Abstract
Background: Atypical antipsychotics decrease irritability in autism. They also affect the cytokine network. Psychological stress, depression, and, possibly, autism spectrum disorder (ASD) are associated with the production of pro-inflammatory cytokines. We sought to determine if risperidone treatment led to changes in plasma cytokine levels.
Methods: Forty-five subjects from an open-label study of risperidone treatment of children and adolescents with ASD, ages 4–18 years, had an analysis of 27 different cytokines at baseline and after 8 weeks of treatment using multiplex assays (Millipore) and read on the Luminex 100™ platform. We examined changes in each of the cytokine levels in the entire group, and also compared changes in cytokines in responders versus nonresponders.
Results: After 8 weeks of risperidone treatment, 2 of the 27 plasma cytokines showed statistically significant decreases in median levels: Eotaxin (p=0.0003) and monocyte chemoattractant protein-1 (MCP-1) (p=0.0024). Six of the 48 subjects met two criteria for responders to risperidone, and the median values of interleukin (IL)-5 were significantly higher (p=0.005) in the overall responder group than in nonresponders.
Conclusions: Two cytokines, eotaxin and MCP-1, which have previously been identified as abnormally elevated in children with autism, decreased during treatment with risperidone. This suggests a possible mechanism of action of risperidone treatment and a balancing of the immune system in affected subjects in this very preliminary study.
Introduction
Atypical antipsychotics, such as risperidone and aripiprazole, are shown to decrease behavioral disturbances, such as irritability, aggression, and anxiety among children with autism (McCracken et al. 2002; Myers and Johnson 2007). There are a growing number of studies reporting that psychological stress, psychosis, or depression are associated with the production of pro-inflammatory cytokines, and that treatment with antipsychotic drugs affects the cytokine network (Himmerich et al. 2011; Na et al. 2014).
To date, reports regarding the effects of antipsychotics on cytokine levels are inconsistent, and no antipsychotic has been shown to have consistent anti-inflammatory action (Drzyzga et al. 2006). Multiple studies of schizophrenia support the anti-inflammatory profile of risperidone through an increase in Th2-type cytokines and a shift toward Th2 responses (Teixeira 2008; Chen et al. 2012). There are a limited number of studies finding an association between treatment and immune response in autism spectrum disorder (ASD) (Ashwood et al. 2006; Enstrom et al. 2010; Gupta et al. 1998). Previous studies investigating inflammatory abnormalities related to ASD have yielded conflicting and inconclusive results (Bent et al. 2011; Onore et al. 2012). However, studies do support a pro-inflammatory and Th1 skewed profile in autistic subjects in CD4+ cells, and an association of these changes with more severe behavioral symptoms (Ashwood 2011a).
Recently, a study reported that clinical improvement in children with ASD following 8 weeks of treatment with risperidone was not associated with changes in plasma levels of cytokines (Tobiasova et al. 2011). In the study we report here, our hypotheses were that risperidone will 1) demonstrate anti-inflammatory properties as shown through a decrease in pro-inflammatory and Th1 cytokines, and an increase in Th2 cytokines, and that 2) these changes will be associated with a favorable treatment response.
Methods
Study design
The primary goal of this project was to determine whether cytokine levels change during risperidone treatment in children and adolescents with autism. The University of California, Davis Institutional Review Board approved all study procedures prior to initiating the study. Informed consent was obtained from parents and assent was obtained from the child, when developmentally appropriate, before any study procedures were performed. This study is registered on clinicaltrials.gov (NCT00584701).
Subjects
Children and adolescents ages 4–18 years were required to have a diagnosis of ASD as confirmed by consensus on the American Psychiatric Association, Diagnostic and Statistical Manual of Mental Disorders, 4th ed. (DSM IV) diagnostic interview and the Autism Diagnostic Observation Schedule (ADOS) (American Psychiatric Association 1994), have an intelligence quotient (IQ)>55, and an Aberrant Behavior Checklist Irritability (ABC-I) subscale rating of ≥18. Subjects were excluded if they had been taking antipsychotics within 8 weeks of entry into the study. Subjects were allowed to continue on other medications or treatments begun 2 months prior to study enrollment. Subjects agreed to keep their current medications and treatments constant during the duration of the study and to abstain from beginning any new treatments. This study also excluded subjects with a diagnosis of bipolar disorder, schizophrenia, ASD of known genetic cause, seizures, metabolic disturbance, or severe illness in the past year, as previously described (Lit et al. 2012).
Dosing schedule
Subjects began with an initial dosage of 0.5 mg at bedtime for 4 days and were uptitrated to 1 mg at the same time for 4 additional days if the previous dose had been tolerated. If the behavioral disturbances continued, 0.5 mg was added to the daily dose in the morning as tolerated, to a maximum daily total of 1.5 mg of risperidone over the duration of the study (Lit et al. 2012).
Statistical analysis
Using the same parameters as McCracken et al. (2002), subjects were defined as overall responders to treatment if they had a decrease in ABC-I ≥25% and a Clinical Global Impressions-Improvement (CGI-I) rating of “very much improved” or “much improved.” Cytokine analysis was performed using multiplex assays (Millipore) according to manufacturer's recommendation and read on the Luminex 100™ platform. The significance of the change in cytokine levels between the responder and nonresponder groups was determined using nonparametric Mann–Whitney tests. Correlations were determined using Spearman's rank correlation coefficient. We did not adjust p values for multiple testing, as this was an exploratory analysis (Thompson 1998).
Results
Data were collected on the plasma levels of 27 different cytokines of 45 subjects (mean age of 114.3 months, SD=52.6). The data included 35 males and 10 females with a mean IQ of 59.9 (SD=25.0) and mean ADOS communication and social interaction total of 16.6 (SD=6.6). Of the 45 subjects, 35 (78%) demonstrated at least a 25% decrease in ABC-I subscale score alone and 11 (24%) received a rating of “very much improved” or “much improved” on the CGI-I. A total of 6 subjects (13%) met both criteria and were identified as responders.
Overall
The mean ABC-I decreased from 24.8 (SD=6.7) to 11.9 (SD=6.1) after the treatment period (95% CI of change: −15.3, −10.4) (p<0.00001). Two of the plasma cytokines showed statistically significant changes (decreases) after risperidone treatment: Eotaxin (p=0.0003) and MCP1 (p=0.002). These decreases were not significantly associated with change in %ABC-I scores (See Table 1).
Table 1.
Cytokines Tested in 45 Subjects Treated with Risperidone
| n=45 | Pretreatment Mean (SD) | Posttreatment Mean (SD) | p | rho (p) |
|---|---|---|---|---|
| TGF-β1 well 1 | 13040.2 (8885.9) | 16407.8 (1973.7) | 0.09 | 0.02 (0.91) |
| TGF-β2 well 2 | 11412.3 (8711.9) | 12794.0 (1485.6) | 0.27 | 0.07 (0.65) |
| BDNF | 1558.4 (292.0) | 1256.5 (129.5) | 0.86 | 0.03 (0.83) |
| Eotaxin | 81.9 (41.5) | 65.8 (30.2) | 0.0003* | −0.17 (0.27) |
| GCSF | 43.6 (75.1) | 43.8 (66.4) | 0.48 | −0.08 (0.63) |
| GMCSF | 84.7 (78.0) | 82.4 (11.7) | 0.60 | 0.02 (0.88) |
| IFN-α2 | 83.1 (202.3) | 75.1 (166.8) | 0.55 | −0.20 (0.20) |
| IFN-γ | 14.1 (20.8) | 12.5 (16.1) | 0.26 | −0.005(0.97) |
| IL-1α | 286.8 (256.4) | 292.6 (226.6) | 0.90 | 0.05 (0.76) |
| IL-1β | 2.9 (5.7) | 2.5 (4.3) | 0.17 | 0.02 (0.91) |
| IL-2 | 4.1 (12.5) | 2.9 (8.8) | 0.51 | 0.02 (0.91) |
| IL-5 | 2.5 (8.1) | 1.8 (4.3) | 0.73 | −0.001(0.95) |
| IL-6 | 5.9 (11.3) | 5.9 (12.7) | 0.31 | 0.25 (0.11) |
| IL-7 | 27.1 (59.4) | 25.8 (54.8) | 0.66 | −0.14 (0.35) |
| IL-8 | 10.3 (17.6) | 9.0 (14.2) | 0.36 | −0.01 (0.93) |
| IL-10 | 5.0 (4.4) | 5.3 (4.6) | 0.50 | 0.20 (0.21) |
| IL-12p40 | 84.4 (102.2) | 79.4 (93.5) | 0.08 | −0.17 (0.28) |
| IL-12p70 | 7.2 (12.0) | 5.2 (7.9) | 0.11 | 0.07 (0.66) |
| IL-13 | 3.0 (9.8) | 2.1 (6.7) | 0.08 | 0.16 (0.30) |
| IL-15 | 4.9 (11.6) | 4.8 (13.0) | 0.50 | −0.12 (0.46) |
| IL-17 | 10.4 (20.3) | 8.5 (15.9) | 0.19 | 0.10 (0.53) |
| IP-10 | 452.7 (413.2) | 444.8 (308.1) | 0.95 | 0.06 (0.72) |
| MCP-1 | 313.0 (143.0) | 265.7 (80.4) | 0.002* | 0.11 (0.48) |
| MIP-1α | 46.8 (46.5) | 44.0 (39.4) | 0.20 | 0.02 (0.92) |
| MIP-1β | 27.4 (27.3) | 25.8 (21.6) | 0.64 | 0.23 (0.15) |
| TNFα | 4.2 (1.6) | 4.0 (1.5) | 0.09 | 0.13 (0.42) |
| TNFβ | 6.9 (17.6) | 8.6 (25.4) | 0.39 | −0.01 (0.95) |
TGF, transforming growth factor; BDNF, brain-derived neurotrophic factor; GCSF, granulocyte colony-stimulating factor; GMCSF, granulocyte macrophage colony-stimulating factor; IFN, interferon; IL, interleukin; IP, Interferon gamma-induced protein; MCP, monocyte chemoattractant protein; MIP, macrophage inflammatory protein; TNF, tumor necrosis factor.
Responder vs. nonresponders
There were no significant difference in baseline characteristics, including age (p=0.92), IQ (p=0.31), ADOS score (p=0.32), and gender (p=0.12) between the responder and nonresponder groups. One cytokine showed a greater change in the six responders on two measures, compared with the nonresponder group. The change in the median values of interleukin (IL)-5 was significantly higher (p=0.005) in the overall responder group than in nonresponders. The mean value of the change in IL-5 increased in the responder group (mean=0.442, SD=0.49) whereas it decreased in the nonresponder group (mean=−0.888, SD=4.5) (See Table 1 and Fig. 1).
FIG. 1.
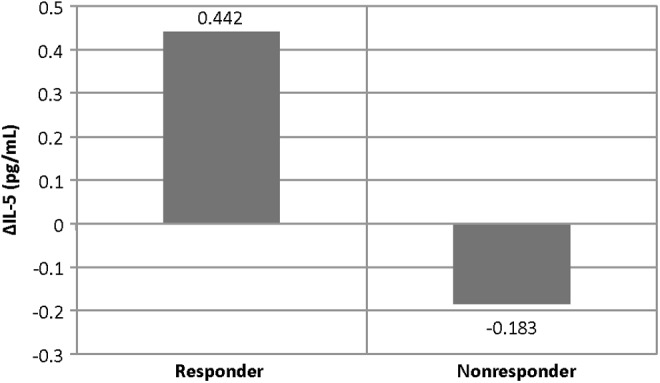
Mean interleukin (IL)-5 change in responder versus nonresponder groups.
Discussion
There was a significant decrease in eotaxin and monocyte chemoattractant protein-1 (MCP-1) levels following 8 weeks of risperidone treatment in children and adolescents with ASD. This finding is in support of Ashwood et al. (2011b) that the production of MCP-1 and eotaxin were significantly higher in children with ASD than in typically developing children and in children with developmental delays other than ASD. Their results are consistent with data from other studies that showed an increase in protein levels of MCP-1 and eotaxin in brain specimens from individuals with ASD (Vargas et al. 2005) or for pro-inflammatory cytokines in the blood of ASD children (Ashwood et al. 2011a). Moreover, a 2005 study by Mundo et al. suggested the role of a polymorphism of the MCP-1 gene (SCYA2) as a resistance factor during antipsychotic treatment of patients with schizophrenia, suggesting an interesting link between chemokine levels and responses to treatment.
Furthermore, the IL-5 increases could suggest that the profile is changing from a Th1 to a Th2- type cytokine and that could suggest benefit as described in previous studies demonstrating the cytokine modulating effects of antipsychotics, including risperidone, in vitro and in vivo. In CD4+ T cells, a shift toward Th2 responses was associated with better cognitive scores in children with ASD (Ashwood et al. 2011a).
Limitations are the small sample size without correction for multiple comparisons.
Conclusion
Together, these data suggest that the immune system may be being balanced as a result of risperidone treatment. Inflammatory processes are thought to play a role in autism, and decreases may benefit symptoms, but replication with larger samples is necessary to determine if this is a direct effect.
Disclosures
Robert L. Hendren received research grants from Autism Speaks, Curemark, BioMarin Pharmaceutical, the Vitamin D Council, Forest Pharmaceuticals, Inc., National Institute of Mental Health (NIMH), Roche, and Shire, and is on Advisory Boards for BioMarin, BioZeus, Coronado Biosciences, Forest, and Janssen. Jae Eun Choi, Felicia Widjaja, Milo Careaga, Stephen Bent, and Paul Ashwood have no institutional or corporate/commercial relationships to disclose.
References
- American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- Ashwood P, Krakowiak P, Hertz-Picciotto I, Hansen R, Pessah IN, Van de Water J: Altered T cell responses in children with autism. Brain Behav Immunol 25:840–849, 2011a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Krakowiak P, Hertz–Picciotto I, Hansen R, Pessah IN, Van de Water J: Associations of impaired behaviors with elevated plasma chemokines in autism spectrum disorders. J Neuroimmunol 232:196–199, 2011b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashwood P, Wills S. and Van De Water J: The immune response in autism: A new frontier for autism research. J Leukoc Biol 80:1–15, 2006 [DOI] [PubMed] [Google Scholar]
- Bent S, Bertoglio K, Ashwood P, Bostrom A, Hendren RL: A pilot randomized controlled trial of omega-3 fatty acids for autism spectrum disorder. J Autism Dev Disord 41:545–554, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ML, Tsai TC, Wang LK, Lin YY, Tsai YM, Lee MC, Tsai FM: Risperidone modulates the cytokine and chemokine release of dendritic cells and induces TNF-α-directed cell apoptosis in neutrophils. Int Immunopharmacol 12:197–204, 2012 [DOI] [PubMed] [Google Scholar]
- Drzyzga L, Obuchowicz E, Marcinowska A, Herman ZS: Cytokines in schizoprenia and the effects of antipsychotic drugs. Brain Behav Immun 20:532–545, 2006 [DOI] [PubMed] [Google Scholar]
- Enstrom AM, Onore CE, Van de Water JA, Ashwood P: Differential monocyte responses to TLR ligands in children with autism spectrum disorders. Brain Behav Immunol 24:64–71, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta S, Aggarwal S, Rashanravan B, Lee T: Th1- and Th2-like cytokines in CD4+ and CD8+ T cells in autism. J Neuroimmunol 85:106–109, 1998 [DOI] [PubMed] [Google Scholar]
- Himmerich H, Schönherr J, Fulda S, Sheldrick AJ, Bauer K, Sack U: Impact of antipsychotics on cytokine production in-vitro. J Psychiatr Res 45:1358–1365, 2011 [DOI] [PubMed] [Google Scholar]
- Lit L, Sharp FR, Bertoglio K, Stamova B, Ander BP, Sossong AD, Hendren RL: Gene expression in blood is associated with risperidone response in children with autism spectrum disorders. Pharmacogenomics J 12:368–371, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken JT, McGough J, Shah B, Cronin P, Hong D, Aman MG, Arnold LE, Lindsay R, Nash P, Hollway J, McDougle CJ, Posey D, Swiezy N, Kohn A, Scahill L, Martin A, Koenig K, Volkmar F, Carroll D, Lancor A, Tierney E, Ghuman J, Gonzalez NM, Grados M, Vitiello B, Ritz L, Davies M, Robinson J, McMahon D; Research Units on Pediatric Psychopharmacology Autism Network: Risperidone in children with autism and serious behavioral problems. N Engl J Med 347:314–321, 2002 [DOI] [PubMed] [Google Scholar]
- Mundo E, Altamura AC, Vismara S, Zanardini R, Bignotti S, Randazzo R, Montresor C, Gennarelli M: MCP-1 gene (SCYA2) and schizophrenia: a case-control association study. Am J Med Genet B Neuropsychiatr Genet 132B:1–4, 2005 [DOI] [PubMed] [Google Scholar]
- Myers SM, Johnson CP: Management of children with autism spectrum disorders. Pediatrics 120, 1162–1182, 2007 [DOI] [PubMed] [Google Scholar]
- Na KS, Jung HY, Kim YK: The role of pro-inflammatory cytokines in the neuroinflammation and neurogenesis of schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 48:277–286, 2014 [DOI] [PubMed] [Google Scholar]
- Onore C, Careaga M, Ashwood P: The role of immune dysfunction in the pathophysiology of autism. Brain Behav Immunol 26:383–92, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira AL, Reis HJ, Nicolato R, Brito–Melo G, Correa H, Teixeira MM, Romano–Silva MA: Increased serum levels of CCL11/eotaxin in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 32:710–714, 2008 [DOI] [PubMed] [Google Scholar]
- Thompson JR: Invited commentary. Re: Multiple comparisons and related issues in the interpretation of epidemiologic data Am J Epidemiol 147:801–806, 1998 [DOI] [PubMed] [Google Scholar]
- Tobiasova Z, van der Lingen KH, Scahill L, Leckman JF, Zhang Y, Chae W, McCracken JT, McDougle CJ, Vitiello B, Tierney E, Aman MG, Arnold LE, Katsovich L, Hoekstra PJ, Volkmar F, Bothwell AL, Kawikova I: Risperidone-related improvement of irritability in children with autism is not associated with changes in serum of epidermal growth factor and interleukin-13. J Child Adolesc Psychopharmacol 21:555–564, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas DL, Nascimbene C, Krishnan C, Zimmerman AW, Pardo CA: Neuroglial activation and neuroinflammation in the brain of patients with autism. Ann Neurol 57:67–81, 2005 [DOI] [PubMed] [Google Scholar]


