Abstract
High numbers of genetically modified hematopoietic stem cells (HSCs) equipped with enhanced engrafting potential are required for successful stem cell gene therapy. By using thalassemia as a model, we investigated the functional properties of hematopoietic stem and progenitor cells (HSPCs) from Hbbth3/45.2+ mice after mobilization with G-CSF, plerixafor, or G-CSF+plerixafor and the engraftment kinetics of primed cells after competitive primary and noncompetitive secondary transplantation. G-CSF+plerixafor yielded the highest numbers of HSPCs, while G-CSF+plerixafor-mobilized Hbbth3/45.2+ cells, either unmanipulated or transduced with a reporter vector, achieved faster hematologic reconstitution and higher levels of donor chimerism over all other types of mobilized cells, after competitive transplantation to B6.BoyJ/45.1+ recipients. The engraftment benefit observed in the G-CSF+plerixafor group was attributed to the more primitive stem cell phenotype of G-CSF+plerixafor-LSK cells, characterized by higher CD150+/CD48 expression. Moreover, secondary G-CSF+plerixafor recipients displayed stable or even higher chimerism levels as compared with primary engrafted mice, thus maintaining or further improving engraftment levels over G-CSF- or plerixafor-secondary recipients. Plerixafor-primed cells displayed the lowest competiveness over all other mobilized cells after primary or secondary transplantation, probably because of the higher frequency of more actively proliferating LK cells. Overall, the higher HSC yields, the faster hematological recovery, and the superiority in long-term engraftment indicate G-CSF+plerixafor-mobilized blood as an optimal graft source, not only for thalassemia gene therapy, but also for stem cell gene therapy applications in general.
Introduction
A considerable number of genetic diseases, including various immunodeficiencies (Cavazzana-Calvo et al., 2000; Aiuti et al., 2009, 2013), lysosomal storage diseases (Cartier and Aubourg, 2010; Biffi et al., 2013), and thalassemia (Cavazzana-Calvo et al., 2010), have been cured during the last decade by stem cell gene therapy. Despite successes, stem cell gene therapy still faces major limitations.
A major issue is the availability of high numbers of hematopoietic stem cells (HSCs) that display high engrafting capacity under competitive conditions. This is especially important when (1) a nonmyeloablative conditioning is preferably applied, (2) the disease background does not provide a selective advantage at the level of stem/progenitor cells to allow an enrichment of gene-corrected cells after infusion, and (3) a low to modest in vivo gene transfer is anticipated. Under these competitive conditions, large numbers of transduced CD34+ cells displaying enhanced engrafting potential may most effectively compete for niche occupancy over the endogenous unmodified bone marrow cells.
In gene therapy of genetic diseases such as thalassemia, Fanconi anemia, Gaucher disease, and chronic granulomatous disease, in which a competitive bone marrow environment exists, the quantity but also the quality of the infused cells are critical for the outcome.
In the present study, we used thalassemia as a disease model, in order to determine the optimal graft source for stem cell gene therapy, as defined by an increased content in HSCs with enhanced long-term repopulating capacity.
We previously addressed the issue of HSC quantity in mobilized grafts in two clinical trials testing G-CSF- and plerixafor-based mobilization approaches in adult patients with thalassemia major (Yannaki et al., 2012, 2013). We concluded from these studies that the G-CSF+plerixafor combination is the optimal mobilization approach for patients with thalassemia, yielding in a synergistic manner, high numbers of CD34+ cells by single-apheresis collections, even when G-CSF is administered at low doses in splenectomized patients to avoid hypeleukocytosis (Yannaki et al., 2013).
Here, we focus on the quality of mouse thalassemic HSCs mobilized by G-CSF, plerixafor, or G-CSF+plerixafor, exploring their functional properties in vitro and in vivo under competitive transplantation settings.
Our results indicate that G-CSF+plerixafor-mobilized HSCs exhibit clear quantitative and qualitative superiority over HSCs obtained by either single-agent mobilization. G-CSF+plerixafor-mobilized cells, either unmanipulated or genetically modified, achieved faster hematologic recovery and the higher chimerism levels after competitive and serial transplantation. Consequently, G-CSF+plerixafor-mobilized blood potentially represents an optimal graft source, the clinical relevance of which extends beyond thalassemia gene therapy, practically applying to the whole stem cell gene therapy field.
Materials and Methods
Mice
B6.129P2-Hbb-b1tm1Unc Hbb-b2tm1Unc/J (Thalassemic, Hbbth-3) and B6.SJL-PtrcaPepcb/BoyJ (B6.BoyJ) mice were purchased from Jackson Laboratory (Bar Harbor, ME), and bred and/or maintained under an individually ventilated cage system and in accordance with the Institutional Animal Care and Use Committee. The thalassemic mouse model (Hbbth-3), developed by Yang et al. (1995), represents a viable form of the disease, which clinically resembles the human β-thalassemia intermedia.
Mobilization
Recombinant hG-CSF (Tevagrastim; TevaGenerics GmbH, Freiburg, Germany) was administered intraperitoneally (ip) at 250 μg/kg, once a day for 6 days. Plerixafor (Mozobil; Genzyme Corp., Cambridge, MA) was administered ip at a dose of 5 mg/kg, once a day for 3 days. In the combination setting, G-CSF was administered in the evening (days 1–6) and plerixafor in the morning (days 5–7). The mice were sacrificed 1 hr after the last plerixafor dose, and the hematopoietic tissues were harvested for analysis. Control mice received no treatment.
Splenectomy
Splenectomy was aseptically performed under general anesthesia. A small incision was made in the peritoneal wall, the blood vessels supporting the spleen were ligated with 3-0 silk sutures, and the spleen was removed. The incision was closed in two layers using 3-0 silk sutures. Mice were left to recover for 15 days before being used in the experiments.
Histopathological and immunohistochemical analysis
Thalassemic spleens were fixed after removal, in 4% formaldehyde buffer for at least 24 hr, dehydrated, and embedded in paraffin. Sections of 2.5 μm were routinely stained with eosin–hematoxylin for histology. For immunohistochemistry, spleen sections were labeled with anti-SDF-1a (FL-93, dilution 1:200; Santa Cruz Biotechnology, Santa Cruz, CA) according to manufacturer's recommendations, and 10 optical fields per section were counted blindly by a pathologist.
Flow cytometry
Cells were labeled with directly fluorescence-conjugated antibodies and subsequently analyzed on a FACS flow cytometer (FACS Calibur; BD, San Jose, CA) with the CELLQuest software, according to standard procedures, unless otherwise stated.
Lin−/sca-1+/c-kit+ cells
Blood, bone marrow, and spleen cells were stained with APC-Mouse Lineage Cocktail (containing anti-CD3, anti-CD11b, anti-B220, anti-GR-1, anti-Ter-119) and FITC-anti-Sca-1 (D7) and PE-anti-c-kit (2B8) (BD Biosciences, San Jose, CA). The absolute number of LSK cells per milliliter of peripheral blood, per two femurs, and per spleen was calculated based on the following formula: LSK%×absolute cell count per milliliter of blood or per tissue×10−2.
Signaling lymphocyte activation marker LSK cells
For evaluation of the percentage of CD150+/CD48− LSKs (signaling lymphocyte activation marker [SLAM] LSKs), peripheral blood cells were depleted of Lin+ cells (Lineage Cell Depletion kit; MiltenyiBiotec, Auburn, CA), stained with FITC-anti-Sca-1/PE-anti-c-kit (BD Biosciences) and with PE-Cy7-anti-CD48 (HM48-1)/APC-anti-CD150 (mShad150) (eBioscience, San Diego, CA), and analyzed via flow cytometry (FACS Aria II, FACSDiva Software).
Cell cycle analysis
Mobilized blood cells were fixed in 70% ethanol and incubated at −20°C for at least 2 hr, before staining with PE-anti-c-kit, APC-Mouse Lineage Cocktail, and propidium iodide. The fraction of cells in the G0, G1, S, and G2/M phases of cell cycle was determined by gating on Lin−/c-kit+ cells.
CXCR4 and CD26
The expression of the CXCR4 and CD26 surface molecules in Lin−/c-kit+ cells (LKs) was evaluated by the frequency and geometric mean fluorescence intensity after staining with FITC-anti-CD184 (2B11/CXCR4)/PE-anti-c-kit/APC-Mouse Lineage Cocktail and FITC-anti-CD26 (H194-112)/PE-anti-c-kit/APC-Mouse Lineage Cocktail (BD Biosciences).
Hematopoietic progenitor cell studies
Peripheral blood cells (105), bone marrow, and spleen single-cell suspensions (5×104) were plated in duplicate according to the manufacturer's instructions in cytokine-supplemented (rmIL-3 and rmSCF; R&D Systems, Minneapolis, MN) methylcellulose medium (Methocult 3134; Stem Cell Technologies Inc., Vancouver, CA). Absolute CFU-GM numbers per milliliter of blood, per two femurs, and per spleen were determined based on the following formula: (CFU-GM×absolute cell count per tissue or per ml of blood)/number of plated cells.
Hematologic parameters and spleen size
White blood cell counts were blindly measured in blood smears stained with May-Grunwalds-Giemsa (Merck, Darmstadt, Germany). Reticulocytes were stained with Thiazole Orange (RetiCount; BD Biosciences) and samples were analyzed by flow cytometry. Spleen size was assessed in the excised spleen by the ratio of spleen weight (mg)/body weight (g).
Migration assays
Migration assays were carried out as previously described (Voermans et al., 1999). The lower compartments of Transwell systems (24-well Transwell plate; Corning, Boston, MA) were supplemented with IMDM+100 ng/ml SDF-1a (R&D Systems). Mobilized and nonmobilized peripheral blood cells (105) were placed in the upper chambers and input control wells. After 4 hr incubation (37°C, 5% CO2), the input and the migrated cells were collected, double-stained with PE-anti-c-kit/APC-Mouse Lineage Cocktail (BD Biosciences), and counted with BD Trucount (BD Biosciences). The percentage of LKs migrated to SDF-1a was calculated as follows: (number of cells migrated/number of input control cells)×100.
Lentiviral vector
We used a reporter vector based on a self-inactivating lentiviral vector backbone containing an internal PGK promoter and an enhanced green fluorescent protein expression cassette (kindly provided by Kiem HP). The vector was produced by 293T cells transient cotransfection with transfer vector, gag–pol construct, and VSV-G envelope construct (kindly provided by Emery DW) according to established protocol (Zufferey et al., 1997). Vector was harvested, filtered, and 1000-fold concentrated. Titer estimates were calculated by flow-cytometric determination of GFP expression in HeLa cells, indicating a titer between 1 and 2×108 transducing units (TU)/ml of concentrated vector supernatant.
Ex vivo culture and transduction
Murine mobilized PB cells containing equal numbers of LSK cells were prestimulated for 10 hr with cytokine combinations of mIL-3 (10 ng/ml), mSCF (50 ng/ml), and Flt-3L (10 ng/ml) (R&D Systems). Transduction was performed as 8 hr vector exposures, by adding vector supernatant at a low multiplicity of infection (MOI 1) to the cells plated in fibronectin fragment-coated (Retronectin; Takara, Shuzo, Japan), nontissue culture-treated six-well plates, in the presence of protamine sulfate at 4 μg/ml plate. Transduction efficiency was evaluated after flow-cytometric determination of GFP expression in bulk culture and in single CFU colonies.
Competitive transplantation and secondary transplantation
For competitive stem cell transplantation experiments, G-CSF-, plerixafor-, G-CSF+plerixafor-mobilized and steady-state blood cells from Hbbth-3/CD45.2 donors were mixed with 5×105 competitor bone marrow cells from B6.BoyJ mice (CD45.1+) and transplanted into lethally irradiated (1050 cGy) B6.BoyJ/CD45.1 recipients. The entire blood volumes, obtained by cardiac puncture, from 3 donor mice were pooled and infused to each recipient (3:1). In other sets of experiments, a peripheral blood volume containing the same LSK cell number (103) was infused to each recipient along with 2×105 competitor bone marrow cells. The absolute number of LSKs to be infused as well as the LKs (Lin−/c-kit+), LSs (Lin−/sca-1+), Lin− cells, and CD150+/CD48−/LSKs contained in the grafts was calculated based on their frequency by FCM and the absolute cell counts within the graft. The absolute number of colony forming cells (CFCs) was calculated based on the number of colonies generated by plating 105 cells and the absolute cell counts within the graft.
At 6 months, primary recipients were sacrificed, bone marrow was collected, and 106 cells were transplanted into lethally irradiated secondary recipients in a noncompetitive fashion.
Hematologic recovery and donor chimerism
Hematologic recovery was assessed weekly for 8 weeks, starting on day 15, by the absolute neutrophil counts in blood smears. Recipients were considered hematologically reconstituted when neutrophils were consistently above 500/μl. Platelet counts were obtained by absolute count beads (BD Trucount) and by flow cytometry based on forward and side scatter characteristics. Platelet recovery was calculated as a percentage to average normal platelet levels. Donor chimerism was determined monthly up to 6 months by flow cytometry (FITC-anti-CD45.2 and PE-anti-CD45.1; Immunostep) as the proportion of CD45.2+ cells in the CD45.1/CD45.2+ cells.
Statistical analysis
Results are expressed as mean±SE, and statistical significance was determined by ANOVA with Bonferroni correction using Minitab v.16; p-value <0.05 was considered statistically significant.
Results
Characteristics of HSC mobilization in thalassemic mice
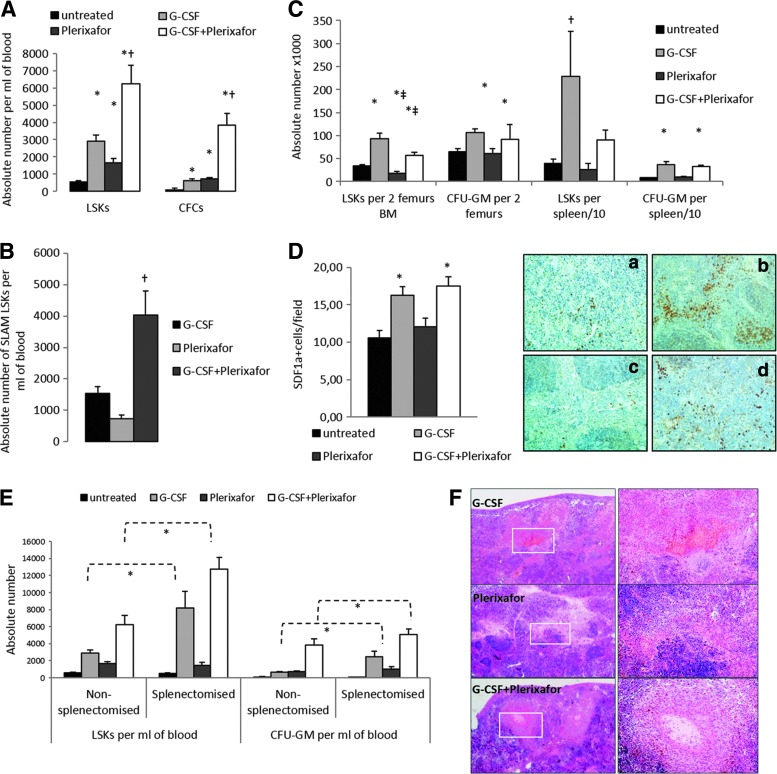
Thalassemic mice were mobilized by G-CSF-alone, plerixafor-alone, or a combination of the two agents. Plerixafor-alone significantly peripheralized LSKs and CFU-GM as compared with steady-state condition; however, it did not further improve hematopoietic stem and progenitor cell (HSPC) mobilization over G-CSF-alone. The G-CSF+plerixafor combination not only induced an 11-fold and a 50-fold increase in the number of LSKs/μl and CFU-GM/ml over steady-state condition, respectively, but also markedly increased the magnitude of mobilization over G-CSF-alone and plerixafor-alone (Fig. 1A). Importantly, the G-CSF+plerixafor combination also significantly increased the numbers of circulating SLAM (signaling lymphocyte activation markers)-LSK cells, defined as CD150+/CD48−/LSKs, over single-agent mobilization (Fig. 1B).
FIG. 1.
Distribution of mobilized HSPCs among hematopoietic compartments and the mobilization effect on the spleen. (A) Absolute numbers of LSK cells and CFCs are presented per milliliter of blood. (B) Absolute numbers of SLAM LSKs are presented per milliliter of blood. (C) Absolute numbers of LSK cells and CFCs are presented per two femurs and spleen (× 10−1). Data are expressed as mean±SEM from untreated (n=13), G-CSF-treated (n=13), plerixafor-treated (n=13), and G-CSF+plerixafor-treated (n=16) thalassemic mice. *p<0.05 vs. untreated, ‡p<0.05 vs. G-CSF, #p<0.05 vs. plerixafor, and †p<0.05 vs. all other. (D) Quantitation and representative images of SDF-1 expression by immunohistochemistry in spleen sections of untreated (a), G-CSF-mobilized (b), plerixafor-mobilized (c), and G-CSF+plerixafor-mobilized (d) mice. Original magnification×200. (E) Absolute numbers of LSK cells and CFU-GM are presented per milliliter of blood of untreated, G-CSF-treated, plerixafor-treated, and G-CSF+plerixafor-treated, nonsplenectomized, and splenectomized mice. Data are expressed as mean±SEM (n=3–6 animals/group per experiment×3 independent experiments). *p<0.05. (F) Splenic infarcts were detected in all mobilization groups. Original magnification×20 (left) and ×100 (right). CFCs, colony forming cells; HSPCs, hematopoietic stem and progenitor cells; SLAM, signaling lymphocyte activation marker.
G-CSF stimulated the proliferation of LSKs and CFU-GM within the bone marrow before their peripheralization, whereas plerixafor rather directly released stem and progenitor cells from their niches to circulation, thus resulting in reduced numbers of LSKs in the plerixafor-mobilized bone marrow over either steady state or G-CSF-mobilized bone marrow. Because of the opposing effects of G-CSF and plerixafor, G-CSF+plerixafor combination increased the bone marrow LSKs over the steady-state condition, but at a lower level than G-CSF-alone (Fig. 1C).
G-CSF or the combination of G-CSF+plerixafor markedly increased LSKs and CFU-GM in the spleen over the steady-state condition, whereas no significant change was observed in the plerixafor spleens (Fig. 1C). Moreover, increased SDF1a expression was demonstrated in the spleens of G-CSF- or G-CSF+plerixafor-mobilized mice, as compared with the untreated and plerixafor-mobilized mice, thus suggesting that G-CSF mobilization generates achemotactic cell motility toward spleen niches, finally resulting in stem and progenitor cell sequestration in the spleen (Fig. 1D). The increased cell migration to the spleen with G-CSF suggests that splenectomy may positively affect G-CSF mobilization. Indeed, splenectomy greatly augmented G-CSF and G-CSF+plerixafor mobilization in terms of LSKs and CFCs, but had no impact on cell yields with plerixafor-alone mobilization (Fig. 1E).
The trapping of HSPCs in the spleen may, at least partially, interpret the significant organ size increase in G-CSF- and G-CSF+plerixafor-mobilized mice over the steady-state condition (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/hgtb) and the higher frequency of G-CSF-induced splenic infarctsin tissue sections (Fig. 1F and Supplementary Table S1). Despite the significant organ enlargement in mice receiving G-CSF, no splenic rupture or death was observed during mobilization in neither G-CSF-treated nor plerixafor-treated groups.
G-CSF-alone- and G-CSF+plerixafor-mobilized mice developed significant leukocytosis, whereas plerixafor-induced leukocytosis was less prominent. These differences were more profound in the mobilized splenectomized mice (Supplementary Table S1). The mobilization per se or the type of mobilization had no effect on reticulocyte counts, whereas splenectomy resulted in all cases in reduced reticulocytosis, probably by ameliorating hemolysis (Supplementary Table S1).
Functional in vitro properties of mobilized thalassemic HSCs
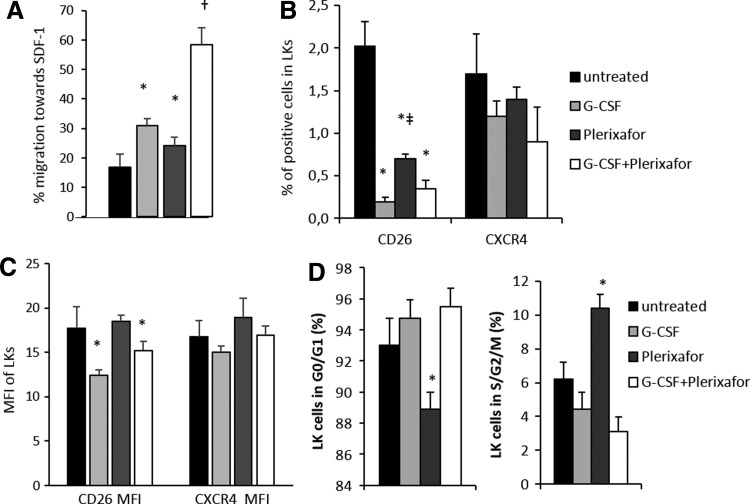
The in vitro chemotaxis of mobilized blood demonstrated enhanced migration of G-CSF+plerixafor-mobilized Lin−c-kit+ (LK) cells toward SDF-1 as compared with all other groups, implying a better homing potential of G-CSF+plerixafor-mobilized cells upon transplantation (Fig. 2A).
FIG. 2.
In vitro properties of differently mobilized LK cells. (A) Migration of Lin−/c-kit+ (LK) mobilized and steady-state blood cells in response to 100 ng/ml SDF-1a. Data are expressed as percentage±SEM of migrated cells (n=3 mice per group assayed in duplicate). Expression of CXCR4 and CD26 on LK cells shown as frequency (B) and as MFI±SEM (C) (n=9 mice per group). (D) Cell cycle status expressed as the average S&G2&M and G0&G1 frequency±SEM of LK cells (n=4 mice per group), *p<0.05 vs. untreated, ‡p<0.05 vs. G-CSF, and †p<0.05 vs. all other.
All mobilized groups exhibited similar LK cell CXCR4 expression and percentage of positive cells, whereas plerixafor-alone-mobilized LK cells showed higher CD26 expression and percentage of positive cells over G-CSF- or G-CSF+plerixafor-mobilized cells (Fig. 2B and C). Cell cycle analysis of the differently mobilized cells showed that plerixafor mobilized more actively cycling LK cells than G-CSF or G-CSF+plerixafor (Fig. 2D).
Accelerated hematologic reconstitution and superior engraftment of G-CSF+plerixafor-mobilized blood cells
To assess the long-term repopulating (LTR) ability of plerixafor-alone-, G-CSF-alone- and G-CSF+plerixafor-mobilized thalassemic HSCs, we used a competitive repopulating assay at a 3:1 ratio, in which, each lethally irradiated CD45.1+ recipient received a whole blood transplant from 3 Hbbth-3/CD45.2+ mobilized donors plus a standard dose of congenic CD45.1+ bone marrow competitor cells. Control mice received steady-state peripheral blood.
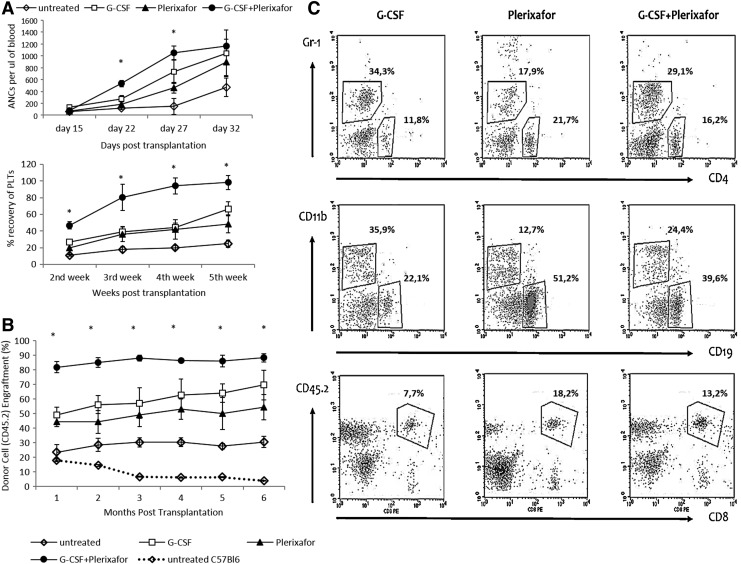
Hematologic recovery was significantly accelerated in G-CSF+plerixafor cell recipients as evidenced by the 1–2-week faster reconstitution of neutrophils and platelets (Fig. 3A).
FIG. 3.
Competitive repopulation in mice after 3:1 transplantation. Whole blood from mobilized thalassemic 45.2+ mice was infused into lethally irradiated B6.BoyJ (45.1+) mice at a 3:1 (donor:recipient) ratio, along with a steady number of competitor 45.1+ cells (0.5×106). (A) Hematologic recovery in terms of platelet recovery (%) is depicted as a percentage to average normal platelet levels and neutrophil recovery as absolute neutrophil cell count (ANC) at different time points after transplantation. Data are expressed as mean±SEM of recipients receiving unmobilized and G-CSF-, plerixafor-, and G-CSF+plerixafor-mobilized grafts. (B) Engraftment based on the % donor's (45.2+) chimera of 45.1+ recipients. (C) Representative dot plots depictive of multilineage reconstitution capacity of differently mobilized CD45.2+ (donor) cells. Each experiment was performed 3 times; n=5 animals per group per experiment. *p<0.05 vs. all other groups. Content of infused grafts in LSK and CFCs, expressed as mean±SEM; *p<0.05 vs. all other groups.
Accordingly, significantly higher donor chimerism was detected in the recipients of cells mobilized with the combination of G-CSF+plerixafor versus plerixafor-alone or G-CSF-alone, at all time points after transplantation and at sacrifice (CD45.2%: 88.34±2.93 vs. 54.40±8.69 vs. 69.69±10.20, p=0.003 and p=0.05, respectively) (Fig. 3B). Balanced multilineage engraftment was achieved in all groups, with plerixafor-engrafted mice showing a tendency toward higher T-lymphocytic representation within the CD45.2+ blood cells (Fig. 3C).
The engraftment potential did not differ between thalassemic and normal mobilized cells, as similar chimerism levels were reached by mobilized C57Bl/6 cells after 3:1 competitive transplantation (Supplementary Fig. S1). Interestingly, steady-state thalassemic blood cell recipients showed modest and persistent chimerism levels (∼20%; Fig. 3B), which could be interpreted by the higher LSK cell and CFU-GM content in the unmobilized blood of thalassemic mice as compared with normal mice (p=0.03) (Fig. 3B and Supplementary Fig. S2).
G-CSF+plerixafor-mobilized HSCs demonstrate a qualitative advantage for engraftment, over plerixafor-alone- or G-CSF-alone-mobilized cells
The higher engraftment levels and faster hematological recovery observed with G-CSF+plerixafor-mobilized blood cells when three donors were used to reconstitute one recipient could simply reflect the presence of higher numbers of LSK cells in the G-CSF+plerixafor-mobilized graft rather than LSK cells with enhanced repopulating ability.
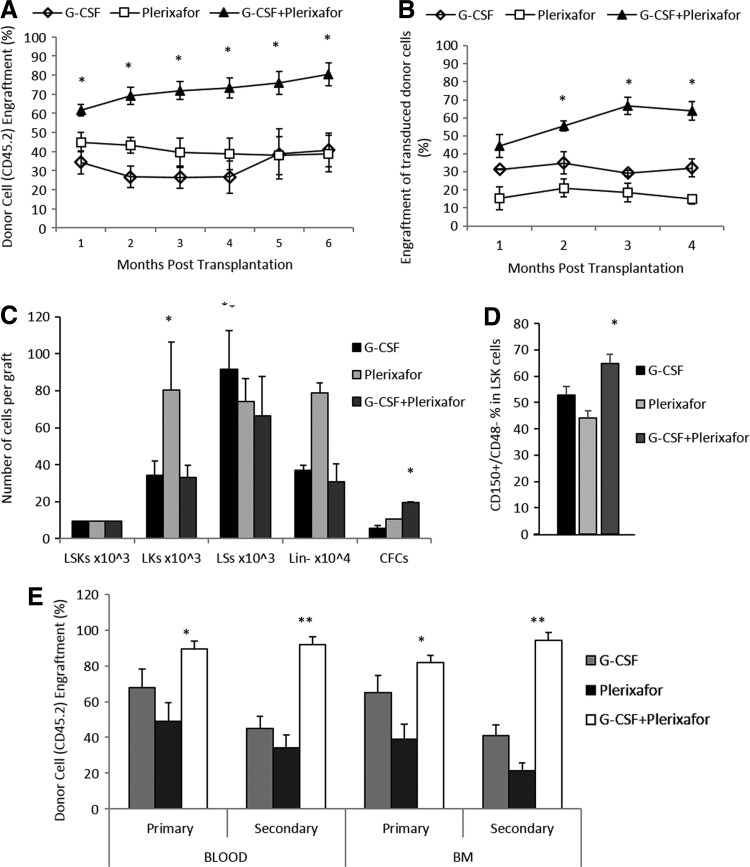
To address this question, we performed competitive transplantations of equal numbers of immunophenotypically characterized mobilized LSK cells to lethally irradiated recipients. As in the 3:1 transplantation, the donor chimerism levels in the G-CSF+plerixafor group were significantly higher over plerixafor-alone or G-CSF-alone groups, at all time points after transplant and at sacrifice (CD45.2%: 80.43±5.99 vs. 38.78±8.73 vs. 40.84±9.57, p=0.01 and p=0.02, respectively) (Fig. 4A), suggesting that G-CSF+plerixafor LSKs are probably qualified with an intrinsic engraftment benefit. Importantly, when G-CSF+plerixafor-mobilized cells were transduced with a GFP reporter lentiviral vector (Supplementary Fig. S3) and transplanted at equal LSK numbers under competitive settings, the long-term engraftment superiority was maintained again, over the differently mobilized cells infused into the recipients after transduction (Fig. 4B).
FIG. 4.
Competitive repopulation after transplantation of equal number of LSKs and secondary transplantation from primary engrafted recipients. (A and B) Mobilized peripheral blood containing equal numbers of immunophenotypically characterized G-CSF-, plerixafor-, G-CSF+plerixafor-mobilized 45.2+ LSKs (1×104) was transplanted in a competitive fashion either unmanipulated (A) or transduced with a reporter lentiviral vector (B), to lethally irradiated recipients, along with 0.25×106 competitor 45.1+ BM cells. Donor's chimera is shown as mean±SEM (n=5 recipients per group). (C) Composition in progenitor cells of infused grafts containing equal number of LSK cells. Data from three grafts per group are expressed as mean±SEM. (D) Frequency of CD150+/CD48− cells (SLAM) within differently mobilized LSK populations. Data from 9 to 11 animals per group, represented as mean±SEM. (E) An amount of 2×106 BM cells from primary recipients of each group were injected in a noncompetitive fashion into lethally irradiated secondary recipients. Primary donor's chimera in secondary recipients is shown as 45.2+ mean frequency±SEM; *p<0.05 and **p<0.0001 vs. all other groups.
The superior long-term engraftment potential of G-CSF+plerixafor-mobilized grafts is associated with a more primitive stem cell phenotype of mobilized cells
To address whether by transplanting equal numbers of nonpurified LSKs we might had infused higher numbers of different primitive or committed cell subpopulations to G-CSF+plerixafor-cell recipients, thus favoring engraftment over plerixafor- or G-CSF-cell recipients, we analyzed the content of the differently mobilized grafts containing equal LSK cells, in terms of Lin−c-kit+ cells (LK), Lin−sca-1+ cells (LS), Lin− cells, and CFCs.
Despite that plerixafor-mobilized grafts contained significantly higher numbers of LK cells and Lin− cells (Fig. 4C), neither short- nor long-term engraftment was enhanced over all other types of grafts. In contrast, the significantly higher numbers of CFCs infused to G-CSF+plerixafor-equal LSK cell recipients (Fig. 4C) were correlated with increased competiveness of the G-CSF+plerixafor-cells over single-agent mobilized cells. However, the higher progenitor cell content cannot robustly support the sustained long-term engraftment superiority of this type of graft.
To further investigate the engraftment privilege of the G-CSF+plerixafor-mobilized cells, we quantitated the frequency of SLAM (CD150+/CD48−) cells within the donor LSKs. A higher fraction of SLAM cells was present in the LSK cell population of G-CSF+plerixafor-mobilized grafts compared to single–agent mobilized grafts (Fig. 4D).
To evaluate if G-CSF+plerixafor-mobilized cells truly have greater inherent long-term repopulating/self-renewal activity, 6 months after primary transplantation, 2×106 bone marrow cells from the competitively engrafted primary mice were injected into lethally irradiated secondary recipients in a noncompetitive manner. Six months after secondary transplant, the comparison of donor chimerism in hematopoietic tissues of secondary engrafted mice versus primary engrafted recipients showed that secondary recipients that received G-CSF+plerixafor-mobilized cells displayed stable or slightly increased chimerism levels, whereas the engraftment superiority was further augmented over the secondary recipients that received G-CSF- or plerixafor-mobilized cells (Fig. 4E).
These data imply that G-CSF+plerixafor-mobilized cells are inherently more competitive than single-agent-mobilized cells.
Discussion
The successful application of stem cell gene therapy requires availability of large numbers of pluripotent repopulating stem cells. G-CSF-mobilized hematopoietic grafts have largely replaced bone marrow in autologous and allogeneic transplantation (Bensinger et al., 2001, 2009), and until recently, G-CSF was the only HSC-mobilizing agent available for clinical use. G-CSF-mobilized grafts were shown to be richer in CD34+ cells and to display faster engraftment kinetics over grafts collected by conventional bone marrow harvest (Bensinger et al., 1993; Heldal et al., 2000; Ringdén et al., 2000). Consequently, G-CSF-mobilized CD34+ cells currently represent the main source of HSCs for transplantation and a preferable graft source for several stem cell gene therapy applications (Ott et al., 2006; Cartier et al., 2009; Boztug et al., 2010).
Successful stem cell gene therapy, however, does not simply rely on the availability of high numbers of CD34+ cells, but also on the quality of the cells to be genetically modified, in order to ensure robust, long-term engraftment after administration to the host.
It is known that the ex vivo transduction process alters the stem/progenitor cell behavior by inducing changes in cell cycle, apoptosis, and adhesion molecules, rendering the gene-modified cells less competitive over their unmanipulated counterparts (Szilvassy et al., 2001; Yong et al., 2002; Ahmed et al., 2004; Mazurier et al., 2004). Moreover, in chronic diseases in which partial myeloablation is preferable to ensure low peritransplant toxicity (i.e., hemoglobinopathies) and/or there is no selective pressure favoring the engraftment of and enrichment in the genetically engineered HSCs (i.e., hemoglobinopathies, Fanconi anemia), the availability of large numbers of not only pluripotent repopulating stem cells but also stem cells with enhanced engrafting properties is mandatorily required.
In recent years, an alternative mobilizing agent, plerixafor, which is a synthetic, reversible inhibitor of the CXCR4 receptor, has been shown to rapidly mobilize human and murine HSCs (Liles et al., 2003; Devine et al., 2004, 2008; Hübel et al., 2004; Larochelle et al., 2006) and to exhibit marked synergism in combination with G-CSF, increasing CD34+ cell yields by several fold (Broxmeyer et al., 2005; Flomenberg et al., 2005; DiPersio et al., 2009; Nademanee et al., 2012).
We have previously explored, in two clinical trials, mobilization by G-CSF or plerixafor-based strategies in adult patients with β-thalassemia major (Yannaki et al., 2012, 2013) and demonstrated that the combination of G-CSF+plerixafor is the optimal mobilization strategy in thalassemia, yielding very high numbers of CD34+ cells by single collections, even when low doses of G-CSF are administered in splenectomized patients to avoid G-CSF-induced hyperleukocytosis.
In the present study, by using thalassemia as a model, we sought to define the optimal graft source for stem cell gene therapy, in terms of both quantity and quality of HSCs. To this end, we tested in a thalassemic mouse model the functional characteristics of LSK cells mobilized by G-CSF-alone, plerixafor-alone, and G-CSF+plerixafor, and the engraftment kinetics of differently mobilized cells, either unmanipulated or genetically modified, in competitive transplantation settings. In addition, the thalassemic mouse model offered us the opportunity to study the different kinetics of HSPCs in the hematopoietic compartments during mobilization by various modes.
Herein, we confirmed the superiority of G-CSF+plerixafor combination as a mobilizer and described a distinct role of the spleen during mobilization by G-CSF-alone and plerixafor-alone. An increased homing/trapping of HSPCs to the spleen, associated with increased splenic SDF-1a expression, was observed during G-CSF mobilization but not plerixafor mobilization. Reasonably, splenectomy greatly augmented G-CSF mobilization, in consistency with our previous study (Yannaki et al., 2010), whereas it had no effect in plerixafor mobilization. In a clinical setting, this could be translated to improved G-CSF mobilization of splenectomized patients over the nonsplenectomized, although in humans where the spleen is not highly active as a hematopoietic organ, this effect may not be so prominent.
G-CSF+plerixafor-mobilized cells demonstrated enhanced engraftment properties over G-CSF-alone- or plerixafor-alone-mobilized cells, which were firstly apparent in the fast kinetics of white blood cell and platelet recovery after transplantation at a 3:1 ratio. The benefit of the earlier hematopoietic reconstitution after full or partial myeloablation is clinically translated to lower incidence of infections or hemorrhagic complications during the early posttransplant period and thus abbreviated hospitalizations (Sheridan et al., 1992; To et al., 1992). The absolute number of transplanted stem and progenitor cells is critical toward accelerated hematologic recovery, and the increased content in LSK cells and CFU-GM of G-CSF+plerixafor-mobilized cells, as compared with single agent-mobilized grafts, was probably associated with this effect.
In addition, the G-CSF+plerixafor-mobilized cells, when transplanted at a 3:1 ratio, demonstrated more competitive long-term repopulation ability throughout the whole posttransplantation period than G-CSF- or plerixafor-mobilized cells. Although the increased stem cell frequency in this graft type may have contributed to this effect, the superior performance of G-CSF+plerixafor-mobilized cells also reflected intrinsic differences in the repopulating potential over the cells mobilized by different modes. Indeed, competitive transplantation of equal numbers of LSK cells, both with unmanipulated or genetically modified cells and with serial transplantation, demonstrated consistently superior LTR/self-renewal ability of G-CSF+plerixafor- over single-agent-mobilized cells, thus suggesting that a qualitative, in addition to quantitative, advantage accounts for the superior performance of G-CSF+plerixafor-mobilized cells. G-CSF+plerixafor-mobilized LSK cells displayed a more primitive HSC phenotype over the differently mobilized LSKs, expressed as CD150+/CD48−. CD150 and CD48 glycoproteins belong to the SLAM family members and have been identified as useful markers for the enrichment of HSCs (Kiel et al., 2005; Kim et al., 2006; Oguro et al., 2013). CD150+/CD48−/LSK cells represent a cell population highly enriched in HSCs and greatly capable of reconstituting a lethally irradiated recipient with almost 50% of single CD150+/CD48−/LSK cells providing multilineage reconstitution (Kiel et al., 2005). Our data show that G-CSF+plerixafor-mobilized LSKs contain CD150+/CD48− cells at a significantly higher frequency over single-agent-mobilized LSKs, thus elucidating the higher engraftment scores in both primary and secondary recipients.
To determine other mechanisms responsible for the enhanced hematopoietic repopulation by G-CSF+plerixafor-mobilized cells, we studied the in vitro migratory capacity to SDF-1a, the expression of CXCR4 and CD26, and the cell cycle status of mobilized LK cells. The SDF-1a/CXCR4 migration axis is considered to play a major role in hematopoietic stem/progenitor cell trafficking and homing (Kim and Broxmeyer, 1998; Ma et al., 1999; Peled et al., 1999; Kollet et al., 2001; Lapidot et al., 2005; Bonig et al., 2006) and the in vitro migratory capacity of CD34+ cells to SDF-1a to be associated with hematopoietic recovery (Voermans et al., 2001; Marquez-Curtis et al., 2009). In addition, CD26, a dipeptidylpeptidase IV (DPPIV), mediates the cleavage of SDF1 and it has been shown that CD26 expression on donor cells negatively regulates their homing and engraftment, whereas inhibition or deletion of CD26 significantly improves the transplantation outcome (Christopherson et al., 2004; Campbell and Broxmeyer, 2008). The cell cycle state can also greatly impact engraftment and homing of HSCs and quiescent HSCs have higher long-term repopulating ability than actively cycling cells (Gothot et al., 1998; Jetmore et al., 2002; Ahmed et al., 2004; Passegué et al., 2005) although this still remains arguable (Goldberg et al., 2013).
In our study, the increased in vitro migratory capacity to SDF-1a of G-CSF+plerixafor-mobilized cells over single-agent-mobilized cells was not associated with differences in CXCR4 or CD26 expression. The increased migration to SDF-1a of G-CSF+plerixafor-mobilized cells could have played a role to the faster hematologic reconstitution and the enhanced engraftment after competitive transplantation. On the other hand, plerixafor-mobilized LK cells displayed higher CD26 expression, and, in agreement with other reports (Larochelle et al., 2006; Welschinger et al., 2013), they were more actively proliferating than G-CSF- or G-CSF+plerixafor-mobilized cells. These features, as well as the lower frequency of SLAM cells in plerixafor-mobilized LSK population, could interpret the lower competiveness of plerixafor-mobilized cells in primary and secondary transplantations as compared with G-CSF- or G-CSF+plerixafor-mobilized cells.
Overall, our data suggest that the G-CSF+plerixafor-mobilized cells represent a superior graft source for thalassemia gene therapy, because of the higher yields of HSCs displaying a more primitive phenotype, the faster hematological recovery, and the superiority in long-term engraftment over single-agent-mobilized cells. Obviously, these data are not restricted to thalassemia but could be clinically translated to all other gene therapy applications where ex vivo HSC manipulation is needed.
Supplementary Material
Acknowledgments
This work was supported by NIH Grant #P01 HL053750-19 and the “Cooperation-Action I” ESPA Program 09SYN-12-1159. We thank Emery DW and Kiem HP for providing us with the plasmid constructs.
Author Disclosure Statement
The authors declare no competing financial interests.
References
- Ahmed F., Ings S.J., Pizzey A.R., et al. (2004). Impaired bone marrow homing of cytokine-activated CD34+ cells in the NOD/SCID model. Blood 103, 2079–2087 [DOI] [PubMed] [Google Scholar]
- Aiuti A., Brigida I., Ferrua F., et al. (2009). Hematopoietic stem cell gene therapy for adenosine deaminase deficient-SCID. Immunol. Res. 44, 150–159 [DOI] [PubMed] [Google Scholar]
- Aiuti A., Biasco L., Scaramuzza S., et al. (2013). Lentiviral hematopoietic stem cell gene therapy in patients with Wiskott-Aldrich syndrome. Science 341, 1233151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensinger W., Singer J., Appelbaum F., et al. (1993). Autologous transplantation with peripheral blood mononuclear cells collected after administration of recombinant granulocyte stimulating factor. Blood 81, 3158–3163 [PubMed] [Google Scholar]
- Bensinger W.I., Martin P.J., Storer B., et al. (2001). Transplantation of bone marrow as compared with peripheral-blood cells from HLA-identical relatives in patients with hematologic cancers. N. Engl. J. Med. 344, 175–181 [DOI] [PubMed] [Google Scholar]
- Bensinger W., DiPersio J.F., and McCarty J.M. (2009). Improving stem cell mobilization strategies: future directions. Bone Marrow Transplant. 43, 181–195 [DOI] [PubMed] [Google Scholar]
- Biffi A., Montini E., Lorioli L., et al. (2013). Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 341, 1233158. [DOI] [PubMed] [Google Scholar]
- Bonig H., Priestley G.V., and Papayannopoulou T. (2006). Hierarchy of molecular-pathway usage in bone marrow homing and its shift by cytokines. Blood 107, 79–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boztug K., Schmidt M., Schwarzer A., et al. (2010). Stem-cell gene therapy for the Wiskott-Aldrich syndrome. N. Engl. J. Med. 363, 1918–1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broxmeyer H.E., Orschell C.M., Clapp D.W., et al. (2005). Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J. Exp. Med. 201, 1307–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell T.B., and Broxmeyer H.E. (2008). CD26 inhibition and hematopoiesis: a novel approach to enhance transplantation. Front. Biosci. 13, 1795–1805 [DOI] [PubMed] [Google Scholar]
- Cartier N., and Aubourg P. (2010). Hematopoietic stem cell transplantation and hematopoietic stem cell gene therapy in X-linked adrenoleukodystrophy. Brain Pathol. 20, 857–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier N., Hacein-Bey-Abina S., Bartholomae C.C., et al. (2009). Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 326, 818–823 [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M., Hacein-bey S., Saint Basile de G., et al. (2000). Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 288, 669–672 [DOI] [PubMed] [Google Scholar]
- Cavazzana-Calvo M., Payen E., Negre O., et al. (2010). Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature 467, 318–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopherson K.W., Hangoc G., Mantel C.R., and Broxmeyer H.E. (2004). Modulation of hematopoietic stem cell homing and engraftment by CD26. Science 305, 1000–1003 [DOI] [PubMed] [Google Scholar]
- Devine S.M., Flomenberg N., Vesole D.H., et al. (2004). Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin's lymphoma. J. Clin. Oncol. 22, 1095–1102 [DOI] [PubMed] [Google Scholar]
- Devine S.M., Vij R., Rettig M., et al. (2008). Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood 112, 990–998 [DOI] [PubMed] [Google Scholar]
- DiPersio J.F., Micallef I.N., Stiff P.J., et al. (2009). Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for. J. Clin. Oncol. 27, 4767–4773 [DOI] [PubMed] [Google Scholar]
- Flomenberg N., Devine S.M., Dipersio J.F., et al. (2005). The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood 1867–1874 [DOI] [PubMed] [Google Scholar]
- Goldberg L.R., Dooner M.S., Johnson K.W., et al. (2013). The murine long-term multi-lineage renewal marrow stem cell is a cycling cell. Leukemia 1–35 [DOI] [PubMed] [Google Scholar]
- Gothot A., van der Loo J.C., Clapp D.W., and Srour E.F. (1998). Cell cycle-related changes in repopulating capacity of human mobilized peripheral blood CD34(+) cells in non-obese diabetic/severe combined immune-deficient mice. Blood 92, 2641–2649 [PubMed] [Google Scholar]
- Heldal D., Tjønnfjord G., Brinch L., et al. (2000). A randomised study of allogeneic transplantation with stem cells from blood or bone marrow. Bone Marrow Transplant. 25, 1129–1136 [DOI] [PubMed] [Google Scholar]
- Hübel K., Liles W.C., Broxmeyer H.E., et al. (2004). Leukocytosis and mobilization of CD34+hematopoietic progenitor cells by AMD3100, a CXCR4 antagonist. Support. Cancer Ther. 1, 165–172 [DOI] [PubMed] [Google Scholar]
- Jetmore A., Plett P.A., Tong X., et al. (2002). Homing efficiency, cell cycle kinetics, and survival of quiescent and cycling human CD34+ cells transplanted into conditioned NOD/SCID recipients. Blood 99, 1585–1593 [DOI] [PubMed] [Google Scholar]
- Kiel M.J., Yilmaz O.H., Iwashita T., et al. (2005). SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell 121, 1109–1121 [DOI] [PubMed] [Google Scholar]
- Kim C.H., and Broxmeyer H.E. (1998). In vitro behavior of hematopoietic progenitor cells under the influence of chemoattractants: stromal cell-derived factor-1, steel factor, and the bone marrow environment. Blood 91, 100–110 [PubMed] [Google Scholar]
- Kim I., He S., Yilmaz O.H., Kiel M.J., and Morrison S.J. (2006). Enhanced purification of fetal liver hematopoietic stem cells using SLAM family receptors. Blood 108, 737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollet O., Spiegel A., Peled A., et al. (2001). Rapid and efficient homing of human CD34(+)CD38(-/low)CXCR4(+) stem and progenitor cells to the bone marrow and spleen of NOD/SCID and NOD/SCID/B2m(null) mice. Blood 97, 3283–3291 [DOI] [PubMed] [Google Scholar]
- Lapidot T., Dar A., and Kollet O. (2005). How do stem cells find their way home? Blood 106, 1901–1910 [DOI] [PubMed] [Google Scholar]
- Larochelle A., Krouse A., Metzger M., et al. (2006). AMD3100 mobilizes hematopoietic stem cells with long-term repopulating capacity in nonhuman primates. Blood 107, 3772–3778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liles W.C., Broxmeyer H.E., Rodger E., et al. (2003). Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood 102, 2728–2730 [DOI] [PubMed] [Google Scholar]
- Ma Q., Jones D., and Springer T.A. (1999). The chemokine receptor CXCR4 is required for the retention of B lineage and granulocytic precursors within the bone marrow microenvironment. Immunity 10, 463–471 [DOI] [PubMed] [Google Scholar]
- Marquez-Curtis L.A., Turner A.R., Larratt L.M., et al. (2009). CD34+ cell responsiveness to stromal cell-derived factor-1alpha underlies rate of engraftment after peripheral blood stem cell transplantation. Transfusion 49, 161–169 [DOI] [PubMed] [Google Scholar]
- Mazurier F., Gan O.I., McKenzie J.L., et al. (2004). Lentivector-mediated clonal tracking reveals intrinsic heterogeneity in the human hematopoietic stem cell compartment and culture-induced stem cell impairment. Blood 103, 545–552 [DOI] [PubMed] [Google Scholar]
- Nademanee A.P., DiPersio J.F., Maziarz R.T., et al. (2012). Plerixafor plus granulocyte colony-stimulating factor versus placebo plus granulocyte colony-stimulating factor for mobilization of CD34(+) hematopoietic stem cells in patients with multiple myeloma and low peripheral blood CD34(+) cell count: results of a subset analysis of a randomized trial. Biol. Blood Marrow Transplant. 18, 1564–1572 [DOI] [PubMed] [Google Scholar]
- Oguro H., Ding L., and Morrison S.J. (2013). SLAM family markers resolve functionally distinct subpopulations of hematopoietic stem cells and multipotent progenitors. Cell Stem Cell 13, 102–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott M.G., Schmidt M., Schwarzwaelder K., et al. (2006). Correction of X-linked chronic granulomatous disease by gene therapy, augmented by insertional activation of MDS1-EVI1, PRDM16 or SETBP1. Nat. Med. 12, 401–409 [DOI] [PubMed] [Google Scholar]
- Passegué E., Wagers A.J., Giuriato S., et al. (2005). Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J. Exp. Med. 202, 1599–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peled A., Petit I., Kollet O., et al. (1999). Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 283, 845–848 [DOI] [PubMed] [Google Scholar]
- Ringdén O., Remberger M., Runde V., et al. (2000). Faster engraftment of neutrophils and platelets with peripheral blood stem cells from unrelated donors: a comparison with marrow transplantation. Bone Marrow Transplant. 25Suppl 2, S6–8 [DOI] [PubMed] [Google Scholar]
- Sheridan W.P., Begley C.G., Juttner C.A., et al. (1992). Effect of peripheral-blood progenitor cells mobilised by filgrastim (G-CSF) on platelet recovery after high-dose chemotherapy. Lancet 339, 640–644 [DOI] [PubMed] [Google Scholar]
- Szilvassy S.J., Meyerrose T.E., Ragland P.L., and Grimes B. (2001). Homing and engraftment defects in ex vivo expanded murine hematopoietic cells are associated with downregulation of beta1 integrin. Exp. Hematol. 29, 1494–1502 [DOI] [PubMed] [Google Scholar]
- To L.B., Roberts M.M., Haylock D.N., et al. (1992). Comparison of haematological recovery times and supportive care requirements of autologous recovery phase peripheral blood stem cell transplants, autologous bone marrow transplants and allogeneic bone marrow transplants. Bone Marrow Transplant. 9, 277–284 [PubMed] [Google Scholar]
- Voermans C., Gerritsen W.R., von dem Borne A.E., and van der Schoot C.E. (1999). Increased migration of cord blood-derived CD34+ cells, as compared to bone marrow and mobilized peripheral blood CD34+cells across uncoated or fibronectin-coated filters. Exp. Hematol. 27, 1806–1814 [DOI] [PubMed] [Google Scholar]
- Voermans C., Kooi M., Rodenhuis S., and Al E. (2001). In vitro migratory capacity of CD34+ cells is related to hematopoietic recovery after autologous stem cell transplantation. Blood 97, 799–804 [DOI] [PubMed] [Google Scholar]
- Welschinger R., Liedtke F., Basnett J., et al. (2013). Plerixafor (AMD3100) induces prolonged mobilization of acute lymphoblastic leukemia cells and increases the proportion of cycling cells in the blood in mice. Exp. Hematol. 41, 293–302.e1 [DOI] [PubMed] [Google Scholar]
- Yang B., Kirby S., Lewis J., et al. (1995). A mouse model for β-thalassemia. Proc. Natl. Acad. Sci. USA 92, 11608–11612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannaki E., Psatha N., Athanasiou E., et al. (2010). Mobilization of hematopoietic stem cells in a thalassemic mouse model: implications for human gene therapy of thalassemia. Hum. Gene Ther. 21, 299–310 [DOI] [PubMed] [Google Scholar]
- Yannaki E., Papayannopoulou T., Jonlin E., et al. (2012). Hematopoietic stem cell mobilization for gene therapy of adult patients with severe β-thalassemia: results of clinical trials using G-CSF or plerixafor in splenectomized and nonsplenectomized subjects. Mol. Ther. 20, 230–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannaki E., Karponi G., Zervou F., et al. (2013). Hematopoietic stem cell mobilization for gene therapy: superior mobilization by the combination of granulocyte-colony stimulating factor plus plerixafor in patients with β-thalassemia major. Hum. Gene Ther. 24, 852–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong K.L., Fahey A., Pizzey A., and Linch D.C. (2002). Influence of cell cycling and cell division on transendothelial migration of CD34+ cells. Br. J. Haematol. 119, 500–509 [DOI] [PubMed] [Google Scholar]
- Zufferey R., Nagy D., Mandel R.J., et al. (1997). Multiply attenuated lentiviral vector achieves efficient gene delivery in vivo. Nat. Biotechnol. 15, 871–875 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.