Abstract
Intensity modulated radiation therapy (IMRT) allows physicians to deliver higher conformal doses to the tumour, while avoiding adjacent structures. As a result the probability of tumour control is higher and toxicity may be reduced. However, implementation of IMRT is highly complex and requires a rigorous quality assurance (QA) program both before and during treatment. The present article describes the process of implementing IMRT for localized prostate cancer in a radiation therapy department. In our experience, IMRT implementation requires careful planning due to the need to simultaneously implement specialized software, multifaceted QA programs, and training of the multidisciplinary team. Establishing standardized protocols and ensuring close collaboration between a multidisciplinary team is challenging but essential.
Keywords: Radiotherapy, IMRT, Prostate cancer
1. Introduction
The concept of intensity-modulated radiotherapy (IMRT) first appeared in 1960, and in 1966 the Sloan Kettering Memorial Cancer Center was the first to treat patients with this new technology.4 IMRT is a significant advancement in the field of Radiation Oncology, as it allows a better conformity of the dose delivered to the tumour and lymph nodes, while avoiding irradiation of adjacent healthy structures. As a result, there is a potential decrease in the likelihood of complications associated with irradiation of these normal surrounding tissues. However, high-quality IMRT requires the support of medical physicists and detailed knowledge of three dimensional (3D) anatomy and patterns of disease spread.1
In recent years, dose escalation has been shown to improve the probability of local tumour control in prostate cancer. As a result, a growing number of radiotherapy departments have incorporated IMRT into their routine work, and many more centres are in the process of doing so.2,3
Our own recent experience in implementing IMRT has shown us that this is a complex, time-consuming, and expensive process. Some centres, particularly smaller ones, may find the process to be daunting. However, despite the obstacles and difficulties, it is feasible to implement IMRT if a well-planned approach is taken.
The aim of the present article is to describe the process of implementing IMRT for prostate cancer in a private radiotherapy centre. Our experience may be useful to other centres that wish to implement IMRT.
2. Hospital description
Médica Sur is a large, privately owned hospital located in Mexico City, Mexico. The hospital has 170 beds and treats approximately 1200 patients per month. The Department of Radiation Oncology is staffed by 20 physicians, 2 nurses, 4 medical physicists, 2 dosimetrists, and 8 technicians. On average, the department treats approximately 600 patients per year. Available equipment includes 2 Varian linear accelerators, one Siemens 16-slice PET-CT unit (shared schedules for virtual simulation), 4 treatment planning systems (TPS) with Eclipse v 7.3 software, and Varis Record and Verify system (R&V) database.
3. IMRT: description
IMRT is a specific radiotherapy technique delivered by a linear accelerator equipped with multileaf collimator (MLC) in which the beams are modulated to produce highly conformal dose distributions. A primary objective of IMRT is to reduce the dose to critical organs to preserve their function by restricting the entire radiotherapy dose only to the treatment volumes. However, the successful delivery of IMRT requires precise, reproducible, and reliable patient positioning, in addition to a rigorous quality assurance (QA) program overseen by medical physicists. IMRT is administered through treatment fields, each of which is individually modulated. The intensity of these variable doses can be managed by the segments in each treatment field.
4. IMRT vs. conventional 3D planning
IMRT planning is conceptually very different from conventional planning. In IMRT, the radiation oncologist must delineate treatment volumes (tumour, lymph nodes) and critical organs (rectum, bladder, spinal cord, saliva glands, eye, etc.), whereas in conventional 3D planning, large fields are used to compensate for daily variations in positioning and the physical characteristics of the radiation beam. The dose distribution in conventional 3D planning is calculated in a planning process called “forward planning” which involves calculating the angle of incidence, the weight or contribution to the total of each beam, and the necessary beam modifier. IMRT, in contrast, requires that the radiation oncologist defines specific doses to the volumes of interest: the gross tumour volume (GTV), clinical target volume (CTV), the planning target volume (PTV), and to critical organs and planning risk volume (PRV) in accordance with ICRU 50/63/82 guidelines.5 The computerized IMRT planning software creates a series of modulation patterns in each incidence angle for the beam and the prescribed doses are obtained by an iterative algorithm. This process is known as “inverse planning”.
5. Development of a protocol
A protocol was created to standardize IMRT treatment procedures for localized prostate cancer. This protocol was developed in accordance with clinical guidelines and a review of the literature to guarantee quality assurance (QA) with regards to technical aspects of treatment and correct physics. The following procedures were implemented.
5.1. Patient preparation
The day prior to the simulation, patients are instructed to eat a bland diet and to take 2 enemas (one at night and the other on the day of the simulation) to avoid movement of the rectal wall caused by the presence of faeces or gas in the rectum.
Thirty minutes prior to the simulation, the patient is instructed to completely empty the bladder, drink 500 ml of water, and retain the urine until the end of the procedure.
5.2. Treatment simulation
After 30 min have passed, the patient is positioned in the dorsal decubitus position on the computed tomography (CT) scanner, with the legs resting on a commercial immobilizing cushion (CIVCO; Kalona, IA, USA), hands crossed over the chest, and the head resting on a support. The radiotherapy technician creates reference points by placing radiopaque markers on the skin of the patient at the abdominal or pelvis: 3 markers are used for axial alignment, one for sagittal alignment, with 2 other markers (one on each knee) to coincide with the cushion markers. CT slices (3 mm) are obtained in an area that ranges from 5 cm above to 5 cm below the treatment site. The reference marker sites are then immediately tattooed and magnetic resonance images (MRI) are acquired under the same positioning conditions, except that the metallic clips are replaced by vitamin E markers. The same segment of the abdominal-pelvic region is scanned with MRI using the same CT parameters in T2-weighted sequence.6
These protocols assure that each treatment is performed under standardized conditions that can be repeated on a daily basis.
5.3. Treatment planning
The CT and MRI images are then imported to the computer system of the Medical Physics department where the 3D image is reconstructed, and both images (CT and MRI) are fused to coincide anatomically. Then, using these images, the radiation oncologist contours the organs at risk (OAR) and treatment volumes (GTV, CTV and PTV) using the Eclipse (v. 7.3) treatment planning software (Varian Medical Systems; Palo Alto, CA, USA).
The contoured OARs include the femoral heads, the whole bladder, and the rectal volume—the complete rectal wall and lumen from the anus to the rectosigmoid junction. When creating the PTV, we initially excluded the anterior wall of the rectum completely. However, to comply with ICRU 83,7 which stipulates the inclusion of a 5 mm margin in the rectal wall to account for uncertainties and internal movements, we later changed our approach. Accordingly, the PTV in our revised protocol now includes this 5 mm margin. In cases in which pelvic lymph nodes need to be irradiated, the small intestine is contoured as an OAR. The penile bulb is delineated on the fused MRI image.
Treatment volumes include the prostate and seminal vesicles, which are contoured according to standard international guidelines.8 The prostate PTV78, defined as the treatment volume that receives at least 95% of the prescribed dose (78 Gy), is the CTV plus additional margins, as follows: a 7 mm margin is added laterally, anteriorly, and in the direction of the head; a 5 mm margin is added posteriorly and towards the feet. The daily dose prescribed to the PTV is 2 Gy for a total dose of 78 Gy (PTV78). The PTV78 is created in consensus with the medical physics department. In cases requiring nodal irradiation, delineation of this area is performed according to the contouring atlas of the Radiotherapy Treatment Group (RTOG).9
Once contouring has been completed, the work is checked and, if necessary, modified. Treatment planning is completed in one week for the prostate and in two weeks in cases in which pelvic lymph nodes are included. Dose limitations to the OARs were established in accordance with the published data for acute and chronic toxicity based on studies of conformal 3D radiotherapy and calculations in dose–volume histograms (DVH). A form with these dose restrictions was implemented to include in each patient record (Fig. 1). The rectum, bladder, femoral heads, small intestine, penile bulb, and dose prescription to the PTV are included on this form.
Fig. 1.

Dose restrictions.
Treatments are planned with the TPS. Although 5 fields were used initially, we found that 7 fields improved the dosimetric results, with gantry angles set at 180°, 135°, 95–100°, 30–35°, 330–325°, 260–265°, and 225° (opposing parallel fields should not be used). The sliding window IMRT technique is used to administer 6 MV photon radiotherapy with a Varian Clinac (model iX). During the course of treatment, digitally reconstructed radiographs (DRR) are created from the CT data and transferred to the patient's medical record for comparison with the orthogonal portal images obtained via an electronic portal imaging device (EPID) set in gantry positions ranging from 0° to 90°. These portal images are taken every third day and deviations from the latero–lateral, superior–inferior and anterior–posterior positions, based on the skin markers and pelvic bone anatomy, are measured. Any deviations greater than 1 mm are corrected and registered on the movement registration form (Fig. 2). If conditions in the rectum differ from the initial conditions (due to the presence of gases or faecal matter) treatment is suspended and the patient is instructed to attempt to empty the rectum within 10 min, without emptying the bladder. If the difference in the rectum remains, the procedure is restarted with bladder empted. Every 10 sessions, a CT scan is performed to assure that the image coincides with the initial CT scan. If large differences are observed, a new plan is created (Fig. 3).
Fig. 2.
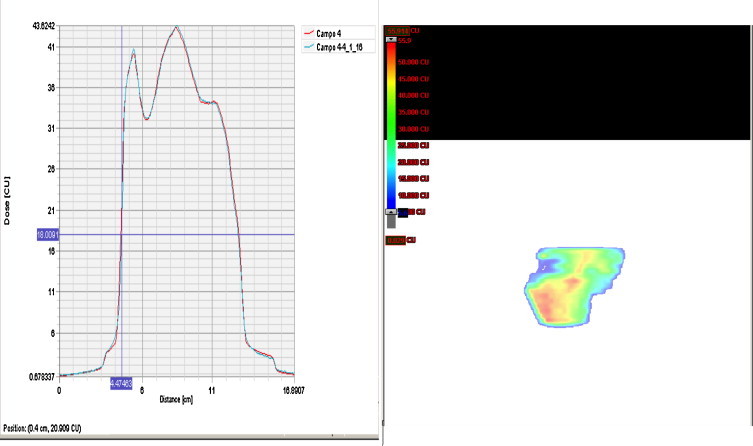
Movement registration.
Fig. 3.
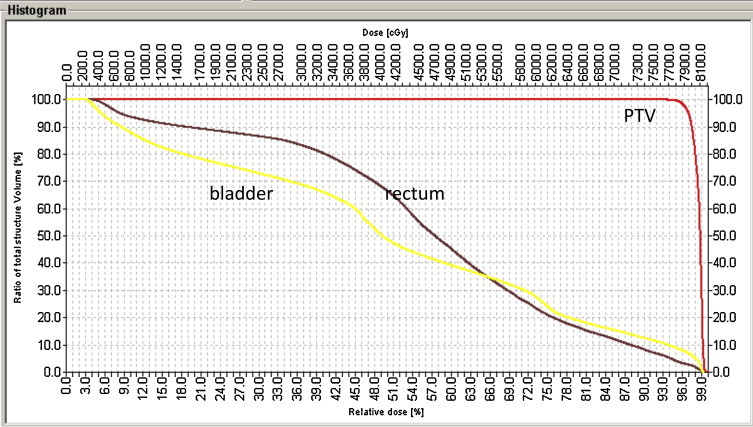
Image overlapping.
5.4. Patient positioning
Correct patient preparation with reproducible bladder filling not only reduces internal movement of the prostate, but can also prevent toxicity to the seminal vesicles. Daily verification of positioning with portal imaging is useful and highly advisable.9–12
5.5. Assessing toxicity
In order to properly determine the results of IMRT, it is essential to assess acute side effects, as one of the main objectives of IMRT is to reduce toxicity as compared to conformal 3D-RT. For this reason, we developed a very simple questionnaire (Fig. 4), which is administered by the nurse during the course of treatment. The nurse is responsible for registering the data from the questionnaire and for informing the radiation oncologist if there are any general, rectal, or urinary symptoms.13,14
5.6. Quality assurance by Medical Physics
The following procedures are performed by the Medical Physics Department:
-
i.
Commissioning of an IMRT planning system: this includes adjustment of the planning system parameters, dosimetric tests with various phantoms, adjustment of the radiation delivery system, and data transfer tests.
-
ii.
Linear accelerator QA procedures: mechanical and dosimetric precision of the dynamic fields. QA of the MLC is also performed. Dosimetric precision depends on the positioning accuracy of each leaf during the entire treatment.
-
iii.
Patient-related QA procedures: verification of the dosimetric plan, and field-by-field dosimetric verification.
-
iv.
Pre-treatment QA: the specific dose for each field in each patient is routinely verified at the start of the morning and afternoon.15 Verification is carried out with a PPMA phantom (type 2967; PTW, Freiburg, Germany).
5.7. Dose distribution: calculation and verification
The dose distribution is calculated on a reference plane that is perpendicular to the central axis, with the absolute dose calculated on a reference point. For each field, the test plan is transferred to the Varis Record and Verify system (R&V) database (Varis Information Systems, Varian Oncology; Palo Alto, CA), together with the leaf movement plan. In the accelerator, the phantom is positioned according to the test plan and a 0.3 cm3 ionization chamber (TW31013, PTW, Freiburg, Germany) is used to measure the absolute dose. The portal dosimetry of the EPID is used to measure the dose distribution on the reference plane.15–17
Administration of the QA fields is performed with the Varis R&V System. This is done not only to corroborate the dosimetric precision of the IMRT fields, but also to assure the correct transfer of data from the TPS to the verification system (Fig. 5).
Next, the dose at various points is measured to confirm that the irradiated fields are correct. To measure the doses, the ionization chamber is positioned in the centre of the axis; however, if a wide gradient is detected in the central axis, a different measuring point is selected.
5.8. Gamma index
The results of the 2D distributions are analysed with an evaluation method called gamma index. This method allows us to compare two dose distributions even in regions with steep gradients in which other methods (such as superimposing isodose distributions or by dose differentials) are not sufficiently precise. The gamma index has to be adjusted by the acceptation criteria provided by the user for the difference between the maximum dose and the distance to agreement (DTA). Only areas with gamma = 1 meet the acceptation criteria. Acceptation criteria were defined as a maximum deviation from the absolute dose of 2% and a DTA of 2 mm.
Superimposition of absolute and calculated isodose measures is represented together with corresponding gamma index distribution. The corresponding gamma index values are generally less than 1, and thus meet the criteria described above. For very small areas, the dose difference is 3%, these deviations can be explained by underdosing due to the tongue-and-groove effect between adjacent leafs, which are not correctly modelled by the Eclipse. For this reason, it seems that a treatment plan is acceptable despite the small areas where the dose differential exceeds 2%.
6. Patients
To date, we have used IMRT to treat 100 prostate cancer patients. In all cases, we used the QA procedures described above. The movement registration form, which was developed to record the movement (in mm) of each patient, was not available initially. A prescription form was implemented in planning to facilitate and record the dose restrictions to the critical organs and the dose to the PTV. Although 5 beam angles were used initially, we found that 7 beam angles resulted in a better dose distribution. Originally, the anterior wall of the rectum was excluded from the PTV; however, following the ICRU 83 recommendations, a 5 mm margin was later included. The DVHs were checked for each patient to confirm the accuracy of the prescription dose and the dose to the OARs (Fig. 6).
The nurses were responsible for administering the treatment symptom tolerance questionnaire; this form was modified to meet the requisites of the hospital's Quality Control Department.
The main elements of a QA program for IMRT treatment of prostate cancer patients are strict patient selection, careful treatment planning and execution, precise positioning, and frequent verifications via regular electronic portal images.18–21
7. Conclusions
Implementation of IMRT into routine clinical practice is feasible but requires careful planning, particularly to achieve quality assurance. The need to simultaneously implement specialized software, multifaceted QA programs for all the new software, and training and education for the multidisciplinary team make IMRT implementation even more complex. An intensive QA program assures the administration of uniform treatments for better tumour control and prevention of acute and chronic toxicity caused by radiation.
In our experience, we found that the key to successful implementation of IMRT is strong leadership, extensive training, creation of a standardized protocol, and a collaborative approach. In addition, it is essential that the centre have sufficient resources (staff, technology, funds) to assure success.
IMRT was found to be feasible and immediately accepted by all staff members, including medical physicists, radiation oncologists, dosimetrists, and radiotherapy technicians.
Conflict of interest
None declared.
Financial disclosure
None declared.
Appendix A. Supplementary data
The following are supplementary data to this article:
References
- 1.Hong T.S., Ritter M.A., Tome W.A., Harari P.M. Intensity-modulated radiation therapy: emerging cancer treatment technology. Br J Cancer. 2005;92:1819–1824. doi: 10.1038/sj.bjc.6602577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cahlon O., Hunt M., Zelefsky M.J. Intensity-modulated radiation therapy supportive data for prostate cancer. Semin Radiat Oncol. 2008;18:48–57. doi: 10.1016/j.semradonc.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Amin N., Konski A.A. Intensity-modulated radiation therapy for prostate cancer is cost effective and improves therapeutic ratio. Expert Rev Pharmacoecon Outcomes Res. 2012;12(4):447–450. doi: 10.1586/erp.12.46. [DOI] [PubMed] [Google Scholar]
- 4.Ling C.C., Burman C., Chui C.S. Conformal radiation treatment of prostate cancer using inversely planned intensity modulated photon beams produced with dynamic multileaf collimation. Int J Radiat Oncol Biol Phys. 1996;35:721–730. doi: 10.1016/0360-3016(96)00174-5. [DOI] [PubMed] [Google Scholar]
- 5.Prescribing, recording, and reporting photon beam therapy (supplement to ICRU report 50). ICRU report 62.
- 6.Hanvey S., Sadozye A.H., McJury M., Glegg M., Foster J. The influence of MRI scan position on image registration accuracy, target delineation and calculated dose in prostatic radiotherapy. Br J Radiol. 2012;85(1020):e1256–e1262. doi: 10.1259/bjr/26802977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Prescribing, recording, and reporting photon-beam intensity-modulated radiation therapy (IMRT) contents. J ICRU. 2010;10(April (1)) [Google Scholar]
- 8.McLaughlin P., Evans C., Feng M., Narayana V. Radiographic and anatomic basis for prostate contouring errors and methods to improve prostate countering accuracy. Int J Radiat Oncol Biol Phys. 2010;76:369–378. doi: 10.1016/j.ijrobp.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto K., Okumura M., Asai Y., Shimomura K., Tamura M., Nishimura Y. Dosimetric properties and clinical application of an a-Si EPID for dynamic IMRT quality assurance. Radiol Phys Technol. 2013;6(1):210–218. doi: 10.1007/s12194-012-0190-1. [DOI] [PubMed] [Google Scholar]
- 10.Boehmera D., Bohsungb J., Eichwurzelb I., Moysb A., Budacha V. Clinical and physical quality assurance for intensity modulated radiotherapy of prostate cancer. Radiother Oncol. 2004;71:319–325. doi: 10.1016/j.radonc.2004.02.016. [DOI] [PubMed] [Google Scholar]
- 11.Bekelman J.E., Hahn S.M. The body of evidence for advanced technology in radiation oncology. J Natl Cancer Inst. 2013;105(1):6–7. doi: 10.1093/jnci/djs508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mendenhall N.P., Schild S., Slater J. Radiation therapy modalities for prostate cancer. JAMA. 2012;308(5):450–451. doi: 10.1001/jama.2012.8112. author reply 451-2. [DOI] [PubMed] [Google Scholar]
- 13.Gay H.A., Barthold H.J., O’Meara E. Pelvic normal tissue contouring guidelines for radiation therapy: a Radiation Therapy Oncology Group consensus panel atlas. Int J Radiat Oncol Biol Phys. 2012;83(3):e353–e362. doi: 10.1016/j.ijrobp.2012.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koh W.Y., Ren W., Mukherjee R.K., Chung H.T. Internal audit of a comprehensive IMRT program for prostate cancer: a model for centers in developing countries? Int J Radiat Oncol Biol Phys. 2009;74(5):1447–1454. doi: 10.1016/j.ijrobp.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 15.MacKay R.I., Staffurth J., Poynter A., Routsis D., Radiotherapy Development Board UK guidelines for the safe delivery of intensity-modulated radiotherapy. Clin Oncol (R Coll Radiol) 2010;22(8):629–635. doi: 10.1016/j.clon.2010.06.017. [DOI] [PubMed] [Google Scholar]
- 16.Stasi M., Bresciani S., Miranti A., Maggio A., Sapino V., Gabriele P. Pretreatment patient-specific IMRT quality assurance: a correlation study between gamma index and patient clinical dose volume histogram. Med Phys. 2012;39(12):7626–7634. doi: 10.1118/1.4767763. [DOI] [PubMed] [Google Scholar]
- 17.Corral Garcia A., Gómez Fervienza J., Marquez Parro R. Dosimetric effect of daily setup correction in prostate IMRT. Rep Pract Oncol Radiother. 2013;18:S379. [Google Scholar]
- 18.Wolff J.M., Mason M. Drivers for change in the management of prostate cancer – guidelines and new treatment techniques. BJU Int. 2012;109(Suppl. 6):33–41. doi: 10.1111/j.1464-410X.2012.11218.x. [DOI] [PubMed] [Google Scholar]
- 19.Bak K., Dobrow M., Hodgson D., Whitton A. Factors affecting the implementation of complex and evolving technologies: multiple case study of intensity-modulated radiation therapy (IMRT) in Ontario, Canada. BMC Health Serv Res. 2011;11:178. doi: 10.1186/1472-6963-11-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin J.M., Frantzis J., Eade T., Chung P. Clinician's guide to prostate IMRT plan assessment and optimization. J Med Imaging Radiat Oncol. 2010;54:569–575. doi: 10.1111/j.1754-9485.2010.02217.x. [DOI] [PubMed] [Google Scholar]
- 21.Adamczyk M., Piotrowski T., Adamiak E. Evaluation of combining bony anatomy and soft tissue position correction strategies for IMRT prostate cancer patients. Rep Pract Oncol Radiother. 2012;17(2):104–109. doi: 10.1016/j.rpor.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


