Abstract
Primary osteogenic sarcoma of the breast is a rare neoplasm, diagnosed mainly by pathohistological and immunohistochemical analysis.
We hereby present a case of primary osteogenic sarcoma in the right breast of a 62-year-old woman with synchronous appearance of an invasive ductal carcinoma. Clinical findings are manifested with two separate painless formations 2.5 cm/2 cm and 1.5 cm/1 cm in size, located on the border of the upper and lower lateral quadrant of the right breast. No axillary lymphadenopathy was diagnosed. The pathohistological and immunohistochemistry findings of both tumors revealed a synchronous manifestation of two distinct neoplasms – epithelial and non-epithelial. Multimodality treatment consisted of Patey's radical mastectomy; 3 cycles of adjuvant chemotherapy; postoperative 50 Gy radiotherapy to the chest wall followed by additional 3 cycles of chemotherapy and anti-estrogen hormonotherapy.
Due to the rarity of osteogenic mammary sarcoma, even more so in a combination with epithelial breast tumors, its clinical features are unclear and optimal treatment remains controversial. Considering the poor prognosis of the combination of both malignomas, we discuss a number of diagnostic and therapeutic issues.
Keywords: Extraskeletal osteogenic mammary sarcoma, Synchronous invasive ductal cancer, Immunohistochemistry, Radiotherapy, Multimodality treatment
1. Background
Extraskeletal osteosarcoma is a rare malignancy that accounts for <1% of all soft-tissue sarcomas. Primary osteogenic sarcoma of the breast occurs in 2–4% of all osteosarcomas1 and in 0.1% of primary neoplasms of the breast.2,3 Extraskeletal sarcoma of the breast is considered an aggressive disease with early local recurrence and distant hematogenic metastases.3–8
We present a casuistic clinical case of a synchronous occurrence of mammary osteosarcoma and invasive ductal carcinoma, localized in one breast.
2. Case presentation
Sixty-two-year-old Caucasian woman has developed an asymptomatic two lumps in the right breast. We have to mention, that 6 months before a regular prophylactic mammography was negative (Fig. 1).
Fig. 1.

The right breast regular prophylactic mammography.
Two tumor lesions were visualized on mammography at the border of both superior outer and inferior outer quadrants of the right breast (Figs. 2 and 3).
Fig. 2.

Mammographic features of both lesions in the right breast. (А) 1 – osteosarcoma and 2 – carcinoma.
Fig. 3.
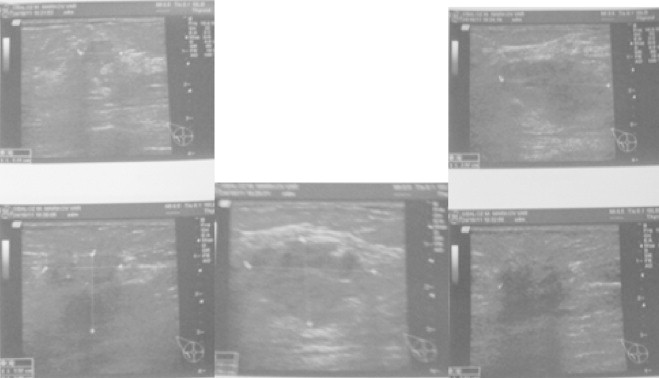
Diagnostic sonography of the right breast.
Detailed mammographic description: Two lesions were seen against the background of profound fat regression: (1) High density lesion 42/30 mm in size with unclear lobular contours and eccentric intra-lesion calcificates. (2) Spicular 20/19 mm lump with defined irregular contours (Fig. 2).
Detailed sonography description of the right breast: First lesion has an irregular lobular contour. Second lesion has also lobular contour with decrease of US signal in its distal part. Additional founding – enlarged right axillary lymph nodes (Fig. 3).
Staging CT scan and skeletal scintigraphy showed no evidence of metastatic disease.
3. Multimodality treatment
The patient was treated with a radical Patey's mastectomy. Detailed pathohistological and imunohistochemical analysis revealed two different malignomas – a rare extraskeletal osteosarcoma synchronous (Fig. 4); with an invasive epithelial invasive ductal cancer (Fig. 5).
Fig. 4.

Photomicrograph showing: primary mammary osteosarcoma of the right breast Hematoxylin and Eosin stain (H&E) 40×.
Fig. 5.
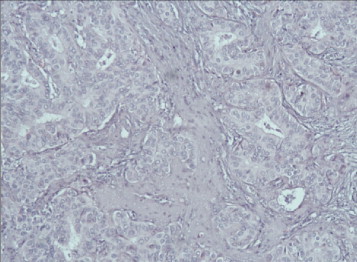
Photomicrograph showing: invasive moderately-differentiated ductal carcinoma of the right breast. Hematoxylin and Eosin stain (H&E) 40×.
Three courses of adjuvant chemotherapy with Pharmarubicin and Endoxan were followed by 50 Gy postoperative radiotherapy to the chest wall and additional 3 courses of same regime chemotherapy. Finally, she was put on Letrozole.
4. Pathohistological findings of both tumors
Macroscopical description revealed a part of the mammary gland to show two separate tumor lesions, found at a distances of 5 cm from each other, without skin engagement and with free resection lines. The first tumor was 1.5 cm/1 cm in size; whitish-yellow in color. The second tumor was 2.5 cm/2 cm in size with thick cut surface. Among the axillary adipose tissue, 11 axillary lymph nodes were dissected.
Classical microscopic description shows an infiltration by elongated and oval multinucleated and monstrous cells surrounded by neoplastic osteoid matter; with increased mitotic activity 50 mitoses/10 HPF; and presence of hemorrhages (Fig. 4). On immunohistochemistry (IHC) tumor cells were Vimentin positive, Ki67 was elevated up to 50%; epithelial markers СK AE1/AE3 and СK7 were negative, as well as estrogen, progesterone receptors and HER2 oncoprotein. The diagnosis was extraskeletal osteosarcoma of the right breast.
The second lumps looks different – in the moderate amount of fibrovascular stroma a proliferation of tubular structures and tumor cell groups with abundant eosinophilic cytoplasm, pleomorphic nuclei and prominent nucleons were seen; cells mitotic activity was moderate 30 mitoses/10 HPF (Fig. 5). On immunohistochemistry (IHC) neoplastic cells were positive for CK7, CKAE1/AE3, ЕR, PR; HER2 oncoprotein was negative and Ki-67 was 20%. Histological diagnosis was invasive moderately-differentiated ductal carcinoma of the right breast – pT2N1G2.
Microscopic description of dissected right axillary lymph nodes: Among the 11 dissected lymph nodes, only one had ductal carcinoma metastases. IHC confirmed the epithelial origin of it: Vimentin (−); ЕR-(+); Cytokeratins (CK) – CK AE1/AE3(+) (Fig. 6).
Fig. 6.

IHC picture of CKAE1/AE3 positive metastasis of invasive ductal carcinoma in only one of axillary lymph nodes of the right breast.
5. Follow up
The left breast sonography and mammography did not show any suspected focal lesions.
Laboratory findings were normal, including the preoperative alkaline phosphatase and tumor marker CA 15-3 was normal/les 30 U/ml serum level.
Post-treatment follow up mammography of the left breast: The residual fibroglandular elements showed mastopatic changes of fibrotic type without suspected solid or cystic distortions. A single benign view classification. No axillary adenopathy. Skin and subskin tissues looked normal (Fig. 7A and B).
Fig. 7.
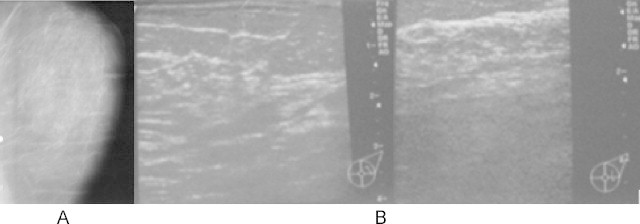
The left breast mammography and sonography.
CT scan and skeletal scintigraphy after 2 years showed no evidence of metastatic disease.
Two years after treatment the patient is disease free and continues her treatment with Letrozole.
6. Discussion
Due to the rare occurrence of primary osteosarcoma of the breast, the oncologist faces a number of diagnostic and therapeutic issues. Pathohistological diagnosis of osteogenic sarcoma of soft tissues requires a combination of three main elements: (1) presence of neoplastic osteoid or bone formation within the tumor cells; (2) exclusion of primary bone neoplasm and (3) absence of malignant epithelial components in the tumor cells.9 In our case the pathohistological and immunohistochemical analysis of the first neoplastic lump proved the presence of a neoplastic osteoid as well as absence of Cytokeratins (СK AE1/AE3 (−) and СK7 (−)), evidencing non-epithelial character of the tumor.1–3,17 The presence of bone formation or osteoid element in epithelial invasive ductal and lobular neoplasia is rare. It is defined as metaplastic carcinoma, characterized by metaplastic epithelial neoplasia after IHC positive to Cytokeratins.10
This clinical case with two distinct neoplasms with epithelial and non-epithelial characteristics occurring synchronously makes the prognosis, which determines adequate postoperative adjuvant therapy, difficult. Primary osteosarcoma of the breast is a biologically aggressive tumor characterized by early local recurrence (43% in the first year) and hematogenous metastases – to the lungs, bone, liver and soft tissue (cutaneous and subcutaneous). The incidence rates of this soft-tissue sarcoma resembles the primary osteosarcoma with a low 5-year overall survival rate – approximately 38%.3,11–13 Literature reports factors defined as significantly worsening the prognosis – tumor size, histological subtype (osteoclastic variant), resection margins pathohistologically positive to tumor cells, number of mitoses and presence of distant metastases.12,13 In this tumor synchronicity, the prognosis is significantly worsened, despite the initial clinical stage of each neoplasm (epithelial and non-epithelial). The cumulative number of adverse factors of the invasive ductal carcinoma (G2, pT2 N1) on the one hand, and of the osteosarcoma of the breast/G3; Кi 67-positive in 50%/, on the other hand, predefine the worsened individual prognosis.14,15
Optimal treatment of osteosarcoma of the breast involve radical surgery (wide local margin of resection with clean resection margins), such as Patey's radical mastectomy in our case.3,6 Adjuvant radiotherapy of the chest wall scar significantly improves local tumor control (LTC) – minimizes local recurrence.16 Despite the presence of two small-size neoplasias, epithelial 2.5 cm/2 cm in diameter and non-epithelial 1.5 cm/1 cm in diameter, and the radical surgery, Patey's mastectomy, given the local aggressiveness of osteosarcoma, we conducted a postoperative radiotherapy of the entire chest wall. As is the case with the other types of soft-tissue extraskeletal sarcomas, in 50% of the cases, hematogenous metastases develop, rarely lymphatic – in the nearest lymph nodes. The progression of the disease leads to exitus letalis in 2–10 months.3,6,11–13 The presence of lymphatic metastases requires cisplatin/ifosfamide-based chemotherapy, which significantly improves the long-term survival rate.6,16 In the case presented here–synchronous occurrence of invasive ductal carcinoma рТ2N1М0 and osteosarcoma of the breast without lymphatic metastases, the chemotherapy is used because of the рN1 of the invasive breast cancer.
7. Conclusion
Primary osteosarcoma of the breast is a rare neoplasm. Synchronous unilateral occurrence of invasive carcinoma of the breast and osteogenic sarcoma of the breast is casuistry.
This clinical case is presented to focus on the significance of pathohistological and immunohistochemical analysis in the differential diagnosis between epithelial and non-epithelial mammary neoplasm. The optimal complex treatment is, on the one hand, based on an accurate assessment of all factors of invasive carcinoma, and on the other hand, on extraskeletal mesenchymal tumor that worsens the prognosis. In the case presented here, we reported 2-year free survival (without local recurrence and distant metastases).
Conflict of interest
None declared.
Financial disclosure
None declared.
References
- 1.Enzinger F.M., Weiss S.W. 5th ed. Mosby; St. Louis, MO: 2008. Enzinger and Weiss's soft tissue tumors; pp. 1051–1059. [Google Scholar]
- 2.Rosen P.P. 2nd ed. vols. 425–453. Lippincott Wilkins; Philadelphia, PA: 2001. pp. 818–819. (Rosen's breast pathology). [Google Scholar]
- 3.Silver S.A., Tavassoli F.A. Primary osteogenic sarcoma of the breast: a clinicopathologic analysis of 50 cases. Am J Surg Pathol. 1998;22:925–933. doi: 10.1097/00000478-199808000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Momoi H., Wada Y., Sarumaru S. Primary osteogenic sarcoma of the breast. Breast Cancer. 2004;11:396–400. doi: 10.1007/BF02968048. [DOI] [PubMed] [Google Scholar]
- 5.Hutton C.W., Strang C. Osteosarcoma of the breast recurring with mediastinal obstruction. Postgrad Med J. 1984;60:159–161. doi: 10.1136/pgmj.60.700.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lin S.-T., Chen C.-H., Kuo C.-L. Primary osteosarcoma of the breast. J Med Sci. 2011;31(5):231–235. [Google Scholar]
- 7.Gull S., Patil P., Spence R.A.J. Primary osteosarcoma of the breast. BMJ Case Rep. 2011 doi: 10.1136/bcr.03.2011.4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jensen M.L., Schumacher B., Jensen O.M. Extraskeletal osteosarcomas: a clinicopathologic study of 25 cases. Am J Surg Pathol. 1998;22(5):588–594. doi: 10.1097/00000478-199805000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Allan C.J., Soule E.H. Osteogenic sarcoma of the somatic soft tissues. Clinicopathologic study of 26 cases and review of literature. Cancer. 1971;27:1121–1133. doi: 10.1002/1097-0142(197105)27:5<1121::aid-cncr2820270519>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 10.Sando N., Oka K., Moriya T. Osteosarcoma arising in the breast. APMIS. 2006;114(7–8):580–586. doi: 10.1111/j.1600-0463.2006.apm_404.x. [DOI] [PubMed] [Google Scholar]
- 11.Khaldi L., Athanasiou E.T., Hadjitheofilou C.T. Primary mammary osteogenic sarcoma. Histol Histopathol. 2007;22:373–377. doi: 10.14670/HH-22.373. [DOI] [PubMed] [Google Scholar]
- 12.Brustugun O.T., Reed W., Poulsen J.P., Bruland O.S. Primary osteosarcoma of the breast. Acta Oncol. 2005;44:767–770. doi: 10.1080/02841860500254897. [DOI] [PubMed] [Google Scholar]
- 13.Vorobiof G., Hariparsad G., Freinkel W. Primary osteosarcoma of the breast: a case report. Breast J. 2003;9:231–233. doi: 10.1046/j.1524-4741.2003.09320.x. [DOI] [PubMed] [Google Scholar]
- 14.Mehdi I., Shah A.H., Moona M.S. Synchronous and metachronous malignant tumours expect the un-expected. J Pak Med Assoc. 2010;60(11):905–909. [PubMed] [Google Scholar]
- 15.Hartman M., Czene K., Reily M. Incidence and prognosis of synchronous and metachronous bilateral breast cancer. J Clin Oncol. 2007;25:4210–4216. doi: 10.1200/JCO.2006.10.5056. [DOI] [PubMed] [Google Scholar]
- 16.McGowan T.S., Cumming B.J., Sillivan O.B. An analysis of 78 breast sarcoma patients without distant metastases at presentation. Int J Radiat Oncol Biol Phys. 2000;46:383–390. doi: 10.1016/s0360-3016(99)00444-7. [DOI] [PubMed] [Google Scholar]
- 17.Yadav B.S., Bansal A., Sharma S.C. A 62 year-old women with osteogenic sarcoma in the contralateral breast 15 years after treatment for breast cancer. Semin Oncol. 2013;40(2):135–144. doi: 10.1053/j.seminoncol.2013.01.010. [DOI] [PubMed] [Google Scholar]


