Abstract
Short-course preoperative radiotherapy (RT) is widely used in northern Europe for locally advanced resectable rectal cancer, but its role in the era of advanced imaging techniques is uncertain. Here, we reviewed articles and abstracts on SCRT published from 1974 through 2013 with the goal of identifying patients who might be best suited for short-course RT. We included relevant articles comparing surgery with or without preoperative radiation published before and after the advent of total mesorectal excision. We also analyzed two randomized trials directly comparing short-course RT with conventionally fractionated chemoradiation (the Polish Colorectal Study Group and the Trans-Tasman Radiation Oncology Group) that compared short-course RT with conventional chemoradiotherapy. We conclude from our review that short-course RT can be generally applied for operable rectal cancer and produces high rates of pelvic control with acceptable toxicity; it reduces local recurrence rates but does not increase overall survival. SCRT seems to be best used for tumors considered “low risk,” i.e., those that are >5 cm from the anal margin, without circumferential margin involvement, and involvement of fewer than 4 lymph nodes. Whether sequential chemotherapy can further improve outcomes remains to be seen, as does the best time for surgery (immediately or 6–8 weeks after RT). We further recommend that selection of patients for short-course RT should be based on findings from magnetic resonance imaging or transrectal ultrasonography.
Keywords: Locally advanced rectal cancer, Short course preoperative radiotherapy, Neoadjuvant radiation therapy, Prognostic factors
1. Background
The optimal treatment for rectal cancer that presents as non-metastatic locally advanced disease remains a topic of debate.1 Although the value of neoadjuvant treatment that could render initially unresectable disease resectable is clear, whether that treatment should be radiation therapy (RT) alone or concurrent chemoradiation therapy remains controversial.2,3 The 2013 guidelines of the U.S. National Comprehensive Cancer Network for the treatment of rectal cancer (available at www.nncn.org) recommend preoperative therapy consisting of RT with fluorouracil or capecitabine chemotherapy for patients with clinical T3 N0 or any-T, N1-2 disease. The other form of preoperative therapy, short-course RT, has been validated by some European studies but is not recommended in the NCCN guidelines. The European Society for Medical Oncology (ESMO)4 describes two options for preoperative RT for rectal cancer: (1) a standard dose of 45–50.4 Gy, given in 1.8- to 2-Gy fractions, with concomitant use of fluoropyrimidines followed 6–8 weeks later by radical surgery, which is more commonly used in the United States and parts of Europe; and (2) short-course RT to a total dose of 25 Gy in five 5-Gy fractions over 1 week followed immediately by surgery (<10 days from the first radiation fraction); this regimen is more common in northern Europe.5,6
We sought here to review and update the literature on the use of short-course RT for the treatment of rectal cancer and to clarify which procedure is the most appropriate when disease staging is based on findings from newer imaging techniques, such as magnetic resonance imaging (MRI).7,8
2. Comparisons of surgery versus neoadjuvant RT plus surgery
2.1. Radiation dose
The dose and schedule of neoadjuvant RT, and the nature of the surgery itself, for rectal cancer have evolved since its earliest use in the 1970s. The first randomized comparison of radiation alone versus radiation followed by surgery, published by Stearns in 1974,9 used an RT dosage of 20 Gy given in 10 fractions of 2 Gy each. In that study, the addition of radiation before surgery did not increase overall survival but did reduce the rate of local recurrence. In 1975, the first results of a trial by the Veterans Administration Surgical Oncology Group10 suggested that preoperative RT to a dose of 20–25 Gy given in 10 fractions given over a 12-day period seemed to reduce both local recurrence (LR) and distant metastasis compared with surgery alone; however, those results could not be reproduced in a second study by the same group.11 Similarly, a contemporaneous report of a large trial by the British Medical Research Council found that preoperative RT given as a single 5-Gy dose or as ten 2-Gy fractions did not affect LR, distant recurrence, or overall survival (OS) relative to surgery alone,12 suggesting that if preoperative RT is to be effective, the dose should be at least 20 Gy. Indeed, a phase III study by the European Organization for Research and Treatment on Cancer (EORTC) reported by Gérard et al.13,14 showed that preoperative RT to a dose of 34.5 Gy, given in 15 fractions over 19 days, did not significantly improve OS, but it did led to marked reductions in LR. The toxicity of this regimen was deemed acceptable, except when it was given to elderly patients (age ≥ 70 years) or to patients with pre-existing cardiovascular disease. The results of these studies suggest that the RT dose to be given as preoperative therapy should be at least 20 Gy.
2.2. Randomized comparisons of radiation schedules
A 1990 report of a large multicenter trial conducted by the Stockholm Rectal Cancer Study Group comparing an RT regimen of 25 Gy to be given over 5–7 days (“short-course” RT) versus surgery alone showed that at a median follow-up time of 53 months, preoperative short-course RT led to lower LR regardless of the stage of the tumor.15 However, again no difference was found among groups in distant control or OS. Postoperative morbidity was noted to be higher in the preoperative RT group, as was postoperative mortality (8% RT vs. 2% no-RT, P < 0.01) (Tables 1 and 2).
Table 1.
Studies of preoperative radiation therapy conducted before the total mesorectal excision era.
| Study and reference | Total dose (Gy) | No. of fractions | Overall survival | Local recurrence | LR |
|
|---|---|---|---|---|---|---|
| Control group | Experimental group | |||||
| Stearns, 19749 | 20 | 10 | No | Yes | ||
| VASOG I, 197510 | 20–25 | 12 | Yes | 37% | 29% | |
| VASOG II, 198611 | 31.5 | 18 | No | 22% | 21% | |
| EORTC, 198813,14 | 5/20 | Unique/10 | No | No | ||
| Stockholm I, 199015 | 25 | 5 | No | Yes | 28% | 15% |
| Swedish Ia, 199016 | 25.5/60 | 7/30 | No | Yes | ||
| Swedish IIb, 199317,19 | 25 | 5 | Yes | Yes | 27% | 12% |
Abbreviation: LR, local recurrence.
Comparison of preoperative RT versus postoperative RT.
Improvement in overall survival at 5 years only reached significance in those patients with Dukes’ C stage disease.
Table 2.
Studies of preoperative radiotherapy conducted during the total mesorectal excision era.
| Study and reference | Total dose (Gy) | No. of fractions | Overall survival | Local recurrence | LR |
|
|---|---|---|---|---|---|---|
| Control group | Experimental group | |||||
| Dutch, 200122 | 25 | 5 | No | Yes | 2.4% | 8.2% |
| Dutch, 2011a30 | 25 | 5 | No | Yes | 11% | 5% |
| CR07, 2009b36 | 25/45 | 5/25 | No | Yes | 10.6% | 4.4% |
Abbreviation: LR, local recurrence.
10-year follow-up.
Results showed that both local control and disease-free interval were positively affected by short-course radiation therapy.
Another report that same year from Uppsala University16 compared preoperative short-course RT (25.5 Gy given over a 1-week period) with conventionally fractionated postoperative RT (60 Gy over 6–8 weeks) for rectal cancer. The LR rate was lower for the group receiving preoperative RT (12% vs. 21%, P = 0.02), but at a mean 6 years of follow-up, the OS rate was no different between groups (42% vs. 38%, P = 0.5).
A subsequent study by the Stockholm Rectal Cancer Study Group17–19 compared LR and OS for patients given surgery alone versus short-course RT followed by surgery 7 days later. The addition of preoperative RT led to a lower LR rate (12% vs. 27%, P < 0.001) and an improved 5-year OS rate (58% vs. 48%, P = 0.004). However, this study has been criticized for imbalances between groups (more patients with Dukes stage A and B in the RT + surgery group, which the authors attributed to downstaging after RT and for the “unacceptably high” LR rate for Dukes stage A tumors in the surgery group).20 Indeed, pathologic review of findings from another trial conducted in the Netherlands (the Dutch TME + RT trial) revealed that short-course RT did not result in tumor downstaging.21 To date, few, if any, studies have replicated the finding of improved OS after preoperative RT.22,23
2.3. Randomized studies of radiation schedules after the standardization of total mesorectal excision
The recognition that involvement of the circumferential resection margin (CRM) by tumor cells is important in LR has led to the general use of total mesorectal excision (TME), in which the entire mesorectum is enveloped and resected by precise, sharp dissection. In the time since TME became the recommended surgical technique for extirpation of rectal cancer,24–26 the question has been raised as to whether RT is necessary when this technique is used. To address this question, the Dutch Colorectal Cancer Group began a study in 1996 to compare surgery (TME) only with short-course preoperative RT (5 Gy given over 5 days) followed by TME.22 A total of 1805 eligible patients were randomly assigned to one of the two treatment groups; among the 1748 patients who underwent a macroscopically complete local resection, the LR rate at 2 years was 5.3%. The 2-year LR rates differed significantly according to treatment, being 2.4% in the RT plus surgery group and 8.2% in the surgery-only group (P < 0.001); however, neither the distant recurrence rate nor the OS rate were different between groups. Outcomes among the group treated with surgery only were compared with a subset of those from a previous Dutch trial, the Cancer Recurrence and Blood Transfusion (CRAB) trial,27 to determine the effect of using TME versus conventional, non-standardized surgery for colorectal cancer. That comparison showed that the use of TME led to substantial reductions in LR rates at 2 years, from 16.6% in the CRAB trial versus 8.6% in the Dutch TME study.28 These results agree with an analysis from the Stockholm Colorectal Cancer Study Group29 comparing the Stockholm I and II randomized trials (which involved conventional surgery with or without RT) and the so-called “TME project,” which involved the use of TME by surgeons who received training in the technique. In that analysis, the 2-year LR rates dropped from 14% to 15% for conventional surgery to 6% for TME.
Longer-term follow-up for the Dutch TME trial23 showed that at a median 6 years of follow-up, the LR rates at 5 years for patients who had a macroscopically complete local resection were 5.6% among those receiving preoperative RT and 10.9% for those receiving TME alone (P < 0.001), but no difference was found in OS (5-year rates: 64.2% RT + TME vs. 63.5% TME only, P = 0.902). Subgroup analyses showed that the receipt of preoperative RT significantly reduced risk of LR among patients with nodal involvement, those with lesions 5–10 cm from the anal verge, and those with uninvolved CRMs. At 12 years’ follow-up,30 the 10-year LR rates had remained roughly the same for each group (5% for RT + TME vs. 11% for TME only, P ≤ 0.001), but neither OS rates (56% vs. 57%) nor distant failure rates (25% vs. 28%) differed between groups. Cancer-specific death rates, however, were different according to treatment (17% for the RT + TME group vs. 22% for the TME-only group, P = 0.04). Other findings from this important trial include the influence of TNM stage, tumor location, and CRM status: although RT was not associated with an increase in OS overall, among patients with stage III disease, tumors in the middle third of the rectum, and negative CRMs, the receipt of RT + TME led to longer OS than the receipt of only TME. The investigators concluded that RT can be most useful for patients with these characteristics.
A subanalysis of acute side effects and surgical complications after RT + TME in the Dutch study31 revealed very little RT-related toxicity. Patients in the RT + TME group lost slightly more blood during surgery (100 mL) than did the TME-only group (P < 0.001) and had more perineal complications in cases requiring abdominoperineal resection (P = 0.008). Although the total number of complications was slightly higher in the RT + TME group (P = 0.008), no differences were found in postoperative mortality rates (4.0% v 3.3%) or in the number of reinterventions needed. Another analysis of long-term functional outcomes from the Swedish Rectal Cancer Trial,32 however, indicated that RT after anterior resection may be associated with more bowel dysfunction and that this effect may persist for 5 years or more after treatment. The authors of that study concluded that predictors of LR should be sought so that some patients who may not need RT can be successfully treated without it.
The successor to the Dutch RT-TME trial, the PROCTOR (“Pre-operative Radiotherapy and/or adjuvant Chemotherapy combined with TME-surgery in Operable Rectal Cancer”) study,33 sought to determine if adding adjuvant chemotherapy after TME or RT + TME would provide any benefit in terms of OS. Preliminary analysis of this trial showed that chemotherapy consisting of fluorouracil and leucovorin given for 6 months after treatment to patients with stage II or III disease after a complete resection (R0) had no survival benefit.34,35 A similar question was asked in the MRC CR07 trial,36 which involved a comparison of preoperative short-course RT followed 7 days later by surgery versus initial surgery followed by postoperative chemoradiation (to 45 Gy in 25 fractions with concurrent fluorouracil, with or without leucovorin) for patients with residual tumor within ≤1 mm of the CRM. In that study, preoperative RT was associated with substantial reductions in risk of LR (relative reduction 61%, absolute difference at 3 years of 6.2%). Preoperative RT was also associated with improved disease-free survival (relative reduction 24% and 6.0% absolute difference at 3 years). Once again, however, OS did not differ between the two treatment groups. The benefit from preoperative short-course RT was similar regardless of the distance of the tumor from the anal verge or the TNM stage, although the absolute difference in local control was worse for patients with stage III disease. At 8 years of follow-up,37 the benefit in LR reduction was still present in the preoperative RT group, but by that time no difference was apparent in disease-free survival or in OS (5-year rates 73% vs. 73.8%). Multivariate analysis indicated that the receipt of abdominoperineal resection, the presence of extravascular invasion, and staging of affected lymph nodes were statistically significant predictors of LR.
In less than complete resections, involvement of the CRM is a key predictor of LR and as such may be useful for identifying patients who may benefit from postoperative chemoradiation. Adam and colleagues at the Centre for Digestive Disease at Leeds (UK)38 showed that involvement of CRM (defined as tumor located ≤1 mm from the CRM) conferred a higher risk of LR and had a negative effect on OS.
A recent meta-analysis by the Cochrane Colorectal Cancer Group39 that included the CR07 and Dutch trials sought to compare OS and LR after preoperative RT followed by surgery versus surgery alone for patients with resectable stage II or III locally advanced rectal cancer. In the five randomized controlled trials (3211 patients) evaluated, the LR rate at 5 years was significantly lower in the combined-therapy group compared to surgery alone (P < 0.001), but no differences were noted in disease-free survival or OS.40
Although the benefit in LR associated with preoperative RT is clear, it should be noted that this treatment is also associated with higher morbidity, after the RT or after surgery, and can affect bowel,41 small intestine, and sexual function.42 The effects tend to vary depending on the RT dosage used, the type of surgery, and the age of the patients. This topic is explored further in Section 2.6.
2.4. Randomized comparisons of short-course RT versus chemoradiation as preoperative therapy
To date, findings from two phase III studies, the French FFCD 9203 and EORTC 22921 trials,43,44 have shown significant reductions in LR from the use of preoperative short-course RT compared with preoperative conventionally fractionated RT given with fluorouracil and leucovorin. However, that experience has not been duplicated in other studies.
The two major studies to directly compare short-course RT and long-course chemoradiation before surgery were done by the Polish Colorectal Study Group45–47 and the Trans-Tasman Radiation Oncology Group.48 In the Polish Group trial, 316 patients with T3 and T4 rectal carcinoma, without sphincter infiltration, and whose lesions were accessible to digital rectal examination were randomly assigned to receive either preoperative RT (25 Gy in five 5-Gy fractions) and surgery within 7 days or chemoradiation (50.4 Gy in 28 1.8-Gy fractions, with bolus 5-fluorouracil and leucovorin) and surgery 4–6 weeks later. The choice of surgical technique depended on the tumor status at the time of surgery and was made at the discretion of the surgeon. The initial report of this trial revealed that although tumor size was 1.9 cm smaller after chemoradiation than after short-course RT (P < 0.001), the sphincter preservation rates were no different between groups (61% in the RT group vs. 58% in the chemoradiation group, P = 0.57).45 A subsequent report indicated that at a median 4 years of follow-up for patients alive at the time of analysis, chemoradiation showed no advantage in terms of survival, local disease control, or late-course toxicity compared with short-course RT.47
The other direct comparison, conducted by the Trans-Tasman Radiation Oncology Group,48 included 326 patients with T3N0-2M0 disease (staged with endorectal ultrasonography or MRI) who were randomly assigned to either short-course RT (5 fractions of 5 Gy each) followed immediately by surgery and then 6 cycles of adjuvant chemotherapy or conventional chemoradiation (50.4 Gy given over 5.5 weeks concurrently with continuous-infusion fluorouracil) followed by surgery 4–6 weeks later and another 4 courses of chemotherapy. At a mean follow-up time of 5.9 years and with 163 patients per treatment group, the 3-year LR rates were no different at 7.5% for the short-course RT group vs. 4.4% for the chemoradiation group (P = 0.24). Chemoradiation may have been more effective at local control of distal tumors (those <5 cm from the anal verge), but that difference was chemoradiation group (P = 0.21). No differences were also found in rates of distant recurrence (27% for short-course RT vs. 30% chemoradiation), OS at 5 years (74% vs. 70%), or late grade 3–4 toxicity (5.8% vs. 8.2%). The only baseline factor that independently predicted LR in multivariate analysis was a high carcinoembryonic antigen (CEA) level. In terms of post-treatment predictors, CRM positivity, nodal positivity, and baseline CEA level all independently predicted LR. However, the distance to the anal margin, analyzed as a continuous variable, did not predict LR.
Another phase III trial by the German Rectal Cancer Study Group (CAO/ARO/AIO-94)49,50 compared preoperative chemoradiation with postoperative chemoradiation in 799 patients and found that the preoperative therapy produced superior local control (10-year LR rates 7.1% vs. 10.1%, P = 0.048), but again, neither treatment was different in terms of distant recurrence or OS. At 46 months’ follow-up, preoperative chemoradiation was associated with better treatment compliance, toxicity, downstaging, sphincter preservation (in patients judged by the surgeon to require an abdominoperineal resection), and local control; by 11 years, multivariate analysis showed that an incomplete local resection, not receiving chemoradiation at all, stage III or IV disease, and having surgery that included intersphincteric or abdominoperineal resection were all associated with poorer local control.
2.5. Ongoing comparative trials
When this review was written, nine studies involving short-course RT were enrolling patients. These studies are evaluating the influence of several variables, including the timing and extent of surgery and the use of various types of chemotherapy, on standard outcomes such as local control, distant control, and OS as well as toxicity, tumor response, and quality of life.51 A few examples of such studies are described briefly below.
A multicenter randomized study by the Berlin Cancer Society group52 randomly assigned patients with T2 N+ or T3 rectal cancer to receive short-course RT followed by TME 5 days later, or conventional-dose 50.4 Gy RT in 28 fractions with continuous-infusion fluorouracil, followed by surgery 5–6 weeks later. All patients were to receive 12 weeks of adjuvant continuous-infusion fluorouracil, and all were to be followed for up to 5 years. The hypothesis for this trial was that preoperative chemoradiation would be superior to short-course RT in terms of local control at 5 years; secondary objectives were rates of OS, disease-free survival, complete resection (R0) and sphincter preservation, and acute and late toxicity. Data from this trial were to be subjected to an interim analysis in 2008, but no results had been published by the time this review was written.
Another phase II multicenter, non-randomized trial53 is evaluating whether extending the interval between preoperative chemoradiation and TME, and giving additional mFOLFOX-6 chemotherapy during that period, will improve tumor response; other endpoints include toxicity and surgical complications. Preliminary analysis of 144 patients revealed that receipt of additional FOLFOX chemotherapy may have improved the tumor pathologic complete response rate (25% vs. 18%) and that chemoradiation-related toxicity and postoperative complications were comparable between groups.
A similar approach is being tested in the RAPIDO trial (NCT01558921; “Radiotherapy and Preoperative Induction Therapy followed by Dedicated Operation”),54 which was to have begun in June 2011. That multicenter study compares two preoperative regimens for locally advanced rectal cancer: 6 weeks of standard chemoradiation (45 Gy in 25 1.8-Gy fractions with capecitabine) followed 6 weeks later by TME; and short-course RT (5 fractions of 5 Gy each, given in 5 days) followed by six cycles of capecitabine and oxaliplatin, followed 6 weeks later by TME. Endpoints of this trial include disease-free survival, OS, LR, toxicity, pathologic response rate, completeness of resection, and quality of life.
Another ongoing trial, the PROCTOR study,33–35 was designed to compare the effects of adding fluorouracil and leucovorin chemotherapy after TME or after RT + TME.
The U.S./Canadian cooperative group PROSPECT trial (“Preoperative Radiation or Selective Preoperative radiation and Evaluation before Chemotherapy and TME”)55,56 is a phase II/III randomized trial to evaluate the selective use of radiation in contrast to consistent use in the neoadjuvant treatment of locally advanced rectal cancer. Patients in the experimental group receive 6 cycles of FOLFOX chemotherapy followed by restaging with MRI or endoscopic ultrasonography. Those who respond to this therapy go on to receive TME followed by postoperative systemic therapy. Those who do not respond to this therapy are given preoperative chemoradiation (with fluorouracil or capecitabine) before surgery. Patients in the control group receive standard long-course chemoradiation (with fluorouracil or capecitabine) followed by TME and adjuvant systemic therapy.
2.6. Toxicity and quality of life after short-course RT or chemoradiation
Findings from the Dutch Colorectal Cancer Group trials30,31,46 suggest that the rate of total adverse events related to short-course RT was 26%, but only 7% were of grade 2 or 3 severity. Surgical mortality rates were 3.5% after RT + TME versus 2.6% for TME alone; the corresponding postoperative complication rates were 48% versus 41%, with the difference attributed mainly to differences in perineal wound healing. The frequencies of bleeding and anastomotic leakage were comparable between groups (13% and 11–12%). Notably, perineal wound complications were more common after abdominoperineal resection in the group that received RT (29% vs. 18%, P = 0.008), but no difference was found in abdominal wound complications.
The Canadian MRC CR07/NCIC CTC C016 trial36,37 also included analyses of quality of life.42 All 1350 patients were asked to complete the 38-item EORTC Quality of Life Questionnaire-Colorectal questionnaire. Increased sexual dysfunction was noted among men immediately after surgery, with a difference in treatment groups emerging at 6 months and continuing on for up to 2 years. Fecal incontinence was also more common among patients who received short-course RT.
The Polish Group study47 (Table 3) reported higher rates of acute toxicity for patients who received chemoradiation (18.2% vs. 3.2% who received short-course RT, P ≤ 0.001). Rates of late toxicity were similar between groups at 10.1% versus 7.0% (P = 0.3). That same group compared long-term toxicity in terms of sexual and anorectal function38 and found no difference in rates of impairment between groups, though it was noted that about two-thirds of all patients had reported impairments in anorectal functions.
Table 3.
Randomized studies comparing short-course radiation therapy versus long-course chemoradiation therapy.
| Studies, references, and treatment regimens |
||||
|---|---|---|---|---|
| Polish Colorectal Cancer Group47 |
Trans-Tasman Radiation Oncology Group48 |
|||
| Five 5-Gy fractions + TME | 50.4 Gy with 5FU bolus + TME | Five 5-Gy fractions + TME 6 cycles adj CT | 50.4 Gy with 5FU ci + TME 4 cycles adj CT | |
| No. of patients | 155 | 157 | 163 | 163 |
| Follow-up time (years) | 4 | 5.9 | ||
| Local failure rate | 9% | 14.2% | 7.5% | 4.4% |
| Disease-free survival rate | 58% | 55% | 73% | 70% |
| Overall survival rate | 67% | 62% | 74% | 70% |
| Grade 3–4 small or large bowel toxicity | 3.2% | 5.1% | ||
| Grade 3–4 late toxicity | 10.1% | 7.1% | 5.8% | 8.2% |
Abbreviations: 5FU, fluorouracil; TME, total mesorectal excision; ci, continuous infusion; CT, chemotherapy.
2.7. Distribution and etiology of local recurrences after treatment
The exact causes of local recurrence after resection of rectal cancer are not well understood but may reflect surgical techniques, tumor location, involvement of the CRM, tumor biology, and other variables.57 Follow-up from the Dutch TME trial reported in 201058 indicated that 114 LRs had occurred among 1417 patients, 81 (11%) in the TME group and 36 (4.6%) in the short-course RT + TME group. The mean time until diagnosis of LR was 2–6 years in the RT-TME group and 1.5 years in the TME group. Presacral recurrences were most common in both groups, and occurred more often after abdominoperineal resections. Receipt of RT reduced the rates of anastomotic LR significantly, except when distal margins after low anterior resection were <5 mm. LR rates were high even among those with a negative CRM after surgery. Presacral and lateral LRs resulted in a poor prognosis, in contrast to anterior or anastomotic LRs with a relatively good prognosis. The rate of anastomotic recurrence at 5 years was higher among those who received TME only (2.7% vs. 0.7% RT + TME, P = 0.003).
To assess if local recurrence was related to differences in tumor biology, tumors were considered as one of two hypothetical types: high risk, i.e., stage IV, T4 N2, or T3-T4 with positive CRM; or low risk, i.e., all other patients. Among the high-risk patients, LR rates were unacceptably high in both treatment groups, being 17.0% in the short-course RT + TME group and 24.6% in the TME-only group. For the low-risk group (48 of 114 recurrences, or 42%), the LR rate at 5 years was 5.1% versus 21% for the high-risk group (P ≤ 0.001).
To summarize, receipt of short-course RT reduced LR in all locations, prevented anastomotic failure, and reduced recurrences caused by a narrow distal resection margin. Abdominoperineal resection mainly resulted in presacral local recurrences; even after resection with a negative circumferential resection margin, local relapse rate was high. Further, suboptimal selection and the inclusion of many ‘advanced’ tumors in the TME trial are likely to have affected these results.
With regard to salvage therapy, short-course RT can be useful for residual disease after surgery, but is often less effective for large disease volumes.57 Predictors of success after salvage surgery vary considerably among studies.59–62 In any event, the development of distant disease after therapy for locally advanced rectal cancer is common, and indeed more patients are likely to die of complications from distant disease.
2.8. Biologically equivalent doses
The biologically equivalent dose of short-course RT, i.e., 5 fractions of 5-Gy each, can be derived from the following formula63:
For late-reacting tissues, for which α/β = 3 and K = 0:
The equivalent dose for conventionally fractionated RT would be:
where N equals approximately 20 sessions.
Thus, this hypofractionated short-course regimen is biologically equivalent to a conventionally fractionated 40 Gy regimen. If acute effects for an α/β ratio of 10 Gy are considered, the final result would be 37.5 Gy.
2.9. Determining clinical tumor volumes
The radiation volumes to be treated in short-course RT are the standard rectal-tumor volumes, with accommodations made for the reproducibility of patient positioning.64 The mesorectal and the posterior pelvic subsites should be included in the clinical target volume (CTV). Inclusion of the inferior pelvic subsite should be considered only if a sphincter-saving procedure is intended and the tumor is <6 cm from the anal margin, or when the tumor invades the anal sphincter and necessitates an abdominoperineal resection. Some investigators propose routine inclusion of the bilateral pelvic lymph nodes as well. The obturator nodes can be omitted only if the tumor is >10 cm from the anal margin. This includes the external iliac lymph nodes if there is an anterior organ involvement and the inguinal lymph nodes if the lower third of the vagina is involved. Some authors recommend lowering the upper margin, particularly for patients with N0 disease.65
To facilitate contouring of the treatment areas, the use of three reference points is mandatory: the proximal mesorectum (Fig. 1), the distal mesorectum at the insertion of the levator ani (Fig. 2), and the obturator artery (Fig. 3).
Fig. 1.
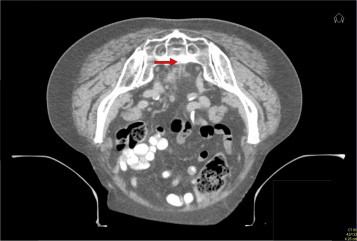
Axial CT scan of a patient with rectal cancer. The red arrow indicates the proximal mesorectum, to be used as one of three reference points for contouring the tumor.
Fig. 2.
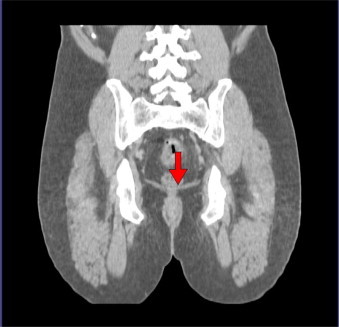
Coronal CT scan of a patient with rectal cancer, with the red arrow indicating the insertion of the levator ani at the distal mesorectum, to be used as a reference point when contouring rectal tumors.
Fig. 3.
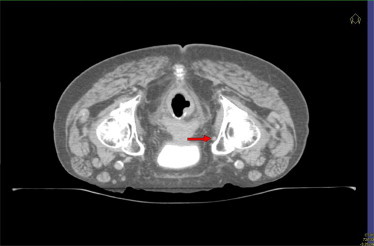
Axial CT scan, with the red arrow indicating the obturator artery, to be used as a reference point when contouring rectal tumors.
2.10. Dose–volume histogram limits
In our experience,66 low toxicity has been observed when the following dose limits for critical organs are used: no more than 12.3 Gy to 150 cm3 of the small bowel; no more than 15–18 Gy to the femoral head; and no more than 15 Gy to <50% of the bladder. Although dose limits for treating cancer of the prostate or pancreas may also be useful, we were unable to find other published dose limits for this technique.
2.11. Contributions of diagnostic imaging
Reliable preoperative imaging is essential for disease staging and choice of treatment based on risk of LR. MRI can visualize the depth of the tumor, its relation to the CRM, and, to some extent, lymph node involvement. The MERCURY study (“Magnetic Resonance Imaging and Rectal Cancer European Equivalence”)67 compared the use of MRI, computed tomography (CT), and histopathologic analysis for the preoperative staging of tumors. In one such analysis of extramural tumor depth and spread, the authors concluded that MR and histologic assessments of tumor spread were considered equivalent to within 0.5 mm. In another multicenter study,68 high-resolution MRI was found to accurately predict involvement versus no involvement of surgical resection margins and, hence, the potential for a failure of surgery or curative resection, respectively. These findings would also be helpful in selection of patients for preoperative treatment with RT versus chemoradiation.69
Members of the Dutch Colorectal Cancer Group classified risk of LR in their study of MRI-based staging as follows: Low-risk, CRM > 2 mm and N0 status; intermediate risk; and high-risk (close or involved CRM, N2 status, or distal tumors). This classification was then used to choose the treatment approach as follows: low-risk, TME or total excision; intermediate risk, short-course RT with TME; and high-risk, conventional long-course chemoradiation followed by surgery 6 weeks later.7 These investigators concluded that linking the imaging technology with the choice of therapy means that LR is no longer the main problem in treatment of locally advanced rectal cancer, but rather that it is early diagnosis and treatment, reducing treatment morbidity, and preventing metastatic disease that become major challenges.9
2.12. What is the optimal timing for surgery after neoadjuvant therapy?
Regardless of whether the neoadjuvant treatment is RT or chemoradiation, the question of what is the optimal interval between surgery after neoadjuvant therapy is still hotly debated. Theoretically, giving RT in larger fractions over a shorter period could result in more late side effects than a conventional, more protracted course of RT. A Dutch group70 recently reported an analysis of whether the interval between preoperative radiotherapy and surgery influences postoperative mortality and recurrence for two groups, the first being the patients who received RT in the Dutch TME trial (n = 642) and the second (the verification set) all patients receiving short-course RT for resectable rectal cancer in two radiotherapy clinics in The Netherlands (n = 600). Endpoints were OS, disease-free survival, LR-free survival and non-cancer-related survival. The results showed that patients in the TME trial aged ≥75 years had worse OS and non-cancer-related survival when the surgery took place 4–7 days after the last RT fraction as compared with 0–3 days. No differences in survival between the interval groups were found in the verification set, leading the investigators to conclude that an interval of 7 days is acceptable, but longer intervals should be avoided.
Another trial addressing the question of the optimum interval between RT and surgery with regard to both toxicity and pathologic response was the Stockholm III study,71 which compared complications after short-course RT and surgery given to 303 patients in one of three schedules: short-course RT (25 Gy in 5-Gy fractions) followed by surgery within 1 week (group 1); short-course RT to (25 Gy in 5-Gy fractions) followed by surgery 4–8 weeks later (group 2); or longer-course RT (50 Gy in 25 2-Gy fractions) followed by surgery 4–8 weeks later (group 3). Preliminary findings from that study showed that 8 patients (2.6%) developed radiation-induced acute toxicity and no differences were found among groups in postoperative complication rates (46.6% in group 1, 40% in group 2, 32% in group 3, P = 0.164). However, postoperative complication rates were the highest for patients undergoing surgery within 11–17 days after the start of RT (P = 0.036); patients who had surgery 3–5 days after beginning RT had fewer complications. This latter finding was also true of the Dutch experience for patients older than 60 years.33 A more definitive answer to this question awaits mature follow-up from the Stockholm III trial. In the meantime, for practical purposes, surgery is recommended to take place either within about 5 days after the last RT fraction or be delayed for 4 weeks or more.
A slightly different question is the effect of RT-to-surgery interval for patients with high-risk rectal cancer who are not candidates for chemoradiation. A group in Leeds (UK)72 retrospectively evaluated the use of short-course RT followed by delayed surgery for patients for whom conventional chemoradiation was indicated but who could not tolerate chemotherapy because of advanced age, poor performance status, or the presence of comorbid conditions. Of the 43 patients (mean age 82 years) selected for short-course RT and delayed surgery, 41 had RT and 26 of those 41 went on to have surgery at a median 8 weeks later. R0 was obtained in 22 patients, and R1 and R2 in 2 patients each. The treatment was relatively well tolerated, with two patients requiring hospitalization for diarrhea and one experiencing delayed small-bowel toxicity attributable to RT. At a mean follow-up interval of 18 months, no LRs were noted in the R0 and R1 groups. The mean OS time for the entire group was 23 months, but 44 months for patients who underwent surgery. The authors concluded that these findings confirm those of Radu et al.,73 described below, and indicate that short-course RT, followed 6–8 weeks later by surgery, can be considered as an option for downstaging tumors when preoperative chemoradiation is contraindicated.
Increase in OS owing to improvements in chemotherapy in recent years have led to surgery being considered even for patients with disseminated disease.
In a retrospective study of 46 patients with non-resectable rectal cancer treated in 2002–2005 reported by Radu et al.,73 Group A (mean age 79 years) had no metastases (T4NXM0), whereas Groups B + C had metastases (T4NXM1). Patients in group B (mean age 76 years) had predominantly locoregional disease and were not candidates for chemoradiation because of age or comorbid conditions; those patients received short-course RT followed by surgery after 4–8 weeks. For group C (mean age 63 years), long-course chemoradiation was given with the intent to perform surgery of both primary and secondary tumors if sufficient regression was seen at 4–8 weeks after the chemoradiation. The short-course treatment was well tolerated by most patients, with only 3 instances of grade 4 diarrhea. One patient in group C died of sepsis with fever and neutropenia. All patients underwent delayed surgery. R0 + R1 was obtained in 22 patients in group A (92%), 4 in group B (44%), and 6 in group C (46%). Pathologic complete response was seen in 4 patients, 2 in group (A) and 2 in group (C). There were no postoperative deaths. The authors of this study concluded that short-course RT followed immediately by surgery tended to result in more postoperative complications than the other regimens, but the increase in risk was mainly among patients who had surgery more than 10 days after the start of RT.
3. Conclusions
Our conclusions from this review are as follows. First, short-course RT can be considered a standard treatment option for intermediate-risk locally advanced rectal tumors. Its ability to control LR does not seem to be different from that of conventional long-course chemoradiation therapy, although the latter may be associated with greater toxicity. Patients being considered for short-course RT should be evaluated with MRI, with or without endorectal ultrasonography, to determine their eligibility for this form of treatment. Short-course RT seems to produce the best results for tumors that are ≥5 cm from the anal margin, have uninvolved CRMs, and present with ≤4 involved lymph nodes. The best interval between short-course RT and surgery is still not clear, but current results suggest that surgery should be done either within 5 days after the initiation of SCRT or 4 weeks afterwards. Because evidence of tumor downstaging is usually not present when short-course RT is followed closely by surgery, it is useful to compare findings from imaging with findings from pathologic evaluations. When short-course RT is being considered for patients with disseminated disease, CRM involvement must be considered before choosing between immediate or delayed surgery. Radiation volumes to be used for SCRT are no different from those used for conventional RT. In elderly patients, short-course RT followed immediately by surgery can prevent some of the morbidity associated with more aggressive treatments, and the current short-term experience suggests that this approach can produce good results. The possibility of sequential administration of short-course RT and new, more active drugs for rectal cancer opens new opportunities for the application of short-course RT. Finally, from a practical standpoint, short-course RT is less expensive than conventional long-course chemoradiation and thus may have a lower cost-benefit ratio.
Conflict of interest
None declared.
Financial disclosure
None declared.
Contributor Information
Juan Pablo Ciria, Email: juanpablo.ciriasantos@osakidetza.net.
Sergio Cafiero, Email: sergio.cafieroballesteros@osakidetza.net.
References
- 1.Minsky B.D., Rödel C., Valentini V. Short-course radiation versus long-course chemoradiation for rectal cancer. J Natl Compr Canc Netw. 2012;10(October (10)):1223–1231. doi: 10.6004/jnccn.2012.0129. [DOI] [PubMed] [Google Scholar]
- 2.Rödel C., Trojan J., Bechstein W.O., Woeste G. Neoadjuvant short- or long-term radio(chemo)therapy for rectal cancer: how and who should be treated? Dig Dis. 2012;30(Suppl. 2):102–108. doi: 10.1159/000342038. [DOI] [PubMed] [Google Scholar]
- 3.Phang P.T., Wang X. Current controversies in neoadjuvant chemoradiation of rectal cancer. Surg Oncol Clin N Am. 2014;23(January (1)):79–92. doi: 10.1016/j.soc.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Glimelius B., Pahlman L., Cervantes A., ESMO Guidelines Working Group Rectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2013;24(Suppl. 6):vi81–vi88. doi: 10.1093/annonc/mdt240. [DOI] [PubMed] [Google Scholar]
- 5.Valentini V., Glimelius B., Haustermans K., Marijnen C.A., Rödel C., Gambacorta M.A. EURECCA consensus conference highlights about rectal cancer clinical management: the radiation oncologist's expert review. Radiother Oncol. 2014;110(1):195–198. doi: 10.1016/j.radonc.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 6.Di Genesio Pagliuca M., Turri L., Munoz F., Melano A., Bacigalupo A., Franzone P. Patterns of practice in the radiation therapy management of rectal cancer: survey of the Interregional Group Piedmont, Valle d’Aosta and Liguria of the “Associazione Italiana di Radioterapia Oncologica (AIRO)”. Tumori. 2013;99(January–February (1)):61–67. doi: 10.1177/030089161309900111. [DOI] [PubMed] [Google Scholar]
- 7.Engelen S.M., Maas M., Lahaye M.J., Leijtens J.W., van Berlo C.L., Jansen R.L. Modern multidisciplinary treatment of rectal cancer based on staging with magnetic resonance imaging leads to excellent local control, but distant control remains a challenge. Eur J Cancer. 2013;49(10):2311–2320. doi: 10.1016/j.ejca.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Sautter-Bihl M.L., Hohenberger W., Fietkau R., Roedel C., Schmidberger H., Sauer R. MRI-based treatment of rectal cancer: is prognostication of the recurrence risk solid enough to render radiation redundant? Ann Surg Oncol. 2014;21(January (1)):197–204. doi: 10.1245/s10434-013-3236-7. [DOI] [PubMed] [Google Scholar]
- 9.Stearns M.W., Jr., Deddish M.R., Quan S.H., Leaming R.H. Preoperative roentgen therapy for cancer of the rectum and rectosigmoid. Surg Gynecol Obstet. 1974;138:584–586. [PubMed] [Google Scholar]
- 10.Higgins G.A., Conn J.H., Jordan P.H., Humphrey E.W., Roswit B., Keehn R.J. Preoperative radiation therapy for rectal cancer. Ann Surg. 1975;181:624–631. doi: 10.1097/00000658-197505000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Higgins G.A., Humphrey E.W., Dwight R.W., Roswit B., Lee L.E., Keehn R.J. Preoperative radiation and surgery for cancer of the rectum. Veterans Administration Surgical Oncology Group Trial II. Cancer. 1986;58:352–359. doi: 10.1002/1097-0142(19860715)58:2<352::aid-cncr2820580226>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 12.Second report of the MRC Working Party. The evaluation of low dose preoperative X-ray therapy in rectal carcinoma: results of a randomly controlled trial. Br J Surg. 1984;71:21–25. doi: 10.1002/bjs.1800710107. [DOI] [PubMed] [Google Scholar]
- 13.Gérard A., Berrod J.L., Pene F., Laughier A., Bruckner R., Camelot G. Interim analysis of phase III study on preoperative radiation therapy in resectable rectal carcinoma. Trial of the Gastrointestinal Tract Cancer Cooperative Group of the European Organization for Research and Treatment of Cancer (EORTC) Cancer. 1985;55(10):2373–2379. doi: 10.1002/1097-0142(19850515)55:10<2373::aid-cncr2820551012>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 14.Gérard A., Buyse M., Nordlinger B., Loygue J., Pène F., Kempf P. Preoperative radiation therapy as adjuvant treatment in rectal cancer. Final results of a randomized study of the European Organization for Research and Treatment of Cancer (EORTC) Ann Surg. 1988;208:606–614. doi: 10.1097/00000658-198811000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stockholm Rectal Cancer Study Group Preoperative short-term radiation therapy in operable rectal carcinoma. A prospective randomised trial. Cancer. 1990;66:49–55. doi: 10.1002/1097-0142(19900701)66:1<49::aid-cncr2820660111>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 16.Pahlman L., Glimelius B. Pre- or postoperative radiotherapy in rectal and rectosigmoid carcinoma: report from a randomized multicentric trial. Ann Surg. 1990;211:187–195. doi: 10.1097/00000658-199002000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Initial report from a Swedish multicentre study examining the role of preoperative irradiation in the treatment of patients with resectable rectal cancer. Swedish Rectal Cancer Trial. Br J Surg. 1993;80:1333–1337. doi: 10.1002/bjs.1800801040. [DOI] [PubMed] [Google Scholar]
- 18.Local recurrence rate in a randomized multicentre trial of preoperative radiotherapy compared to surgery alone in resectable rectal cancer. Swedish Rectal Cancer Trial. Eur J Surg. 1996;162:397–402. [PubMed] [Google Scholar]
- 19.Improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med. 1997;336(14):980–987. doi: 10.1056/NEJM199704033361402. [Erratum in N Engl J Med 1997;336(21):1539] [DOI] [PubMed] [Google Scholar]
- 20.Lavery I.C., Fazio V.W., Lopez-Kostner F. Radiotherapy for rectal cancer. Comment on improved survival with preoperative radiotherapy in resectable rectal cancer. Swedish Rectal Cancer Trial. N Engl J Med. 1997;337(5):346–347. doi: 10.1056/NEJM199707313370514. [author reply 347–8] [DOI] [PubMed] [Google Scholar]
- 21.Marijnen C.A.M., Nagtegaal I.D., Klein Kranenbarg E., Hermans J., Van de Velde C.J.H., Leer J.W.H. No downstaging after short-term preoperative radiotherapy in rectal cancer patients. J Clin Oncol. 2001;19:1976–1984. doi: 10.1200/JCO.2001.19.7.1976. [DOI] [PubMed] [Google Scholar]
- 22.Kapiteijn E., Marijnen C.A., Nagtegaal I.D., Putter H., Steup W.H., Wiggers T. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med. 2001;345:638–646. doi: 10.1056/NEJMoa010580. [DOI] [PubMed] [Google Scholar]
- 23.Peeters K.C., Marijnen C.A., Nagtegaal I.D., Kranenbarg E.K., Putter H., Wiggers T. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg. 2007;246(5):693–701. doi: 10.1097/01.sla.0000257358.56863.ce. [DOI] [PubMed] [Google Scholar]
- 24.Heald R.J., Ryall R.D. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;1:1479–1482. doi: 10.1016/s0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 25.MacFarlane J.K., Ryall R.D., Heald R.J. Mesorectal excision for rectal cancer. Lancet. 1993;341:457–460. doi: 10.1016/0140-6736(93)90207-w. [DOI] [PubMed] [Google Scholar]
- 26.Heald R.J., Moran B.J., Ryall R.D.H., Sexton R., MacFarlane J.K. The Basingstoke experience of total mesorectal excision, 1978–1997. Arch Surg. 1998;133:894–899. doi: 10.1001/archsurg.133.8.894. [DOI] [PubMed] [Google Scholar]
- 27.Houbiers J.G., Brand A., van de Watering L.M., Hermans J., Verwey P.J., Bijnen A.B. Randomized controlled trial comparing transfusion of leukocyte-depleted or buffy-coat-depleted blood in surgery for colorectal cancer. Lancet. 1994;334:573–578. doi: 10.1016/s0140-6736(94)91965-8. [DOI] [PubMed] [Google Scholar]
- 28.Kapiteijn E., Putter H., van de Velde C.J., Cooperative investigators of the Dutch ColoRectal Cancer Group Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg. 2002;89(9):1142–1149. doi: 10.1046/j.1365-2168.2002.02196.x. [DOI] [PubMed] [Google Scholar]
- 29.Martling A.L., Holm T., Rutqvist L.E., Moran B.J., Heald R.J., Cedemark B. Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Stockholm Colorectal Cancer Study Group, Basingstoke Bowel Cancer Research Project. Lancet. 2000;356:93–96. doi: 10.1016/s0140-6736(00)02469-7. [DOI] [PubMed] [Google Scholar]
- 30.Van Gijn W., Marijnen C.A., Nagtegaal I.D., Kranenbarg E.M., Putter H., Wiggers T. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12 year follow-up of the multicenter, randomized controlled TME trial. Lancet Oncol. 2011;12(6):575–582. doi: 10.1016/S1470-2045(11)70097-3. [DOI] [PubMed] [Google Scholar]
- 31.Marijnen C.A., Kapiteijn E., van de Velde C.J., Martijn H., Steup W.H., Wiggers T. Acute side effects and complications after short-term preoperative radiotherapy combined with mesorectal excision in primary rectal cancer: report of a multicenter randomized trial. J Clin Oncol. 2002;20:817–825. doi: 10.1200/JCO.2002.20.3.817. [DOI] [PubMed] [Google Scholar]
- 32.Dahlberg M., Glimelius B., Graf W., Påhlman L. Preoperative irradiation affects functional results after surgery for rectal cancer. Results from a randomized study. Dis Colon Rectum. 1998;41:543–551. doi: 10.1007/BF02235256. [DOI] [PubMed] [Google Scholar]
- 33.Peeters K.C., Kapiteijn E., van de Velde C.J., Dutch ColoRectal Cancer Group Managing rectal cancer: the Dutch experience. Colorectal Dis. 2003;5:423–425. doi: 10.1046/j.1463-1318.2003.00513.x. [DOI] [PubMed] [Google Scholar]
- 34.Guimelius B. Adjuvant chemotherapy in rectal cancer: an issue or a nonissue? [Comment on Does adjuvant fluoropyrimidine-based chemotherapy provide a benefit for patients with resected rectal cancer who have already received neoadjuvant radiochemotherapy? A systematic review of randomised trials (Ann Oncol 2010)] Ann Oncol. 2010;21(9):1739–1741. doi: 10.1093/annonc/mdq054. [DOI] [PubMed] [Google Scholar]
- 35.Breugom A.J., van den Broek C.B.M., van Gijn W. The value of adjuvant chemotherapy in rectal cancer patients after preoperative radiotherapy or chemoradiation followed by TME-surgery: The PROCTOR/SCRIPT study. 2013 European Cancer Congress. Abstract 1; September 28; 2013. [Google Scholar]
- 36.Sebag-Montefiore D., Stephens R.J., Steele R., Monson J., Grieve R., Khanna S. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet. 2009;373:811–820. doi: 10.1016/S0140-6736(09)60484-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sebag-Montefiore D., Steele R., Monson J., Couture J., de Metz C., Pugh C. The MRC C016 [MRC CR07/NCIC-CTG C016] trial after a median follow-up of 8 years (abstract OC-0219) Radiother Oncol. 2012;103(Suppl. 1):S85. [Google Scholar]
- 38.Adam I.J., Mohamdee M.O., Martin I.G., Scott N., Finan P.J., Johnston D. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344:707–711. doi: 10.1016/s0140-6736(94)92206-3. [DOI] [PubMed] [Google Scholar]
- 39.De Caluwé L., Van Nieuwenhove Y., Ceelen W.P. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev. 2013;2(February):CD006041. doi: 10.1002/14651858.CD006041.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stephens R.J., Thompson L.C., Quirke P., Steele R., Grieve R., Couture J. Impact of short course preoperative radiotherapy for rectal cancer patients’ quality of life: data from the Medical Research Council CR07/National Cancer Institute of Canada Clinical Trials Group C016 randomized clinical trial. J Clin Oncol. 2010;28:4233–4239. doi: 10.1200/JCO.2009.26.5264. [DOI] [PubMed] [Google Scholar]
- 41.Guckenberger M., Saur G., Wehner D., Thalheimer A., Kim M., Germer C.T. Long-term quality-of-life after neoadjuvant short-course radiotherapy and long-course radiochemotherapy for locally advanced rectal cancer. Radiother Oncol. 2013;108(August (2)):326–330. doi: 10.1016/j.radonc.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 42.Ciammella P., Ruggieri M.P., Galeandro M., D’Abbiero N., Giunta A., Iotti C. Short-course preoperative radiotherapy combined with chemotherapy in resectable locally advanced rectal cancer: local control and quality of life. Radiol Med. 2013;118(December (8)):1397–1411. doi: 10.1007/s11547-013-0939-6. [DOI] [PubMed] [Google Scholar]
- 43.Gerard J.P., Conroy T., Bonnetain F., Bouché O., Chapet O., Closon-Dejardin M.T. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol. 2006;24:4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 44.Bosset J.F., Collette L., Calais G., Mineur L., Maingon P., Radosevic-Jelic L. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355:1114–1123. doi: 10.1056/NEJMoa060829. [DOI] [PubMed] [Google Scholar]
- 45.Bujko K., Nowacki M.P., Nasierowska-Guttmejer A., Michalski W., Bebenek M., Pudełko M. Sphincter preservation following preoperative radiotherapy for rectal cancer: report of randomised trial comparing short-term radiotherapy vs conventionally fractionated radiochemotherapy. Radiother Oncol. 2004;72:15–24. doi: 10.1016/j.radonc.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 46.Pietrzak L., Bujko K., Nowacki M.P., Kepka L., Oledzki J., Rutkowski A. Quality of life, anorectal and sexual functions after preoperative radiotherapy for rectal cancer: report of a randomised trial. Radiother Oncol. 2007;84:217–225. doi: 10.1016/j.radonc.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 47.Bujko K., Nowacki M.P., Nasierowska-Guttmejer A., Michalski W., Bebenek M., Kryj M. Long-term results of a randomised trial comparing short-course radiotherapy vs preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg. 2006;93:1215–1223. doi: 10.1002/bjs.5506. [DOI] [PubMed] [Google Scholar]
- 48.Ngan S.Y., Burmeister B., Fisher R.J., Solomon M., Goldstein D., Joseph D. Randomized trial of short course radiotherapy versus long course chemoradiation comparing rates of local recurrence in patients with t3 rectal cancer. Trans-Tasman Radiation Oncology Group trial 01-04. J Clin Oncol. 2012;30(31):3827–3833. doi: 10.1200/JCO.2012.42.9597. [Erratum in J Clin Oncol 2013;31(3):399] [DOI] [PubMed] [Google Scholar]
- 49.Sauer R., Becker H., Hohenberger W., Rödel C., Wittekind C., Fietkau R. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 50.Sauer R., Liersch T., Merkel S., Fietkau R., Hohenberger W., Hess C. Preoperative versus postoperative chemoradiotherapy for locally advanced rectal cancer: results of the German CAO/ARO/AIO-94 randomized phase III trial after a median follow-up of 11 years. J Clin Oncol. 2012;30(16):1926–1933. doi: 10.1200/JCO.2011.40.1836. [DOI] [PubMed] [Google Scholar]
- 51.Yeo S.G., Oh J.H., Kim D.Y., Baek J.Y., Kim S.Y., Park J.W. Preoperative short-course concurrent chemoradiation therapy followed by delayed surgery for locally advanced rectal cancer: a phase 2 multicenter study (KROG 10-01) Int J Radiat Oncol Biol Phys. 2013;86(May (1)):34–39. doi: 10.1016/j.ijrobp.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 52.Siegel R., Burock S., Wernecke K.D., Kretzschmar A., Dietel M., Loy V. Preoperative short-course radiotherapy versus combined radiochemotherapy in locally advanced rectal cancer: a multi-centre prospectively randomised study of the Berlin Cancer Society. BMC Cancer. 2009;9:50. doi: 10.1186/1471-2407-9-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Garcia-Aguilar J., Smith D.D., Avila K., Bergsland E.K., Chu P., Krieg R.M., for the Timing of Rectal Cancer Response to Chemoradiation Consortium Optimal timing of surgery after chemoradiation for advanced rectal cáncer: preliminary results of a multicenter, non-randomized phase II prospective trial. Ann Surg. 2011;254(1):97–102. doi: 10.1097/SLA.0b013e3182196e1f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nilsson P.J., van Etten B., Hospers G.A.P., Pahlman L., van de Velde C.J.H., Beets-Tan R.G.H. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer—the RAPIDO trial. BMC Cancer. 2013;13:279–287. doi: 10.1186/1471-2407-13-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schrag D. Evolving role of neoadjuvant therapy in rectal cancer. Curr Treat Options Oncol. 2013;14:350–364. doi: 10.1007/s11864-013-0242-8. [DOI] [PubMed] [Google Scholar]
- 56.Schrag D., Weiser M.R., Goodman K.A., Gonen M., Hollywood E., Cercek A. Neoadjuvant chemotherapy without routine use of radiation therapy for patients with locally advanced rectal cancer: a pilot trial. J Clin Oncol. 2014;32(6):513–518. doi: 10.1200/JCO.2013.51.7904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beets G.L., Beets-Tan R.G.H., Lahaye M., Nagtegaal I.D., van del Velde C.J.H., Marijnen C.A.M. Mechanisms of local recurrence after rectal cancer resection. What can we learn from the Dutch TME Trial? (abstract no. 011.04). Oral session presented at the European Multidisciplinary Colorectal Cancer Congress, 12–14 Feb 2006, Berlin, Germany. Ann Oncol. 2006;17(Suppl. 1):ii4. [Google Scholar]
- 58.Kusters M., Marijnen C.A., van de Velde C.J., Rutten H.J., Lahaye M.J., Kim J.H. Patterns of local recurrence in rectal cancer; a study of the Dutch TME trial. Eur J Surg Oncol. 2010;36(5):470–476. doi: 10.1016/j.ejso.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 59.López-Kostner F., Fazio V.W., Vignali A., Rybicki L.A., Lavery I.C. Locally recurrent rectal cancer: predictors and success of salvage surgery. Dis Colon Rectum. 2001;44:173–178. doi: 10.1007/BF02234289. [DOI] [PubMed] [Google Scholar]
- 60.García-Aguilar J., Cromwell J.W., Marra C., Lee S.H., Madoff R.D., Rothenberger D.A. Treatment of locally recurrent rectal cancer. Dis Colon Rectum. 2001;44:1743–1748. doi: 10.1007/BF02234449. [DOI] [PubMed] [Google Scholar]
- 61.Yamada K., Ishizawa T., Niwa K., Chuman Y., Akiba S., Aikon T. Patterns of pelvic invasion are prognostic in the treatment of locally recurrent rectal cancer. Br J Surg. 2001;88:988–993. doi: 10.1046/j.0007-1323.2001.01811.x. [DOI] [PubMed] [Google Scholar]
- 62.Asoglu O., Karanlik H., Muslumanoglu M., Igci A., Emek E., Ozmen V. Prognostic and predictive factors after surgical treatment for locally recurrent rectal cancer: a single institute experience. Eur J Surg Oncol. 2007;33(10):1199–1206. doi: 10.1016/j.ejso.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 63.Cox J.D., Ang K.K., editors. Radiation oncology: rationale, technique, results. 8th ed. Mosby; St Louis, MO: 2003. p. 27. [Google Scholar]
- 64.Roels S., Duthoy W., Haustermans K., Penninckx F., Vandecaveye V., Boterberg T. Definition and delineation of the clinical target volume for rectal cancer. Int J Radiat Oncol Biol Phys. 2006;65(4):1129–1142. doi: 10.1016/j.ijrobp.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 65.Nijkamp J., Kusters M., Beets-Tan R.G., Martijn H., Beets G.L., van de Velde C.J. Three-dimensional analysis of recurrence patterns in rectal cancer: the cranial border in hypofractionated preoperative radiotherapy can be lowered. Int J Radiat Oncol Biol Phys. 2011;80(1):103–110. doi: 10.1016/j.ijrobp.2010.01.046. [DOI] [PubMed] [Google Scholar]
- 66.Cerezo L., Ciria J.P., Arbea L., Cafiero S., Cellini F. Current treatment of rectal cancer adapted to the individual patient. Rep Pract Oncol Radiother. 2013;18(6):353–362. doi: 10.1016/j.rpor.2013.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mercury Study Group Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer results of the MERCURY study. Radiology. 2007;243:132–139. doi: 10.1148/radiol.2431051825. [DOI] [PubMed] [Google Scholar]
- 68.Mercury Study Group Diagnostic accuracy of preoperative magnetic resonance imaging in predicting curative resection of rectal cancer: prospective observational study. Br Med J. 2006;333:779. doi: 10.1136/bmj.38937.646400.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Curvo-Semedo L., Lambregts D.M., Maas M., Beets G.L., Caseiro-Alves F., Beets-Tan R.G. Diffusion-weighted MRI in rectal cancer: apparent diffusion coefficient as a potential noninvasive marker of tumor aggressiveness. J Magn Reson Imaging. 2012;35(June (6)):1365–1371. doi: 10.1002/jmri.23589. [DOI] [PubMed] [Google Scholar]
- 70.Van den Broek C.B., Vermeer T.A., Bastiaannet E., Rutten H.J., van de Velde C.J., Marijnen C.A. Impact of the interval between short-course radiotherapy and surgery on outcomes of rectal cancer patients. Eur J Cancer. 2013;49(15):3131–3139. doi: 10.1016/j.ejca.2013.05.025. [DOI] [PubMed] [Google Scholar]
- 71.Pettersson D., Cedermark B., Holm T., Radu C., Påhlman L., Glimelius B. Interim analysis of the Stockholm III trial of preoperative radiotherapy regimens for rectal cancer. Br J Surg. 2010;97:580–587. doi: 10.1002/bjs.6914. [DOI] [PubMed] [Google Scholar]
- 72.Hatfield P., Hingorani M., Radhakrishna G., Cooper R., Melcher A., Crellin A. Short-course radiotherapy, with elective delay prior to surgery, in patients with unresectable rectal cancer who have poor performance status or significant co-morbidity. Radiother Oncol. 2009;92(2):210–214. doi: 10.1016/j.radonc.2009.04.007. [DOI] [PubMed] [Google Scholar]
- 73.Radu C., Berglund A., Påhlman L., Glimelius B. Short-course preoperative radiotherapy with delayed surgery in rectal cancer: a retrospective study. Radiother Oncol. 2008;(87):343–349. doi: 10.1016/j.radonc.2007.11.025. [DOI] [PubMed] [Google Scholar]


