Abstract
The genetic deficiency of the C1 inhibitor is responsible for hereditary angioedema (HAE), which is a disease transmitted as an autosomal dominant trait. More than 200 point mutations in the C1 inhibitor gene have been found to be associated with HAE. Patients with this disease suffer from recurrent angioedema, which is mediated by bradykinin derived from activation of the contact system. This system is physiologically controlled at several steps by the C1 inhibitor. In this review, we describe known mechanisms for the development of angioedema in patients with C1 inhibitor deficiency.
The Genetics of Hereditary Angioedema
William Osler first comprehensively described the clinical picture of hereditary angioedema (HAE) in 18881 and, acknowledging the severity of the disease, urged the community to search for a scientific solution. In 1963, Donaldson and Evans discovered that HAE was caused by a genetic deficiency of the C1 inhibitor (C1-INH).2 Other forms of HAE have since been described, but the pathogenesis of those diseases remains obscure. This review will therefore focus on HAE that develops as a result of C1-INH deficiency.3 C1-INH is a serine protease inhibitor (SERPIN), which controls different proteases involved in the complement, kinin/contact, fibrinolytic, and coagulation systems.4 Similar to other SERPINs, C1-INH consists of α-helices and β-sheets, as well as an exposed mobile reactive center loop that is cleaved upon contact with a target protease. This reaction results in the formation of a stable, covalent bond. Protease binding causes dramatic conformational changes in C1-INH, which crushes the protease against its lower pole resulting in inactivation of the enzyme.5 Mutations in C1-INH disrupt the structure of the protein, but oftentimes these changes cannot be detected in plasma or, when a mutated C1-INH is present, has no functional activity. These two situations account for the genetic variants of HAE described by Rosen et al. They found that all patients have low functional plasma levels of C1-INH: most of the times with antigenic deficiency (HAE type I), and in 20% of affected subjects with normal or elevated antigenic levels (HAE type II).6
The genetic transmission of HAE as an autosomal dominant trait was first described in 1917.7 Discovery and subsequent sequencing of the C1-INH gene (SERPING1)8,9 confirmed that the disease occurs when one of the two alleles is mutated.10,11 Rare homozygous C1-INH deficient patients have also been described.12–14 The presence of one mutated allele affects mRNA transcription and protein secretion, which eventually leads to C1-INH plasma levels ranging from 10% to 30% of normal.15 The mechanisms that prevent the remaining wild-type allele from generating C1-INH plasma levels around 50% remain unknown. Patients with HAE catabolize C1-INH faster than normal subjects,16 but concomitant downregulation of the normal allele may also occur, at least with some mutations.17–19 Most mutations that cause HAE are point mutations resulting in single amino acid substitutions.10 Independent families rarely possess identical mutations. More than 200 different mutations have been associated with HAE, and only one protein polymorphism resulting in an amino acid substitution not associated with a disease state has been described to date.20 Moreover, no correlation between genotype and clinical phenotype has been found.
Mechanisms of Edema Formation
Patients with genetic deficiency of C1-INH constantly present increased activation of the classical complement pathway with depletion of C4 and, to a lesser extent, of C2.21,22 Measurement of these parameters may help in the laboratory diagnosis of HAE. Patients with this condition have lifelong C1-INH deficiency and episodic, localized subcutaneous and/or submucosal angioedema. It was originally proposed that complement activation may play a principle role in the pathogenesis of these symptoms, but this was later discredited.23–27 It is known that minor local trauma, stress, and other events may trigger angioedema, and it is now well established that bradykinin (BK) is the principal mediator of symptoms of C1-INH deficiency.28,29 Accordingly, specific inhibition of BK-B2 receptors can revert angioedema.30
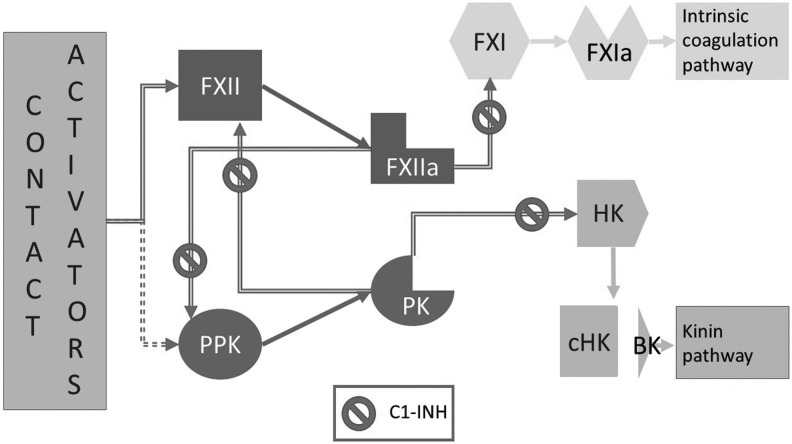
Previous studies of plasma from these patients have indicated that attacks are marked by signs of activation of the contact system.31,32 This system is comprised of a substrate, high molecular weight kininogen (HK), and two zymogens, plasma prekallikrein (PPK) and factor XII (FXII). The system is closely linked to intrinsic coagulation (FXI and onward) and kinin generation pathways (Fig. 1).33 In vitro activation of the contact system is used to measure activated partial thromboplastin time (aPTT), while the best characterized event associated with its in vivo activation is angioedema due to C1-INH deficiency. It has been shown that the increase in BK plasma levels during attacks is due to local production of BK as a result of inappropriate activation of systems controlled by C1-INH.34,35 However, the molecular events triggering angioedema remain unclear, and the reasons for the extreme variability in symptom recurrences among patients and within the same subject from time to time are unknown. Since increased generation of BK appears to be critical for angioedema formation, specific emphasis has been placed on the study of HK, which is the substrate that releases BK when it is cleaved at two sites by plasma kallikrein (PK).36 The cleaved form of HK remains in plasma after protease cleavage, and measurement of this form has shown that the breakdown product not only increases during attacks, but also correlates with disease severity.37 It is known that BK release is the final event of contact system activation, and that activation begins when the two zymogens of this system, FXII and PPK, are proteolytically cleave into FXIIa and PK to acquire enzymatic activity, respectively. These two enzymes reciprocally act on their zymogens through a positive feedback loop to generate BK efficiently. Binding to a negatively charged surface changes the conformation of FXII, which attains limited proteolytic activity and becomes increasingly susceptible to cleavage by PK and FXIIa.38 Small amounts of FXIIa have the capacity to cleave more of its own precursor to produce efficient contact activation.39 The mechanisms for in vitro activation of the contact system by negatively charged surfaces are well established. The process begins with the formation of FXIIa, which in turn generates the active enzymes PK and FXIa that eventually form BK and thrombin. However, the sequence of events that occurs in vivo is less clear, and pathways of contact activation that skip FXII and begin with PPK and/or PK have been described. Membrane-expressed enzyme prolylcarboxypeptidase (PRCP) and/or the protein HSP90 can directly activate prekallikrein bound to endothelial cell surfaces.40,41 PPK itself has enzymatic activity that can initiate PK generation and contact activation under specific circumstances.42 Based on these findings, a FXII-independent mechanism of contact activation initiating symptoms in HAE patients has been suggested.43 Experimental results have shown that FXII may pivot contact system activation in vivo similar to the mechanism observed in vitro, suggesting that the enzymatic activity of FXII may initiate contact activation.33,44 Moreover, other studies have shown that FXII can be activated in vivo by substances such as platelets polyphosphates and heparin-activated mast cells, thus ensuring efficient activation of the contact system.45,46 Thus, it is possible that these mechanisms of contact activation specifically contribute to angioedema formation.47
FIG. 1.
Schematic representation of the mechanism of activation of the contact system. In the presence of contact system activators (see text for details), the zymogens Factor XII (FXII) and/or possibly plasma prekallikrein (PPK) are converted into active enzymes FXIIa and plasma kallikrein (PK), respectively. These enzymatic forms reciprocally activate their zymogens and thus generate a positive feedback loop. In the presence of sufficient amounts of active enzyme, FXIIa generates active Factor XI (FXIa), which initiates the intrinsic coagulation pathway. PK proteolyzes high molecular weight kininogen (HK) at two sites thus generating cleaved HK (cHK) and the nonapeptide bradykinin in the kinin pathway. C1 inhibitor (C1-INH) controls the system by intervening at several locations.
In any consideration of the molecular events leading to episodic BK release in patients deficient in C1-INH, the role of plasmin, which is a key enzyme of the fibrinolytic cascade, should be considered. Plasmin is generated from plasminogen by physiologic activators tPA and uPA, but plasmin is also involved in contact system activation and can form kinins through the activation of kallikrein.48 In addition, the contact system can also lead to the generation of fibrinolytic activity in vivo.49 Early experiments from Virginia Donaldson's group showed that the presence of plasmin is necessary for in vitro generation of kinin activity in plasma from patients with HAE, and that the presence of plasmin facilitates the release of BK from HK.24,50 Moreover, patients with HAE have increased levels of plasmin–antiplasmin complexes during attacks, and antifibrinolytic agents have been shown to be efficacious in the treatment of these patients.51–53 Thus, although the data are not conclusive and the in vivo relevance of plasmin activity on the contact system remains to be defined, a role for plasmin should be carefully considered when trying to solve the puzzle of angioedema pathogenesis.
Other proteases, such as MASP-1, which is activated during episodes of angioedema, might also contribute to bradykinin production.54 Under physiologic conditions, BK either binds specific receptors or is degraded by proteolytic enzymes comprising angiotensin-converting enzyme (ACE), aminopeptidase P (APP), neutral endopeptidase 24.11 (NEP, neprilysin), and carboxypeptidases M and N (CPM, CPN).55 During angioedema attacks, HAE patients release large amounts of BK into their plasma, and it has been found that these patients can have up to a 10-fold increase in concentration over resting conditions.28 Two types of receptors can bind BK: B2, which is constitutively expressed, and B1, which is induced by inflammatory stimuli (mainly IL-1β and tumor necrosis factor [TNF]-α).56 Animal studies assessing the therapeutic efficacy of specific B2 antagonists have shown that angioedema is predominantly dependent on stimulation of B2 receptors.29,30 Nevertheless, experiments using a transwell model of endothelial cell permeability and intravital microscopy in animals suggest a potential role also for B1 receptors.57 Considering that expression of these receptors requires an inflammatory stimulus and that efficiently respond to BK metabolites as well, it has been suggested that they may intervene during the late phase of the attacks and possibly extend the duration.
The receptors for BK are coupled to G-proteins, which comprise a family of intracellular signal transmitting proteins that signal through intracellular calcium mobilization, release of arachidonic acid, and stimulation of the endothelial nitric-oxide synthase (eNOS).58 These intracellular events increase paracellular permeability opening through endothelial cell–cell junctions, which are largely composed of vascular endothelial cadherin (VE-cadherin).59 BK has a fast and potent effect on increasing the permeability in veins by inducing the acute and generally reversible disorganization of junctional VE-cadherin through tyrosine phosphorylation, which depends on activation of the tyrosine kinase Src.60 Bouillet et al. demonstrated that BK- and PK-mediated stimulation of a confluent endothelial cell monolayer induced a time-dependent release of soluble VE-cadherin fragments, as well as an increase in vascular permeability secondary to the disruption of cell–cell junctions. They also detected a 90 kDa VE-cadherin extracellular domain fragment in a patient's serum during the attack that was barely detectable when the attack was not occurring.61 Moreover, Kajdacsi et al. found that markers of endothelial cell activation, including serum levels of von Willebrand factor antigen and collagen-binding activity, soluble E-selectin, and endothelin-1, were significantly increased during HAE attacks.62 Taken together, these studies indicate that endothelial cells actively participate in angioedema attacks and may represent new potential targets for diagnosis and therapy.
Starting from Osler's solicitation to discover a scientific solution for HAE, this review has detailed studies that advance our understanding of the mechanisms leading to angioedema. Importantly, these studies have led to therapies that are already available for treatment of HAE, as well as others that are currently being assessed in clinical trials. As a consequence, the lives of patients with HAE have drastically improved. However, this review underscores the possibility of developing new diagnostic and therapeutic approaches that may be better tailored to patients. Moreover, we still face a marginal understanding of newly described forms of angioedema that await novel diagnostic and therapeutic approaches. Thus, Osler's request still remains today: science has to proceed and find solutions.
Acknowledgments
This review was supported by Telethon grant no. GGP08223.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Osler W. Hereditary angio-neurotic oedema. Am J Med Sci 1888;95:362–367 [DOI] [PubMed] [Google Scholar]
- 2.Donaldson VH, Evans RR. A biochemical abnormality in hereditary angioneurotic edema: absence of serum inhibitor of C′1-esterase. Am J Sci 1963;31:37–44 [DOI] [PubMed] [Google Scholar]
- 3.Cicardi M, Aberer W, Banerji A, et al. Classification, diagnosis, and approach to treatment for angioedema: consensus report from the Hereditary Angioedema International Working Group. Allergy 2014;69:602–616 [DOI] [PubMed] [Google Scholar]
- 4.Davis AE, 3rd, Mejia P, Lu F. Biological activities of C1 inhibitor. Mol Immunol 2008;45:4057–4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gooptu B, Lomas DA. Conformational pathology of the serpins: themes, variations, and therapeutic strategies. Annu Rev Biochem 2009;78:147–176 [DOI] [PubMed] [Google Scholar]
- 6.Rosen FS, Pensky J, Donaldson V, Charache P. Hereditary angioneurotic edema: two genetic variants. Science 1965;148:957–958 [DOI] [PubMed] [Google Scholar]
- 7.Crowder JR, Crowder TR. Five generations of angioneurotic edema. Arch Inter Med. 1917;20:840–852 [Google Scholar]
- 8.Bock SC, Skriver K, Nielsen E, et al. Human C1 inhibitor: primary structure, cDNA cloning, and chromosomal localization. Biochemistry 1986;25:4292–4301 [DOI] [PubMed] [Google Scholar]
- 9.Carter PE, Duponchel C, Tosi M, Fothergill JE. Complete nucleotide sequence of the gene for human C1 inhibitor with an unusually high density of Alu elements. Eur J Biochem 1991;197:301–308 [DOI] [PubMed] [Google Scholar]
- 10.Cicardi M, Igarashi T, Kim MS, Frangi D, Agostoni A, Davis AE., 3rd.Restriction fragment length polymorphism of the C1 inhibitor gene in hereditary angioneurotic edema. J Clin Invest 1987;80:1640–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoppa-Lyonnet D, Tosi M, Laurent J, Sobel A, Lagrue G, Meo T. Altered C1 inhibitor genes in type I hereditary angioedema. N Engl J Med 1987;317:1–6 [DOI] [PubMed] [Google Scholar]
- 12.Blanch A, Roche O, Urrutia I, Gamboa P, Fontan G, Lopez-Trascasa M. First case of homozygous C1 inhibitor deficiency. J Allergy Clin Immunol 2006;118:1330–1335 [DOI] [PubMed] [Google Scholar]
- 13.Lopez-Lera A, Favier B, de la Cruz RM, Garrido S, Drouet C, Lopez-Trascasa M. A new case of homozygous C1-inhibitor deficiency suggests a role for Arg378 in the control of kinin pathway activation. J Allergy Clin Immunol 2010;126:1307–1310 e3 [DOI] [PubMed] [Google Scholar]
- 14.Bafunno V, Divella C, Sessa F, et al. De novo homozygous mutation of the C1 inhibitor gene in a patient with hereditary angioedema. J Allergy Clin Immunol 2013;132:748–750 [DOI] [PubMed] [Google Scholar]
- 15.Cicardi M, Igarashi T, Rosen FS, Davis AE., 3rd.Molecular basis for the deficiency of complement 1 inhibitor in type I hereditary angioneurotic edema. J Clin Invest 1987;79:698–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Quastel M, Harrison R, Cicardi M, Alper CA, Rosen FS. Behavior in vivo of normal and dysfunctional C1 inhibitor in normal subjects and patients with hereditary angioneurotic edema. J Clin Invest 1983;71:1041–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kramer J, Rosen FS, Colten HR, Rajczy K, Strunk RC. Transinhibition of C1 inhibitor synthesis in type I hereditary angioneurotic edema. J Clin Invest 1993;91:1258–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ernst SC, Circolo A, Davis AE, 3rd, Gheesling-Mullis K, Fliesler M, Strunk RC. Impaired production of both normal and mutant C1 inhibitor proteins in type I hereditary angioedema with a duplication in exon 8. J Immunol 1996;157:405–410 [PubMed] [Google Scholar]
- 19.Pappalardo E, Zingale LC, Cicardi M. C1 inhibitor gene expression in patients with hereditary angioedema: quantitative evaluation by means of real-time RT-PCR. J Allergy Clin Immunol 2004;114:638–644 [DOI] [PubMed] [Google Scholar]
- 20.Kalmar L, Hegedus T, Farkas H, Nagy M, Tordai A. HAEdb: a novel interactive, locus-specific mutation database for the C1 inhibitor gene. Hum Mutat 2005;25:1–5 [DOI] [PubMed] [Google Scholar]
- 21.Austen KF, Sheffer AL. Detection of hereditary angioneurotic edema by demonstration of a reduction in the second component of human complement. N Engl J Med 1965;272:649–656 [DOI] [PubMed] [Google Scholar]
- 22.Carpenter CB, Ruddy S, Shehadeh IH, Muller-Eberhard HJ, Merrill JP, Austen KF. Complement metabolism in man: hypercatabolism of the fourth (C4) and third (C3) components in patients with renal allograft rejection and hereditary, angioedema (HAE). J Clin Invest 1969;48:1495–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klemperer MR, Donaldson VH, Rosen FS. Effect of C′1 esterase on vascular permeability in man: studies in normal and complement-deficient individuals and in patients with hereditary angioneurotic edema. J Clin Invest 1968;47:604–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donaldson VH, Rosen FS, Bing DH. Role of the second component of complement (C2) and plasmin in kinin release in hereditary angioneurotic edema (H.A.N.E.) plasma. Trans Assoc Am Physicians 1977;90:174–183 [PubMed] [Google Scholar]
- 25.Fields T, Ghebrehiwet B, Kaplan AP. Kinin formation in hereditary angioedema plasma: evidence against kinin derivation from C2 and in support of “spontaneous” formation of bradykinin. J Allergy Clin Immunol 1983;72:54–60 [DOI] [PubMed] [Google Scholar]
- 26.Strang CJ, Cholin S, Spragg J, et al. Angioedema induced by a peptide derived from complement component C2. J Exp Med 1988;168:1685–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shoemaker LR, Schurman SJ, Donaldson VH, Davis AE., 3rd.Hereditary angioneurotic oedema: characterization of plasma kinin and vascular permeability-enhancing activities. Clin Exp Immunol 1994;95:22–28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nussberger J, Cugno M, Amstutz C, Cicardi M, Pellacani A, Agostoni A. Plasma bradykinin in angio-oedema. Lancet 1998;351:1693–1697 [DOI] [PubMed] [Google Scholar]
- 29.Han ED, MacFarlane RC, Mulligan AN, Scafidi J, Davis AE., 3rd.Increased vascular permeability in C1 inhibitor-deficient mice mediated by the bradykinin type 2 receptor. J Clin Invest 2002;109:1057–1063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cicardi M, Banerji A, Bracho F, et al. Icatibant, a new bradykinin-receptor antagonist, in hereditary angioedema. N Engl J Med 2010;363:532–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schapira M, Silver LD, Scott CF, et al. Prekallikrein activation and high-molecular-weight kininogen consumption in hereditary angioedema. N Engl J Med 1983;308:1050–1053 [DOI] [PubMed] [Google Scholar]
- 32.Cugno M, Cicardi M, Coppola R, Agostoni A. Activation of factor XII and cleavage of high molecular weight kininogen during acute attacks in hereditary and acquired C1-inhibitor deficiencies. Immunopharmacology 1996;33:361–364 [DOI] [PubMed] [Google Scholar]
- 33.de Maat S, Tersteeg C, Herczenik E, Maas C. Tracking down contact activation—from coagulation in vitro to inflammation in vivo. Int J Lab Hematol 2014;36:374–381 [DOI] [PubMed] [Google Scholar]
- 34.Nussberger J, Cugno M, Cicardi M, Agostoni A. Local bradykinin generation in hereditary angioedema. J Allergy Clin Immunol 1999;104:1321–1322 [DOI] [PubMed] [Google Scholar]
- 35.Cugno M, Cicardi M, Bottasso B, et al. Activation of the coagulation cascade in C1-inhibitor deficiencies. Blood 1997;89:3213–3218 [PubMed] [Google Scholar]
- 36.Schmaier AH. Assembly, activation, and physiologic influence of the plasma kallikrein/kinin system. Int Immunopharmacol 2008;8:161–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suffritti C, Zanichelli A, Maggioni L, Bonanni E, Cugno M, Cicardi M. High-molecular-weight kininogen cleavage correlates with disease states in the bradykinin mediated angioedema due to hereditary c1-inhibitor deficiency. Clin Exp Allergy 2014February19. doi: 10.1111/cea.12293. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Griffin JH. Role of surface in surface-dependent activation of Hageman factor (blood coagulation factor XII). Proc Natl Acad Sci U S A 1978;75:1998–2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Citarella F, Wuillemin WA, Lubbers YT, Hack CE. Initiation of contact system activation in plasma is dependent on factor XII autoactivation and not on enhanced susceptibility of factor XII for kallikrein cleavage. Br J Haematol 1997;99:197–205 [DOI] [PubMed] [Google Scholar]
- 40.Shariat-Madar Z, Mahdi F, Schmaier AH. Identification and characterization of prolylcarboxypeptidase as an endothelial cell prekallikrein activator. J Biol Chem 2002;277:17962–17969 [DOI] [PubMed] [Google Scholar]
- 41.Joseph K, Tholanikunnel BG, Kaplan AP. Heat shock protein 90 catalyzes activation of the prekallikrein-kininogen complex in the absence of factor XII. Proc Natl Acad Sci U S A 2002;99:896–900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Joseph K, Tholanikunnel BG, Kaplan AP. Factor XII-independent cleavage of high-molecular-weight kininogen by prekallikrein and inhibition by C1 inhibitor. J Allergy Clin Immunol 2009;124:143–149 [DOI] [PubMed] [Google Scholar]
- 43.Joseph K, Tholanikunnel BG, Bygum A, Ghebrehiwet B, Kaplan AP. Factor XII-independent activation of the bradykinin-forming cascade: Implications for the pathogenesis of hereditary angioedema types I and II. J Allergy Clin Immunol 2013;132:470–475 [DOI] [PubMed] [Google Scholar]
- 44.Renne T, Schmaier AH, Nickel KF, Blomback M, Maas C. In vivo roles of factor XII. Blood 2012;120:4296–4303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oschatz C, Maas C, Lecher B, et al. Mast cells increase vascular permeability by heparin-initiated bradykinin formation in vivo. Immunity 2011;34:258–268 [DOI] [PubMed] [Google Scholar]
- 46.Muller F, Mutch NJ, Schenk WA, et al. Platelet polyphosphates are proinflammatory and procoagulant mediators in vivo. Cell 2009;139:1143–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bjorkqvist J, Sala-Cunill A, Renne T. Hereditary angioedema: a bradykinin-mediated swelling disorder. Thromb Haemost 2013;109:368–374 [DOI] [PubMed] [Google Scholar]
- 48.Vogt W. Kinin formation by plasmin, an indirect process mediated by activation of kallikrein. J Physiol 1964;170:153–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levi M, Hack CE, de Boer JP, Brandjes DP, Buller HR, ten Cate JW. Reduction of contact activation related fibrinolytic activity in factor XII deficient patients. Further evidence for the role of the contact system in fibrinolysis in vivo. J Clin Invest 1991;88:1155–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kleniewski J, Blankenship DT, Cardin AD, Donaldson V. Mechanism of enhanced kinin release from high molecular weight kininogen by plasma kallikrein after its exposure to plasmin. J Lab Clin Med 1992;120:129–139 [PubMed] [Google Scholar]
- 51.Cugno M, Hack CE, de Boer JP, Eerenberg AJ, Agostoni A, Cicardi M. Generation of plasmin during acute attacks of hereditary angioedema. J Lab Clin Med 1993;121:38–43 [PubMed] [Google Scholar]
- 52.Frank MM, Sergent JS, Kane MA, Alling DW. Epsilon aminocaproic acid therapy of hereditary angioneurotic edema. A double-blind study. N Engl J Med 1972;286:808–812 [DOI] [PubMed] [Google Scholar]
- 53.Sheffer AL, Austen KF, Rosen FS. Tranexamic acid therapy in hereditary angioneurotic edema. N Engl J Med 1972;287:452–454 [DOI] [PubMed] [Google Scholar]
- 54.Dobo J, Major B, Kekesi KA, et al. Cleavage of kininogen and subsequent bradykinin release by the complement component: mannose-binding lectin-associated serine protease (MASP)-1. PLoS One 2011;6:e20036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moreau ME, Garbacki N, Molinaro G, Brown NJ, Marceau F, Adam A. The kallikrein-kinin system: current and future pharmacological targets. J Pharmacol Sci 2005;99:6–38 [DOI] [PubMed] [Google Scholar]
- 56.Marceau F, Regoli D. Bradykinin receptor ligands: therapeutic perspectives. Nat Rev Drug Discov 2004;3:845–852 [DOI] [PubMed] [Google Scholar]
- 57.Bossi F, Fischetti F, Regoli D, et al. Novel pathogenic mechanism and therapeutic approaches to angioedema associated with C1 inhibitor deficiency. J Allergy Clin Immunol 2009;124:1303–1310 e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leeb-Lundberg LM, Marceau F, Muller-Esterl W, Pettibone DJ, Zuraw BL. International union of pharmacology. XLV. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev 2005;57:27–77 [DOI] [PubMed] [Google Scholar]
- 59.Dejana E, Orsenigo F, Lampugnani MG. The role of adherens junctions and VE-cadherin in the control of vascular permeability. J Cell Sci 2008;121:2115–2122 [DOI] [PubMed] [Google Scholar]
- 60.Orsenigo F, Giampietro C, Ferrari A, et al. Phosphorylation of VE-cadherin is modulated by haemodynamic forces and contributes to the regulation of vascular permeability in vivo. Nat Commun 2012;3:1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bouillet L, Mannic T, Arboleas M, et al. Hereditary angioedema: key role for kallikrein and bradykinin in vascular endothelial-cadherin cleavage and edema formation. J Allergy Clin Immunol 2011;128:232–234 [DOI] [PubMed] [Google Scholar]
- 62.Kajdacsi E, Jani PK, Csuka D, et al. Endothelial cell activation during edematous attacks of hereditary angioedema types I and II. J Allergy Clin Immunol 2014;133:1686–1691 [DOI] [PubMed] [Google Scholar]