific task, are less suited to identifying small changes in activity that may reflect spontaneous cognitive processes. Despite this limitation, the relative ease of obtaining simultaneous EEG and rsMRI in healthy volunteers has made it a valuable tool in elucidating the neural basis of functional networks and has begun providing insights to the origin of their dynamics as well. Chang and associates (2013) found an inverse relationship between connectivity between the default mode network (DMN) and dorsal attention network (DAN) and alpha power, using 40 s segments. Higher alpha power was also linked to a larger extent of anticorrelation. A similar study by Tagliazucchi and associates (2012) found that increased alpha and beta power generally corresponded to decreased functional connectivity, while gamma power increases were linked to increased connectivity. This pattern was altered in subjects who were undergoing changes in their level of vigilance (i.e., falling asleep), where slower oscillations were linked to increased connectivity. Similar to the animal studies that used anesthesia level as a perturbation to link changes in neural activity to changes in connectivity (Lu et al., 2007; Pan et al., 2011), changing levels of vigilance may alter both EEG and network activity without necessarily identifying a causal relationship.
The subject's relative level of drowsiness or alertness over the course of a scan may explain many of the large-scale changes observed in functional networks. Allen and associates clustered correlation matrices made using sliding window analysis from a large group of subjects (405) and found that particular states were common across participants. Intriguingly, the frequency of the occurrence of one particular state (state 3) increased over time, suggesting that it might be linked to drowsiness (Allen et al., 2014). The state exhibited thalamocortical disconnection and weakened connectivity in the DMN, consistent with the transition between wakefulness and sleep. Changes in vigilance may also account for some of the links observed between alpha power and connectivity in the DMN/DAN (Chang et al., 2013).
In addition to vigilance levels, other changes can also affect large areas of the brain. Wu and associates (2010) found that posterior alpha power produced wide-spread hemodynamic responses with high functional connectivity between the areas involved while subjects were resting with eyes closed, but many of the hemodynamic responses disappeared and functional connectivity was decreased when eyes were opened. Presumably even without an accompanying change in mental state, the state of the brain can be significantly altered.
One of the side effects of the use of anesthesia in animal studies is that no change in vigilance levels would be expected during stable conditions. Thompson and associates (2013a) compared sliding window correlations in band-limited power from left and right somatosensory cortex to sliding window correlation in the simultaneously acquired BOLD signal in the rat and found that theta, beta, and gamma bands contributed to the BOLD variability. The link between BOLD and BLP is an encouraging indication that the dynamic analysis of rsMRI may be sensitive to more subtle changes in correlation in addition to those caused by changes in wakefulness.
Much interest has been expressed in EEG microstates as building blocks of cognitive processes and potential underpinnings for the BOLD fluctuations used to map connectivity. These microstates manifest as EEG scalp topography that exhibits a stable configuration for a time on the order of 100 msec. Typically, a small number of these stable topographies are revisited over time. The time course of the microstates, when correlated with the BOLD response, produces networks similar to the resting-state networks typically obtained from independent component analysis (ICA) (Britz et al., 2010; Musso et al., 2010), which suggests that the microstates themselves may at least partly underlie the network dynamics observed with BOLD.
Betzel and associates examined the dynamic properties of the EEG, but using synchronization likelihood to measure the time-varying connectivity across electrodes. As with microstates, patterns were found to be stable for tens to hundreds of milliseconds, and a small number of “families” of patterns were continually revisited (Betzel et al., 2012). However, the patterns were not similar to previously observed microstates, which are based on power at a given electrode rather than measures of coordination across electrodes. Their reliance on synchronization rather than power makes these patterns particularly appealing as potential contributors to time-varying BOLD correlations.
MEG studies are not readily combined with MRI, but similar functional connectivity analysis provides intriguing results. In the paper by de Pasquale and associates (2010), it was shown that theta, alpha, and beta bands produced maps most similar to those obtained from MRI when nonstationary analysis was used. Gamma power tended to be more localized, whether because it is locally generated or because of loss of sensitivity due to low SNR is unknown. These results echo the original EEG/rsMRI studies which found that multiple frequencies contributed to each network.
The Influence of Network Events
One way to think of spontaneous cognition is as a string of discrete thoughts or events, whether these are linked to EEG microstates or not. Researchers in rsMRI have begun examininge these factors as potential drivers for the network dynamics observed in the BOLD signal. A work by Petridou and associates (2013) using a deconvolution approach to identify single events has shown that spontaneous events detected in rsMRI account for much of the correlation within networks. A similar study by Liu and Duyn (2013) found coactivation patterns based on high signals in a seed region. The patterns of co-activated areas identified are somewhat similar to those obtained using temporal ICA (Smith et al., 2012). These studies suggest that at least some of the variation in functional connectivity in awake human subjects is driven by transient events rather than slow modulations of vigilance.
The detection of spontaneous events also suggests the possibility of “brain states,” or common configurations of connectivity that recur over time. Keilholz and associates utilized simple thresholding to separate the time courses from somatosensory areas, motor cortex, and the caudate putamen in the anesthetized rat into three states: correlated, uncorrelated, and anticorrelated. They found that a few states accounted for most of the variation throughout the scan (Keilholz et al., 2013). A more complex version of brain state analysis in human subjects was reported by Allen and associates (2014), who first identified network components within a large group of subjects using ICA, then applied sliding window analysis to examine the correlation between each pair of regions as a function of time. The resulting time-varying functional connectivity matrices were then clustered to identify common brain states and transitions. Seven common states were identified across subjects, with the most common state similar to the steady-state connectivity matrix and the others representing substantial deviations from the steady-state values.
Quasi-Periodic Patterns of Brain Activity
“Brain states” are typically considered patterns of instantaneous activity, which may repeat over time but in a sporadic fashion. Recent studies suggest that there are also quasiperiodic spatiotemporal modulations of brain activity which occur on a slower time scale. The first evidence of repeated spatiotemporal patterns of spontaneous BOLD fluctuations came from anesthetized rats. Majeed and associates (2009) reported patterns of high activity that propagated from lateral cortical areas to medial cortical areas, followed by a similar propagating wave of relatively low activity. This first study relied on visual inspection and observed the patterns at discrete intervals. The subsequent development of a correlation-based pattern-finding algorithm not only identified occurrences of the pattern, but also showed that the process was nearly periodic, occurring even when visual detection was difficult due to the presence of other signals (Majeed et al., 2011). Similar patterns were observed in CBV-weighted data (Magnuson et al., 2010).
A natural question was whether these patterns were only present due to the use of anesthesia in the rodent. A group-based analysis of human data using the same pattern-finding algorithm, however, identified a spatiotemporal pattern that was consistent in spatial location and timing across all subjects (Majeed et al., 2011). Interestingly, it involved signal propagation and alternation in several areas of the DMN and task-positive network (TPN). In a different experiment using different methodology, Grigg and Grady (2010) observed a variable pattern of DMN and TPN connectivity that was consistent with the patterns obtained by Majeed and associates. Similar patterns of alternating activation in the DMN and TPN were also observed by Chow and associates (2013) but only during REM sleep. The pattern-finding algorithm used by Majeed and associates makes use of extensive averaging to increase SNR, which may account for their detection of the pattern in data from awake humans. It may be that additional activity overlays the pattern during wakefulness, decreasing its prominence in comparison to REM sleep.
Some of the quasi-periodic patterns (QPPs) appear to follow the vascular structure of the brain, and a study by Tong and Frederick (2010) using near-infrared to map hemodynamics found a pattern similar to QPPs, although the time scales seem to be different. Nevertheless, electrophysiological analogues of these patterns have also been observed. Ko and associates (2011) used intracortical electrical recording to show that infraslow (<1 Hz) changes in BLP coordinate activity in the DMN, with high gamma coherence in a narrow band of low frequencies centered around 0.015 Hz, suggesting a neural basis for the quasiperiodic processes seen in the BOLD signal. Using MEG, de Pasquale and associates (2010) found that the total interdependence measure of internodal coherence had a local maximum at 0.1 Hz, though the infraslow frequencies were not directly measured. These findings fit well with previous work showing that infraslow fluctuations occur in both electrical recordings and psychophysical performance as well as the BOLD signal, with the phase rather than the amplitude of the infraslow activity typically coupled to performance (Khader et al., 2008; Palva and Palva, 2012). Interestingly, both infraslow electrical activity and DMN/TPN correlation have been linked to attention and performance (Fox et al., 2007; Helps et al., 2010; Kelly et al., 2008; Monto et al., 2008; Thompson et al., 2012).
Further evidence of a neural basis for the QPPs comes from localized, invasive measurements of infraslow activity and simultaneous rsMRI in the rat. Infraslow activity was tightly correlated to the BOLD signal from the area near the electrode tip and the homologous region in the contralateral cortex (Pan et al., 2013). Time-lagged correlation between the two signals gave rise to a pattern of propagation that was similar to the QPPs under two different anesthetics, even though the timing of the QPPs depends on the anesthetic used (Thompson et al., 2013b). Other studies in animals also support a direct neural analogue for low-frequency BOLD oscillations. For example, Li and associates (2014a) used voltage-sensitive dye of slow cortical potentials (1–4 Hz) in mice to map functional networks similar to those obtained with hemodynamic measures.
Nested Scales of Electrical Activity and BOLD Correlates
It is hypothesized that high frequencies organize local activity, while lower frequencies are involved in long-range coordination (Buzsaki, 2006). Relationships between different bands of activity create a multiscale, nested structure. Studies in animals have often found relationships between the phase of lower frequencies and the amplitude of higher frequencies, a phenomenon known as phase-amplitude coupling (Tort et al., 2010). It is likely that this sort of hierarchical relationship carries over to the BOLD signal. An excellent study by Magri and associates (2012) found that while gamma power is typically related to the amplitude of the BOLD signal, alpha and beta power carry additional information that modulates the amplitude and latency of the BOLD response to changes in gamma power. In monkeys, Wang and associates (2012) found that oscillations below 20 Hz modulated local gamma activity. Similar results were seen in humans performing a visual attention task (Scheeringa et al., 2011).
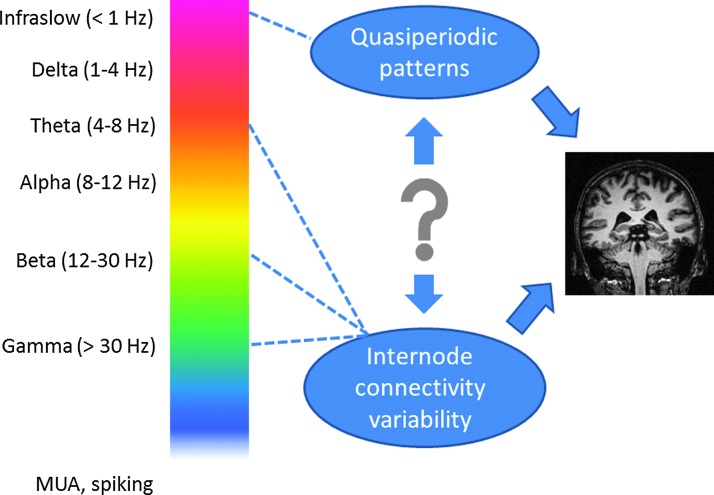
In the context of different levels of coordination mediated by low frequencies as compared with high frequencies, it is tempting to speculate that the demonstrated links between QPPs and infraslow activity and between sliding window BOLD correlation and higher-frequency activity may allow the contributions of the two types of activity to be teased out of the BOLD signal (Fig. 1). The QPPs are large-scale patterns, relatively periodic, and fairly slow, while the changes in sliding window correlation can theoretically be shorter and localized to a few small areas. It has been hypothesized that the infraslow oscillations are driven by changes in power in the higher frequencies or themselves modulate higher-frequency power. However, an analysis of simultaneous microelectrode recording and rsMRI in the rat found that the two processes contributed separately to the BOLD signal (Thompson et al., 2014). Studies in humans are also consistent with the idea that networks may exhibit meaningful variability overlaid on a consistent pattern of coordinated activity. For example, Grigg and Grady (2010) found that the DMN exhibits two modes, one a stable network and the other a variable pattern of connection which is more affected by task performance.
FIG. 1.
The spectrum of electrical activity in the brain ranges from the very low frequencies of infraslow waves to multiunit spiking activity (MUA) measured in hundreds of hertz. Simultaneous imaging and recording experiments in humans and animals have shown that variations in blood-oxygenation-level-dependent (BOLD)-based functional connectivity over time are related to underlying changes in neural activity in multiple frequency bands. As summarized in this review, at least two types of dynamic activity in the brain contribute to the changes in network connectivity. Quasi-periodic patterns of BOLD fluctuations are a large-scale phenomenon linked to infraslow electrical signals, while transient, irregular variations in correlation between sites appear to be more closely tied to electrical activity in higher frequency bands. Studies suggest that subjects' varying levels of wakefulness over the course of a scan also have widespread effects on network connectivity. These processes may exist independently or interact with each other. Spontaneous fluctuations of the BOLD signal clearly contain a wealth of information about dynamic processes in the brain, and the application of appropriate analysis techniques holds the potential to enhance sensitivity to particular aspects of the brain's ongoing activity.
Summary
A substantial body of evidence indicates that at least some portion of the network dynamics measured with rsMRI reflects time-varying coordination of neural activity. In particular, sliding window correlation methods appear to be sensitive to large-scale changes in brain state due to fluctuations in levels of alertness or drowsiness. The variations in connectivity between areas are superimposed on coordinated patterns of activation and deactivation that animal studies have linked to infraslow electrical activity.
The precise mechanisms that underlie the changes in connectivity remain unknown. The data are consistent with the idea that multiple neural processes on different time scales contribute to the network dynamics observed with rsMRI. An exciting prospect is that these processes may be separable, enhancing the BOLD signal sensitivity to particular neural events. One possible approach would be to first obtain the QPP template and use the time course showing template power to regress out the QPPs. Sliding window correlation could be performed on the signal after this regression. The overall result would be a template that maps patterns predominated by infraslow activity; a time course of the strength of those patterns; and a correlation time course which should reflect primarily changes in higher frequency activity. If the QPPs truly reflect a pattern of infraslow modulation, they may prove a fruitful ground for new studies on the diagnosis of attention disorders or in the quest to monitor and improve attentional performance in normal subjects. We speculate that the more transient changes which are often observed with sliding window techniques, on the other hand, may prove more sensitive to changes related to cognitive alterations. Substantial further work will be needed to explore these possibilities.
Future dynamic rsMRI studies stand to benefit from progress on multiple fronts. On the hardware side, engineering advances are providing increased SNR, better temporal resolution, and larger numbers of samples per unit time. New analytical tools incorporating wavelet decomposition may provide better sensitivity to state changes and improved segmentation that reduces the temporal blurring which sliding windows create. Approaches based on graph theory have potential to summarize relevant parameters of the large amounts of data resulting from dynamic analysis. Along with these advances, future experiments that further elucidate the link between changes in connectivity and neural activity or relevant behavioral outputs will place dynamic rsMRI on a firm footing as a tool for basic and clinical science.
Author Disclosure Statement
No competing financial interests exist.
References
- Allen EA, Damaraju E, Plis SM, Erhardt EB, Eichele T, Calhoun VD. 2014. Tracking whole-brain connectivity dynamics in the resting state. Cereb Cortex 24:663–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes A, Bullmore ET, Suckling J. 2009. Endogenous human brain dynamics recover slowly following cognitive effort. PLoS One 4:e6626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betzel RF, Erickson MA, Abell M, O'Donnell BF, Hetrick WP, Sporns O. 2012. Synchronization dynamics and evidence for a repertoire of network states in resting EEG. Front Comput Neurosci 6:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal B, Yetkin FZ, Haughton VM, Hyde JS. 1995. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magn Reson Med 34:537–541 [DOI] [PubMed] [Google Scholar]
- Britz J, Van De Ville D, Michel CM. 2010. BOLD correlates of EEG topography reveal rapid resting-state network dynamics. Neuroimage 52:1162–1170 [DOI] [PubMed] [Google Scholar]
- Bruyns-Haylett M, Harris S, Boorman L, Zheng Y, Berwick J, Jones M. 2013. The resting-state neurovascular coupling relationship: rapid changes in spontaneous neural activity in the somatosensory cortex are associated with haemodynamic fluctuations that resemble stimulus-evoked haemodynamics. Eur J Neurosci 38:2902–2916 [DOI] [PubMed] [Google Scholar]
- Buzsaki G. 2006. Rhythms of the Brain. New York: Oxford University Press [Google Scholar]
- Chang C, Glover GH. 2010. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage 50:81–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Liu Z, Chen MC, Liu X, Duyn JH. 2013. EEG correlates of time-varying BOLD functional connectivity. Neuroimage 72:227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow HM, Horovitz SG, Carr WS, Picchioni D, Coddington N, Fukunaga M, Xu Y, Balkin TJ, Duyn JH, Braun AR. 2013. Rhythmic alternating patterns of brain activity distinguish rapid eye movement sleep from other states of consciousness. Proc Natl Acad Sci U S A 110:10300–10305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Pasquale F, Della Penna S, Snyder AZ, Lewis C, Mantini D, Marzetti L, Belardinelli P, Ciancetta L, Pizzella V, Romani GL, Corbetta M. 2010. Temporal dynamics of spontaneous MEG activity in brain networks. Proc Natl Acad Sci U S A 107:6040–6045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME. 2007. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron 56:171–184 [DOI] [PubMed] [Google Scholar]
- Gonçalves SI, de Munck JC, Pouwels PJW, Schoonhoven R, Kuijer JPA, Maurits NM, Hoogduin JM, Van Someren EJW, Heethaar RM, Lopes da Silva FH. 2006. Correlating the alpha rhythm to BOLD using simultaneous EEG/fMRI: inter-subject variability. Neuroimage 30:203–213 [DOI] [PubMed] [Google Scholar]
- Grigg O, Grady CL. 2010. Task-related effects on the temporal and spatial dynamics of resting-state functional connectivity in the default network. PLoS One 5:e13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handwerker DA, Roopchansingh V, Gonzalez-Castillo J, Bandettini PA. 2012. Periodic changes in fMRI connectivity. Neuroimage 63:1712–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Zempel JM, Smyth MD, Raichle ME. 2008. Electrophysiological correlates of the brain's intrinsic large-scale functional architecture. Proc Natl Acad Sci U S A 105:16039–16044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helps SK, Broyd SJ, James CJ, Karl A, Chen W, Sonuga-Barke EJ. 2010. Altered spontaneous low frequency brain activity in attention deficit/hyperactivity disorder. Brain Res 1322:134–143 [DOI] [PubMed] [Google Scholar]
- Huang L, Liu Y, Li M, Hu D. 2014. Hemodynamic and electrophysiological spontaneous low-frequency oscillations in the cortex: Directional influences revealed by Granger causality. Neuroimage 85:810–822 [DOI] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Allen EA, Bandettini PA, Calhoun VD, Corbetta M, Della Penna S, Duyn JH, Glover GH, Gonzalez-Castillo J, Handwerker DA, Keilholz S, Kiviniemi V, Leopold DA, de Pasquale F, Sporns O, Walter M, Chang C. 2013a. Dynamic functional connectivity: promise, issues, and interpretations. Neuroimage 80:360–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison RM, Womelsdorf T, Gati JS, Everling S, Menon RS. 2013b. Resting-state networks show dynamic functional connectivity in awake humans and anesthetized macaques. Human Brain Mapp 34:2154–2177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilholz SD, Magnuson ME, Pan WJ, Willis M, Thompson GJ. 2013. Dynamic properties of functional connectivity in the rodent. Brain Connect 3:31–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller CJ, Bickel S, Honey CJ, Groppe DM, Entz L, Craddock RC, Lado FA, Kelly C, Milham M, Mehta AD. 2013. Neurophysiological investigation of spontaneous correlated and anticorrelated fluctuations of the BOLD signal. J Neurosci 33:6333–6342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Uddin LQ, Biswal BB, Castellanos FX, Milham MP. 2008. Competition between functional brain networks mediates behavioral variability. Neuroimage 39:527–537 [DOI] [PubMed] [Google Scholar]
- Khader P, Schicke T, Roder B, Rosler F. 2008. On the relationship between slow cortical potentials and BOLD signal changes in humans. Int J Psychophysiol 67:252–261 [DOI] [PubMed] [Google Scholar]
- Kiviniemi V, Vire T, Remes J, Elseoud AA, Starck T, Tervonen O, Nikkinen J. 2011. A sliding time-window ICA reveals spatial variability of the default mode network in time. Brain Connect 1:339–347 [DOI] [PubMed] [Google Scholar]
- Ko AL, Darvas F, Poliakov A, Ojemann J, Sorensen LB. 2011. Quasi-periodic fluctuations in default mode network electrophysiology. J Neurosci 31:11728–11732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer MA, Eden UT, Lepage KQ, Kolaczyk ED, Bianchi MT, Cash SS. 2011. Emergence of persistent networks in long-term intracranial EEG recordings. J Neurosci 31:15757–15767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H, Krakow K, Sterzer P, Eger E, Beyerle A, Salek-Haddadi A, Kleinschmidt A. 2003. Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proc Natl Acad Sci U S A 100:11053–11058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HL, Zahneisen B, Hugger T, LeVan P, Hennig J. 2013. Tracking dynamic resting-state networks at higher frequencies using MR-encephalography. Neuroimage 65:216–222 [DOI] [PubMed] [Google Scholar]
- Leonardi N, Richiardi J, Gschwind M, Simioni S, Annoni J-M, Schluep M, Vuilleumier P, Van De Ville D. 2013. Principal components of functional connectivity: A new approach to study dynamic brain connectivity during rest. Neuroimage 83:937–950 [DOI] [PubMed] [Google Scholar]
- Li B, Liu R, Huang Q, Lu J, Luo Q, Li P. 2014a. Coherent slow cortical potentials reveal a superior localization of resting-state functional connectivity using voltage-sensitive dye imaging. Neuroimage 91:162–168 [DOI] [PubMed] [Google Scholar]
- Li X, Zhu D, Jiang X, Jin C, Zhang X, Guo L, Zhang J, Hu X, Li L, Liu T. 2014b. Dynamic functional connectomics signatures for characterization and differentiation of PTSD patients. Hum Brain Mapp 35:1761–1778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Duyn JH. 2013. Time-varying functional network information extracted from brief instances of spontaneous brain activity. Proc Natl Acad Sci U S A 110:4392–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Zhu X-H, Zhang Y, Chen W. 2012. Neural origin of spontaneous hemodynamic fluctuations in rats under burst-suppression anesthesia condition. Cereb Cortex 21:374–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H, Zuo Y, Gu H, Waltz JA, Zhan W, Scholl CA, Rea W, Yang Y, Stein EA. 2007. Synchronized delta oscillations correlate with the resting-state functional MRI signal. Proc Natl Acad Sci U S A 104:18265–18269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnuson MM, Majeed W, Keilholz SD. 2010. Functional connectivity in BOLD and CBV weighted resting state fMRI in the rat brain. J Magn Reson Imaging 32:584–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magri C, Schridde U, Murayama Y, Panzeri S, Logothetis NK. 2012. The amplitude and timing of the BOLD signal reflects the relationship between local field potential power at different frequencies. J Neurosci 32:1395–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed W, Magnuson M, Hasenkamp W, Schwarb H, Schumacher EH, Barsalou L, Keilholz SD. 2011. Spatiotemporal dynamics of low frequency BOLD fluctuations in rats and humans. Neuroimage 54:1140–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majeed W, Magnuson M, Keilholz SD. 2009. Spatiotemporal dynamics of low frequency fluctuations in BOLD fMRI of the rat. J Magn Reson Imaging 30:384–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantini D, Perrucci MG, Del Gratta C, Romani GL, Corbetta M. 2007. Electrophysiological signatures of resting state networks in the human brain. Proc Natl Acad Sci U S A 104:13170–13175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeller S, Yacoub E, Olman CA, Auerbach E, Strupp J, Harel N, Ugurbil K. 2010. Multiband multislice GE-EPI at 7 tesla, with 16-fold acceleration using partial parallel imaging with application to high spatial and temporal whole-brain fMRI. Magn Reson Med 63:1144–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monto S, Palva S, Voipio J, Palva JM. 2008. Very slow EEG fluctuations predict the dynamics of stimulus detection and oscillation amplitudes in humans. J Neurosci 28:8268–8272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musall S, von Pföstl V, Rauch A, Logothetis NK, Whittingstall K. 2014. Effects of neural synchrony on surface EEG. Cereb Cortex 24:1045–1053 [DOI] [PubMed] [Google Scholar]
- Musso F, Brinkmeyer J, Mobascher A, Warbrick T, Winterer G. 2010. Spontaneous brain activity and EEG microstates. A novel EEG/fMRI analysis approach to explore resting-state networks. Neuroimage 52:1149–1161 [DOI] [PubMed] [Google Scholar]
- Nir Y, Mukamel R, Dinstein I, Privman E, Harel M, Fisch L, Gelbard-Sagiv H, Kipervasser S, Andelman F, Neufeld MY, Kramer U, Arieli A, Fried I, Malach R. 2008. Interhemispheric correlations of slow spontaneous neuronal fluctuations revealed in human sensory cortex. Nat Neurosci 11:1100–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palva JM, Palva S. 2012. Infra-slow fluctuations in electrophysiological recordings, blood-oxygenation-level-dependent signals, and psychophysical time series. Neuroimage 62:2201–2211 [DOI] [PubMed] [Google Scholar]
- Pan W, Thompson G, Magnuson M, Majeed W, Jaeger D, Keilholz S. 2011. Broad-band LFPs correlate with spontaneous fluctuations in fMRI signals in the rat somatosensory cortex under isoflurane anesthesia. Brain Connect 1:119–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan WJ, Thompson GJ, Magnuson ME, Jaeger D, Keilholz S. 2013. Infraslow LFP correlates to resting-state fMRI BOLD signals. Neuroimage 74:288–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petridou N, Gaudes CC, Dryden IL, Francis ST, Gowland PA. 2013. Periods of rest in fMRI contain individual spontaneous events which are related to slowly fluctuating spontaneous activity. Human Brain Mapp 34:1319–1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posse S, Ackley E, Mutihac R, Rick J, Shane M, Murray-Krezan C, Zaitsev M, Speck O. 2012. Enhancement of temporal resolution and BOLD sensitivity in real-time fMRI using multi-slab echo-volumar imaging. Neuroimage 61:115–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakoglu U, Pearlson GD, Kiehl KA, Wang YM, Michael AM, Calhoun VD. 2010. A method for evaluating dynamic functional network connectivity and task-modulation: application to schizophrenia. MAGMA 23:351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheeringa R, Fries P, Petersson K-M, Oostenveld R, Grothe I, Norris DG, Hagoort P, Bastiaansen MCM. 2011. Neuronal dynamics underlying high- and low-frequency EEG oscillations contribute independently to the human BOLD signal. Neuron 69:572–583 [DOI] [PubMed] [Google Scholar]
- Scholvinck ML, Maier A, Ye FQ, Duyn JH, Leopold DA. 2010. Neural basis of global resting-state fMRI activity. Proc Natl Acad Sci U S A 107:10238–10243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuel A, Leopold DA. 2008. Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: implications for functional connectivity at rest. Hum Brain Mapp 29:751–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Miller KL, Moeller S, Xu J, Auerbach EJ, Woolrich MW, Beckmann CF, Jenkinson M, Andersson J, Glasser MF, Van Essen DC, Feinberg DA, Yacoub ES, Ugurbil K. 2012. Temporally-independent functional modes of spontaneous brain activity. Proc Natl Acad Sci U S A 109:3131–3136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tagliazucchi E, von Wegner F, Morzelewski A, Brodbeck V, Laufs H. 2012. Dynamic BOLD functional connectivity in humans and its electrophysiological correlates. Front Hum Neurosci 6:339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G, Magnuson M, Merritt M, Schwarb H, Pan W, McKinley A, Tripp L, Schumacher E, Keilholz S. 2012. Short time windows of correlation between large scale functional brain networks predict vigilance intra-individually and inter-individually. Hum Brain Mapp 34:3280–3298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson G, Pan W, Billings J, Grooms JK, Shakil S, Jaeger D, Keilholz S. 2014. Phase-amplitude coupling and infraslow (<1 Hz) frequencies in the rat brain: relationship to resting state fMRI. Front Integr Neurosci 8:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Merritt MD, Pan W-J, Magnuson ME, Grooms JK, Jaeger D, Keilholz SD. 2013a. Neural correlates of time-varying functional connectivity in the rat. Neuroimage 83:826–836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson GJ, Pan WJ, Magnuson ME, Jaeger D, Keilholz SD. 2013b. Quasi-periodic patterns (QPP): Large-scale dynamics in resting state fMRI that correlate with local infraslow electrical activity. Neuroimage 84:1018–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Y, Frederick BD. 2010. Time lag dependent multimodal processing of concurrent fMRI and near-infrared spectroscopy (NIRS) data suggests a global circulatory origin for low-frequency oscillation signals in human brain. Neuroimage 53:553–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort ABL, Komorowski R, Eichenbaum H, Kopell N. 2010. Measuring phase-amplitude coupling between neuronal oscillations of different frequencies. J Neurophysiol 104:1195–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dijk KRA, Hedden T, Venkataraman A, Evans KC, Lazar SW, Buckner RL. 2010. Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol 103:297–321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Saalmann YB, Pinsk MA, Arcaro MJ, Kastner S. 2012. Electrophysiological low-frequency coherence and cross-frequency coupling contribute to BOLD connectivity. Neuron 76:1010–1020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Eichele T, Calhoun VD. 2010. Reactivity of hemodynamic responses and functional connectivity to different states of alpha synchrony: a concurrent EEG-fMRI study. Neuroimage 52:1252–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Craddock RC, Margulies DS, Yan C-G, Milham MP. 2014. Common intrinsic connectivity states among posteromedial cortex subdivisions: Insights from analysis of temporal dynamics. Neuroimage 93:124–137 [DOI] [PMC free article] [PubMed] [Google Scholar]