Abstract
Background: Diabetic foot wounds are a highly morbid and costly complication of diabetes mellitus. Targeted amino acid supplementation, by increasing tissue hydroxyproline concentrations, has been implicated in improved wound outcomes in surgical incisions and chronic wounds, and after radiation injury. A major component of collagen, hydroxyproline is a surrogate marker used commonly for tissue collagen concentrations. This paper reviews the literature pertaining to amino acid supplementation and wound healing, and also evaluates our pilot data relating to supplementation with arginine, glutamine, and beta-hydroxy-beta-methylbutyrate (HMB) in the treatment of diabetic foot ulcers.
Methods: For the pilot study, nine patients scheduled to undergo wound debridement for diabetic foot ulcers were randomized prospectively to be a part of either a placebo group or a treatment group that received supplementation twice daily for 2 wks. Tissue samples were collected both before and after 2 wk of supplementation. The results of assay of the samples for hydroyproline were then analyzed via a one tailed Student t-test to evaluate tissue concentrations of hydroxyproline. For the literature review in the study, the MEDLINE/PubMed database was reviewed, using search terms contained in the Medical Subject Headings (MeSH).
Results: The treatment group in the study exhibited a significantly greater hydroxyproline concentration after supplementation than before it (p=0.03). The mean percent change in the tissue hydroxyproline concentration for arginine, glutamine, and HMB group was +67.8%, with a standard deviation (SD) of 129.89. The mean percent change for the corresponding amino acids in the placebo group was −78.4%, with an SD of 20.55. The review of the MEDLINE/PubMed literature revealed only two human studies of amino acid supplementation in patients with diabetic foot wounds, one of which found a significant improvement in wound-depth and wound-appearance scores.
Conclusions: Given the results of our pilot study, and on the basis of a review of the literature, the administration of a simple amino acid supplement may improve the healing of diabetic foot wounds via increased collagen production.
The incidence of diabetes mellitus and diabetes-related complications is increasing globally. Ulcers affecting the lower limbs of diabetic individuals are a frequent and costly clinical complication of the disease. Approximately $6- to $15 billion annually is spent caring for all chronic wounds in the United States [1,2]. Among diabetic individuals, approximately 1%–4% will develop a foot ulcer per year, with up to 25% of persons with diabetes developing an ulcer during their lifetimes. Many of these wounds will result in substantial morbidity, leading to amputations, disfigurement, and sepsis, and potentially to death. On average, diabetic foot ulcers exist for a mean±standard deviation (SD) of 87±83 d [3]. In approximately 15% of patients in whom they develop, these slow-healing wounds progress to osteomyelitis, and roughly 16% of patients with osteomyelitis eventually require amputation [4].
In this paper we review the literature pertaining to amino acid supplementation for wounds in general, with specific attention to diabetic foot ulcers. Additionally, we describe our group's pilot data for findings relating to amino acid supplementation for nine patients with diabetic foot ulcers. The aim of this pilot study was to determine whether dietary amino acid supplementation with arginine, glutamine, and beta-hydroxy-beta-methylbutyrate (HMB) caused an increase in the tissue hydroxyproline concentration as compared with the administration of a placebo.
Patients and Methods
The study was a prospective triple-blind study that was approved by the institutional review board (IRB) of our facility before patient recruitment and sample collection were done. Patients with chronic diabetic foot wounds present for more than 30 d were selected as participants if their foot wounds would require multiple debridement on an outpatient basis at our clinic. Additionally, patients selected for the study were older than 18 y of age and had a history of at least two visits to our outpatient center. Patients excluded from the study were those with documented lower extremity wounds of other than diabetic origin, those who received hyperbaric oxygen therapy, and those who had had collagen-based topical wound therapy or recent (within 3 mo) skin grafting. Also excluded from the study were patients with a history of poor followup and current “uncontrolled diabetes” as documented in physician records reviewed within 3 mo of the beginning of the study, inpatients, and patients from outside Clark County, Nevada. Demographics of patients included in the study are presented in Tables 1 and 2.
Table 1.
Patient Demographics in Arginine, Glutamine, and Beta-Hydroxy-Beta Methylbutyrate Treatment Group
| Patient number | Age | Gender | Race | Recent BGa | Recent HbA1cb | Smoker | Insurance |
|---|---|---|---|---|---|---|---|
| 1 | 44 | Male | White | NA | 9.3 | No | Yes |
| 2 | 45 | Male | White | 125 | 7.2 | No | Yes |
| 3 | 37 | Female | White | 250 | NA | No | No |
| 4 | 53 | Male | Black | 250 | NA | No | Yes |
| 5 | 37 | Male | White | NA | NA | No | Yes |
| 6 | 60 | Male | White | NA | 9.0 | No | Yes |
| Median | 44.5 | – | – | 250 | 9.0 | – | – |
| Range | 37–60 | – | – | 125–250 | 7.2–9.3 | – | – |
Recent blood glucose (BG) defined as highest value obtained within 3 mo of entrance into study.
Recent hemoglobin A1c concentration defined as the value obtained within 3 mo of entrance into study.
BG=blood glucose conceutration; NA=not available.
Table 2.
Demographics of Patients in Placebo Group
| Patient number | Age | Gender | Race | Recent BGa | Recent HbA1cb | Smoker | Insurance |
|---|---|---|---|---|---|---|---|
| 6 | 53 | Female | Hispanic | NA | 7.8 | No | No |
| 7 | 54 | Male | White | NA | 6.4 | No | Yes |
| 9 | 43 | Female | Hispanic | NA | 14.0 | No | Yes |
| Median | 53 | – | – | – | 7.8 | – | – |
| Range | 43–54 | – | – | – | 6.4–14.0 | – | – |
Recent blood glucose concentration (BG) defined as highest value obtained within 3 mo of entrance into study. bRecent hemoglobin A1c concentration (HbA1c) defined as value obtained within three months of entrance into study.
NA=not available.
After informed consent was obtained from each of the nine study participants, they were assigned randomly to receive, from a blinded third party, placebo or the arginine, glutamine, and HMB supplement used in the study. Physicians and laboratory technicians who worked with the study participants, the participants themselves, and researchers involved in the study were also blinded to whether the patients were assigned to treatment or placebo. Samples of wound tissue were collected at wound debridements scheduled previously. The first tissue sample was taken before nutritional supplementation and another sample was taken after the nutritional supplement or placebo had been given orally, twice per day, for 2 wks. The patients were educated about how to take the supplement. We chose two weeks as a time point for this pilot study because it was the time period used in the other major study involving this supplement [5]. The supplement is available commercially (Abbott Laboratories, Abbott Park, IL) in powder form, and may be mixed into a beverage or liquid of choice. The supplement contains 7 g each of arginine and glutamine and 1.5 g of HMB.
At each collection time point, from 0.8–3.0 g of tissue was collected from each patient's foot wound. The specimens obtained from the wound were sent to the laboratory in their entirety. For wounds that were necrotic and had voluminous debrided material, only less necrotic-appearing, indeterminately viable tissue was sampled so as to get a representative sample of the patient's true tissue hydroxyproline concentration. The hydroxyproline concentration of the tissue samples was determined with the BioVision Hydroxyproline Colorimetric Assay Kit (Biovision, Milpitas, CA). A random pooled sample, composed of 10 of the homogenized tissue samples, was used as an experimental control to ensure that each run of the assay was consistent with previous runs. Statistical analysis of hydroxyproline concentrations was then done with a one-tailed Student t-test using the SPSS version 20 software system (SPSS, Chicago, IL).
The literature review in the study was done by searching the medical subject headings (MeSH) of the MEDLINE/PubMed data base using the following terms “wound healing,” “glutamine,” “dietary supplements,” “amino acids,” “foot ulcer,” and “diabetes mellitus.” These particular MeSH search terms identified only two sources as investigating dietary amino acid supplementation in patients with diabetic foot ulcers [6,7].
Results
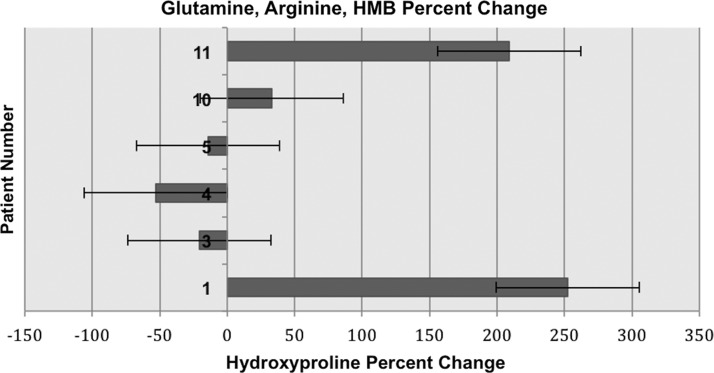
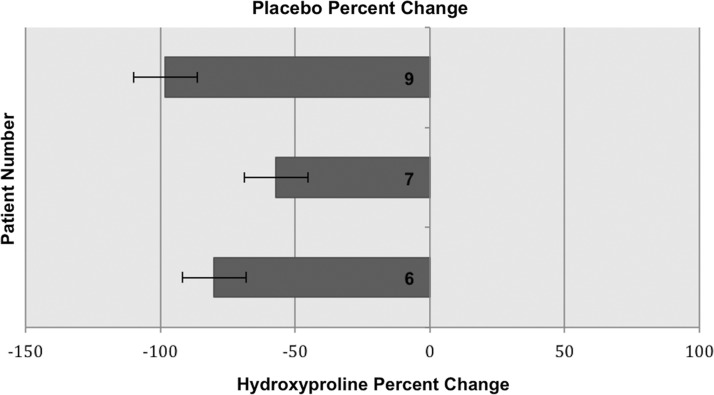
Analysis was conducted of the tissue samples in the active treatment group (n=6) and the placebo group (n=3) through one-tailed Student t-testing. A statistically significant increase in the tissue hydroxyproline concentration after as compared with before supplementation was found in the treatment group, with a value of p=0.03, as compared with a value of p=0.22 in the placebo group. The mean percent change in the tissue hydroxyproline concentration for the treatment group was +67.8%, with a standard deviation (SD) of 129.89. The mean percent change for the placebo group was −78.4%, with an SD of 20.55. A one-tailed Student t-test was used for this pre- and post-supplementation comparison because we expected only to see an increase and not a decrease in tissue hydroxyproline concentrations. A two-tailed Student t-test was conducted for completeness, and yielded insignificant values of p. Certain wounds in patients given the arginine, glutamine, and HMB supplement exhibited decreased catabolism of these three amino acids (a decreased negative percent change in their tissue concentrations) as compared with the placebo group, with respective percent change values of +252.4, +209.1, +33, and −14.3, −20.6, and −52.8 (Fig. 1). There was less of a decrement in tissue hydroxyproline concentration identified among patients in the treatment group (Fig. 1). For three patients in the treatment group, there was an anabolic effect. Diminished tissue hydroxyproline concentration was identified in patients' tissue samples from the placebo group compared to the patients' tissue samples from the treatment group. Percent changes in hydroxyproline concentration for each placebo group patient are listed in Fig. 2. In summary, a decrease in catabolism and occasional anabolism was identified in the treatment group, a phenomenon that was not observed in the placebo group (see Fig. 2).
FIG. 1.
Post-treatment percent change in tissue concentrations of hydroxyproline in patients receiving glutamine, arginine, and beta-hydroxy-beta-methylbutyrate (HMB) supplement.
FIG. 2.
Post-treatment percent change in tissue concentration of hydroxyproline in patients receiving placebo.
Discussion
The pilot data in our study have limitations, the most important of which is the size of the pilot group. Despite their small sample size, the two groups in the study were reasonably similar in terms of age, smoking status, and insurance status (Tables 1 and 2). The median ages of the patients in the placebo and treatment groups are within 10 y of each other, at 53 y and 44.5 y, respectively. All of the patients in both groups were non-smokers, and all but one patient in each group had health insurance. The median hemoglobin A1c (HbA1c) concentrations in the placebo and treatment groups were 7.8% and 9.0%, respectively. Althugh the HbA1c concentrations were slightly closer to normal in the placebo than in the treatment group, the importance of this is unclear in view of the small population sample size of the two groups. It may be found ultimately that in patients with poorer glycemic control, the amino acid supplement used in the present study has a benefit in promoting the healing of diabetic foot wounds, but we cannot yet attribute our positive findings in the amino acid supplement group to this difference. With a larger population sample size, we anticipate more balanced demographic factors in a control and amino acid supplementation group, and will record descriptive statistics for these groups. Another major limitation of our data is that we sent patients in the amino acid supplement group back to their own homes to continue taking the supplement, and their treatment compliance was not monitored in any direct and immediate manner, making it impossible to assure that they were following the study protocol in a prescribed and consistent manner. Additionally, the placebo group was not given a non-specific nitrogen load equivalent to that produced by the amino acid supplement, which could have skewed our results if the patients in this group were lacking in dietary protein. A power analysis derived from previous study of this active compound [5] showed that to obtain a power of 0.80 (alpha<0.05), a minimum of 26 patients in each of the placebo and supplementation groups will be needed for future study. We are now working toward obtaining this subset of patients for further analysis.
Literature Review: Pilot Data in Context
Immunonutrients are nutrients (both micro and macro) that may in some way augment immune activity [8]. Nutrients identified most commonly as immunonutrients include glutamine, arginine, branched-chain amino acids, n-3 polyunsaturated fatty acids, and nucleotides. Many commercial nutritional supplements and feeding solutions containing all or a few of these nutrients are available [8]. Amino acid supplementation has been implicated in improved outcomes after many types of metabolic insult, although this has had only limited study in the case of chronic diabetic foot ulcers. Of importance to such study are the three particular amino acids glutamine, arginine, and HMB. Glutamine is a non-essential amino acid, found in abundance in human beings. Its tissue concentrations can be diminished by more than 50% and plasma concentrations may be diminished by more than 25% after a major catabolic insult [9]. Arginine is a semi-essential amino acid that becomes essential in critical illness. It is known to increase T-cell proliferation and the generation of lymphokine-activated killer cells, both of which are important factors in the human immune response. Beta-hydroxy-beta-methylbutyrate (HMB) is a metabolite of leucine and has also been implicated in improved outcomes of wound healing under conditions of physiologic stress [5,9].
A relative lack of data exist for the effect of immunonutrition on diabetic foot wounds, but our group has taken a particular interest in studying the commercially available amino acid supplement used in the present study, which has already proved useful in promoting the healing of surgical wounds [5]. However, although the amino acids used for this have been shown as beneficial in wound healing in otherwise healthy subjects, the present pilot study is the first prospective, randomized study of these amino acids in diabetic individuals. In a key 2002 study by Williams et al., a mixture of arginine, glutamine, and HMB was compared with placebo in a study of wound healing in 35 patients. Wound healing was evaluated in healthy non-smoking human volunteers after supplementation through the explantation and analysis of empty polytetrafluoroethylene (PTFE) tubes. A statistically significant increase in plasma arginine and ornithine concentrations was noted in patients given the specialized amino acid mixture, as well as a statistically significant increase in their plasma hydroxyproline concentrations [5].
Our literature review identified only two studies reporting amino acid supplementation in patients with diabetic foot wounds. One was a retrospective review of 11 diabetic patients with foot ulcers who were given supplementation with arginine, glutamine, and HMB. In those 11 patients who were evaluated, degeneration was not identified in any wounds assessed with standardized scoring systems for wound depth and wound appearance [6], but on the contrary, statistically significant improvements in scores for wound depth and wound appearance were identified. This implies a decrease in diabetic wound catabolism leading to diminished wound degeneration. In a study done in 2013 by Kesici et al., whose results contrasted with those of the study just discussed, in which supplementation was associated with improved scores for wound depth and appearance, amino acid supplementation in rats was not associated with significant increases in hydroxyproline concentrations. This finding is important in identifying a potential difference in the biochemical milieu of healing wounds in diabetic patients as compared with healthy controls [9]. The second human study identified in our literature review focused on arginine supplementation in patients with diabetic foot wounds. The authors examined 30 patients receiving either dietary arginine or placebo. Surrogates for quality of microcirculation were assessed, including transcutaneous oxygen partial pressure in the foot, neuropathic disability score, and vibration-perception threshold. The study found no significant difference between the treatment group and the placebo group in terms of the quality of microcirculation [7]. Although this latter study did not focus directly on wound healing per se, it implies that select amino acid supplementation does not improve wound healing from a microvascular standpoint. Yavas et al., in 2013, identified glutamine, arginine, and HMB supplementation as being associated with significant differences in terms of epithelial thickness, subepithelial edema, inflammation, and congestion via histologic analyses of rat oral mucosa. Differences were identified in the supplement-treated control group of rats. In the non-radiated supplement-treated group, mean epithelial thickness was increased, whereas subepithelial edema, inflammation, and congestion scores were decreased in rats treated with the supplement. Given the overall decrement in inflammatory sequelae or irradiation found in the supplement-treated rats in this study, it is possible that glutamine, arginine, and HMB will improve wound healing in diabetic individuals via immunomodulatory and anti-inflammatory pathways.
Conclusions
Our preliminary findings in the study reported here are important in implicating arginine, glutamine, and HMB in improving protein deposition in diabetic patients. We found an overall decrement in the catabolic effect of diabetes in patients given amino acid supplementation, which is the most essential point to draw from our data. Understanding the metabolic response to injury, especially in diabetic patients, is a challenge. Complicating this is the role of insulin sensitivity in obese versus non-obese diabetic individuals. Is the inflammatory milieu evident in obese diabetic patients with metabolic syndrome analogous to that present in lean diabetic subjects? How exactly does the tissue microenvironment in diabetic individuals alter wound healing and collagen deposition? Obese yet malnourished patients appear to exhibit a generally catabolic state, with a low lean body mass and the probable depletion of key skeletal and peripheral stores of amino acids including glutamine, arginine, and HMB. The relationship between these nutrient deficits and immunity has yet to be clarified fully, but a pro-inflammatory biochemical environment in diabetic individuals has been established [11]. More studies focused specifically on the effects of the supplement mix of glutamine, arginine, and HMB in diabetic individuals need to be conducted to identify the mechanism by which it improves wound healing at the cellular level. In future studies, we plan to examine, in the chronically ulcerated feet of diabetic patients, the particular biochemical environment during amino acid supplementation at particular time points during the administration of a glutamine, arginine, and HMB supplement. In the data of the present study, we found a wide range of catabolic decrements in diabetic foot wounds treated with amino acid supplementation. We hypothesize that there may be a particular time during the course of a wound at which supplementation may have a more marked benefit, and thus during this time may lead to a more uniform increase in the percent change in the local concentration of hydroxyproline in the wound.
Overall, it is remarkable that after only two weeks of supplementation, we were able to identify a preparation that improved markedly the in vivo hydroxyproline concentration in a patient population fraught with complications following wound debridement [1]. This preparation is safe, easy to administer, and less costly than many other complex wound-care treatments. We predict that patients given this arginine, glutamine, and HMB preparation supplementally over the long term will exhibit decreased times to wound healing and improved outcomes in terms of both patient-related and cost-related parameters. Through continued analysis of this particular treatment modality, we will glean important conclusions about supplementation with glutamine, arginine, and HMB in diabetic patients with foot ulcers. With time and research, we will be able to target amino acid therapies based on particular patient characteristics, for a safe, reliable, and cost-effective manner of improving wound-healing outcomes.
Acknowledgments and Author Disclosure Statement
This research was supported by National Institute of General Medical Sciences grant 8 P20 GM103440-11). The authors have no conflicts of interest in the work reported here.
References
- 1.Gordois A, Scuffham P, Shearer A, et al. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care 2003;26:1790–1795 [DOI] [PubMed] [Google Scholar]
- 2.Markova A, Mostow EN. US skin disease assessment: Ulcer and wound care. Dermatol Clin 2012;30:107–111, ix. [DOI] [PubMed] [Google Scholar]
- 3.Stockl K, Vanderplas A, Tafesse E, et al. Costs of lower-extremity ulcers among patients with diabetes. Diabetes Care 2004;27:2129–2134 [DOI] [PubMed] [Google Scholar]
- 4.Driver VR, Fabbi M, Lavery LA, et al. The costs of diabetic foot: The economic case for the limb salvage team. J Vasc Surg 2010;52(3 Suppl):17S–22S [DOI] [PubMed] [Google Scholar]
- 5.Williams JZ, Abumrad N, Barbul A. Effect of a specialized amino acid mixture on human collagen deposition. Ann Surg 2002;236:369–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sipahi S, Gungor O, Gunduz M, et al. The effect of oral supplementation with a combination of beta-hydroxy-beta-methylbutyrate, arginine and glutamine on wound healing: a retrospective analysis of diabetic haemodialysis patients. BMC Nephrol 2013;14:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jude EB, Dang C, Boulton AJ. Effect of L-arginine on the microcirculation in the neuropathic diabetic foot in Type 2 diabetes mellitus: A double-blind, placebo-controlled study. Diabet Med 2010;27:113–116 [DOI] [PubMed] [Google Scholar]
- 8.Suchner U, Kuhn KS, Fürst P. The scientific basis of immunonutrition. Proc Nutr Soc 2000;59:553–563 [DOI] [PubMed] [Google Scholar]
- 9.Kesici U, Kesici S, Ulusoy H, et al.. Effects of glutamine on wound healing. Int Wound J 2013June6. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yavas C, Yavas G, Acar H, et al. Amelioration of radiation-induced acute inflammation and mucosal atrophy by beta-hydroxy-beta-methylbutyrate, L-glutamıne, and L-argınıne: results of an experimental study. Support Care Cancer 2013;21:883–888 [DOI] [PubMed] [Google Scholar]
- 11.Eming SA, Hammerschmidt M, Krieg T, et al. Interrelation of immunity and tissue repair or regeneration. Semin Cell Dev Biol 2009;20:517–527 [DOI] [PubMed] [Google Scholar]