Abstract
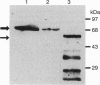
The saliva of hematophagous insects contains a variety of pharmacologically active substances that counteract the normal hemostatic response to injury in vertebrate hosts. The yellow-fever mosquito, Aedes aegypti, secretes an apyrase that inhibits ADP-dependent platelet aggregation. Apyrase was purified as an active enzyme from adult female salivary glands and subjected to tryptic digestion, and the resulting peptides were sequenced. The amino acid sequences obtained match the conceptual translation product of a cDNA clone isolated from an adult female salivary gland library. Sequence comparisons indicate similarities with a ubiquitous family of 5'-nucleotidases. The mosquito protein differs from other members of the family by lacking a carboxyl-terminal hydrophobic domain. The apparent conversion of a gene encoding an enzyme involved in a common metabolic event at the cellular level to a gene involved in the antihemostatic response of mosquitoes illustrates one way this particular insect has adapted to the challenges of bloodfeeding.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Beacham I. R., Wilson M. S. Studies on the UDP-sugar hydrolases from Escherichia coli and Salmonella typhimurium. Arch Biochem Biophys. 1982 Oct 15;218(2):603–608. doi: 10.1016/0003-9861(82)90385-x. [DOI] [PubMed] [Google Scholar]
- Burns D. M., Beacham I. R. Nucleotide sequence and transcriptional analysis of the E. coli ushA gene, encoding periplasmic UDP-sugar hydrolase (5'-nucleotidase): regulation of the ushA gene, and the signal sequence of its encoded protein product. Nucleic Acids Res. 1986 May 27;14(10):4325–4342. doi: 10.1093/nar/14.10.4325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté Y. P., Ouellet S., Beaudoin A. R. Kinetic properties of type-II ATP diphosphohydrolase from the tunica media of the bovine aorta. Biochim Biophys Acta. 1992 Dec 28;1160(3):246–250. doi: 10.1016/0167-4838(92)90084-q. [DOI] [PubMed] [Google Scholar]
- Glaser L., Melo A., Paul R. Uridine diphosphate sugar hydrolase. Purification of enzyme and protein inhibitor. J Biol Chem. 1967 Apr 25;242(8):1944–1954. [PubMed] [Google Scholar]
- Grossman G. L., James A. A. The salivary glands of the vector mosquito, Aedes aegypti, express a novel member of the amylase gene family. Insect Mol Biol. 1993;1(4):223–232. doi: 10.1111/j.1365-2583.1993.tb00095.x. [DOI] [PubMed] [Google Scholar]
- Korangy F., Julin D. A. Alteration by site-directed mutagenesis of the conserved lysine residue in the ATP-binding consensus sequence of the RecD subunit of the Escherichia coli RecBCD enzyme. J Biol Chem. 1992 Jan 25;267(3):1727–1732. [PubMed] [Google Scholar]
- Law J. H., Ribeiro J. M., Wells M. A. Biochemical insights derived from insect diversity. Annu Rev Biochem. 1992;61:87–111. doi: 10.1146/annurev.bi.61.070192.000511. [DOI] [PubMed] [Google Scholar]
- Misumi Y., Ogata S., Hirose S., Ikehara Y. Primary structure of rat liver 5'-nucleotidase deduced from the cDNA. Presence of the COOH-terminal hydrophobic domain for possible post-translational modification by glycophospholipid. J Biol Chem. 1990 Feb 5;265(4):2178–2183. [PubMed] [Google Scholar]
- Misumi Y., Ogata S., Ohkubo K., Hirose S., Ikehara Y. Primary structure of human placental 5'-nucleotidase and identification of the glycolipid anchor in the mature form. Eur J Biochem. 1990 Aug 17;191(3):563–569. doi: 10.1111/j.1432-1033.1990.tb19158.x. [DOI] [PubMed] [Google Scholar]
- Moodie F. D., Baum H., Butterworth P. J., Peters T. J. Purification and characterisation of bovine spleen ADPase. Eur J Biochem. 1991 Dec 18;202(3):1209–1215. doi: 10.1111/j.1432-1033.1991.tb16492.x. [DOI] [PubMed] [Google Scholar]
- Nagano M., Kelly P. A. Absence of a putative ATP/GTP binding site in the rat prolactin receptor. Biochem Biophys Res Commun. 1992 Mar 16;183(2):610–618. doi: 10.1016/0006-291x(92)90526-q. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. M. Role of saliva in blood-feeding by arthropods. Annu Rev Entomol. 1987;32:463–478. doi: 10.1146/annurev.en.32.010187.002335. [DOI] [PubMed] [Google Scholar]
- Ribeiro J. M., Sarkis J. J., Rossignol P. A., Spielman A. Salivary apyrase of Aedes aegypti: characterization and secretory fate. Comp Biochem Physiol B. 1984;79(1):81–86. doi: 10.1016/0305-0491(84)90081-6. [DOI] [PubMed] [Google Scholar]
- Rossignol P. A., Ribeiro J. M., Spielman A. Increased intradermal probing time in sporozoite-infected mosquitoes. Am J Trop Med Hyg. 1984 Jan;33(1):17–20. doi: 10.4269/ajtmh.1984.33.17. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkis J. J., Guimarães J. A., Ribeiro J. M. Salivary apyrase of Rhodnius prolixus. Kinetics and purification. Biochem J. 1986 Feb 1;233(3):885–891. doi: 10.1042/bj2330885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- Traverso-Cori A., Traverso S., Reyes H. Different molecular forms of potato apyrase. Arch Biochem Biophys. 1970 Mar;137(1):133–142. doi: 10.1016/0003-9861(70)90420-0. [DOI] [PubMed] [Google Scholar]
- Valenzuela M. A., López J., Depix M., Mancilla M., Kettlun A. M., Catalán L., Chiong M., Garrido J., Traverso-Cori A. Comparative subcellular distribution of apyrase from animal and plant sources. Characterization of microsomal apyrase. Comp Biochem Physiol B. 1989;93(4):911–919. doi: 10.1016/0305-0491(89)90066-7. [DOI] [PubMed] [Google Scholar]
- Volknandt W., Vogel M., Pevsner J., Misumi Y., Ikehara Y., Zimmermann H. 5'-nucleotidase from the electric ray electric lobe. Primary structure and relation to mammalian and procaryotic enzymes. Eur J Biochem. 1991 Dec 18;202(3):855–861. doi: 10.1111/j.1432-1033.1991.tb16443.x. [DOI] [PubMed] [Google Scholar]
- Wieland F. T., Gleason M. L., Serafini T. A., Rothman J. E. The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell. 1987 Jul 17;50(2):289–300. doi: 10.1016/0092-8674(87)90224-8. [DOI] [PubMed] [Google Scholar]
- Willadsen P., Riding G. A., Jarmey J., Atkins A. The nucleotidase of Boophilus microplus and its relationship to enzymes from the rat and Escherichia coli. Insect Biochem Mol Biol. 1993 Mar;23(2):291–295. doi: 10.1016/0965-1748(93)90010-p. [DOI] [PubMed] [Google Scholar]
- Yagi K., Kato N., Shinbo M., Shimba L. S., Miura Y. Purification and characterization of adenosine diphosphatase from human umbilical vessels. Chem Pharm Bull (Tokyo) 1992 Aug;40(8):2143–2146. doi: 10.1248/cpb.40.2143. [DOI] [PubMed] [Google Scholar]
- Zimmermann H. 5'-Nucleotidase: molecular structure and functional aspects. Biochem J. 1992 Jul 15;285(Pt 2):345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]