Abstract
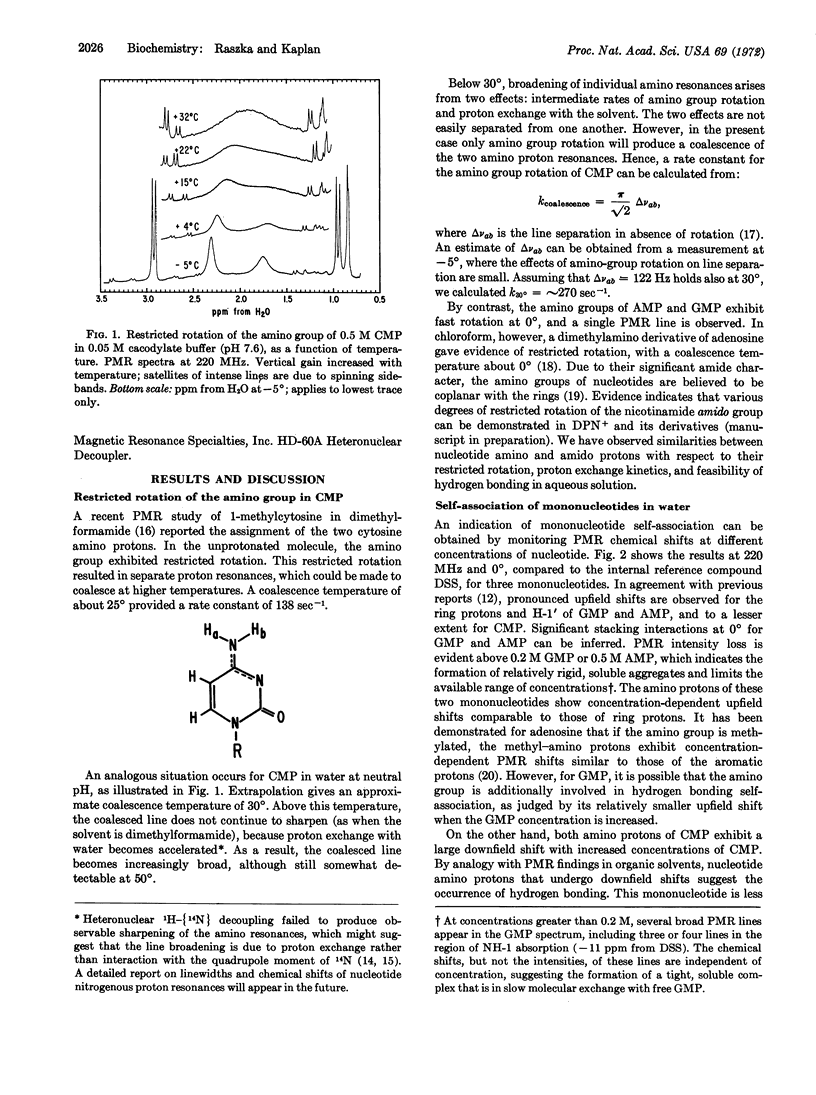
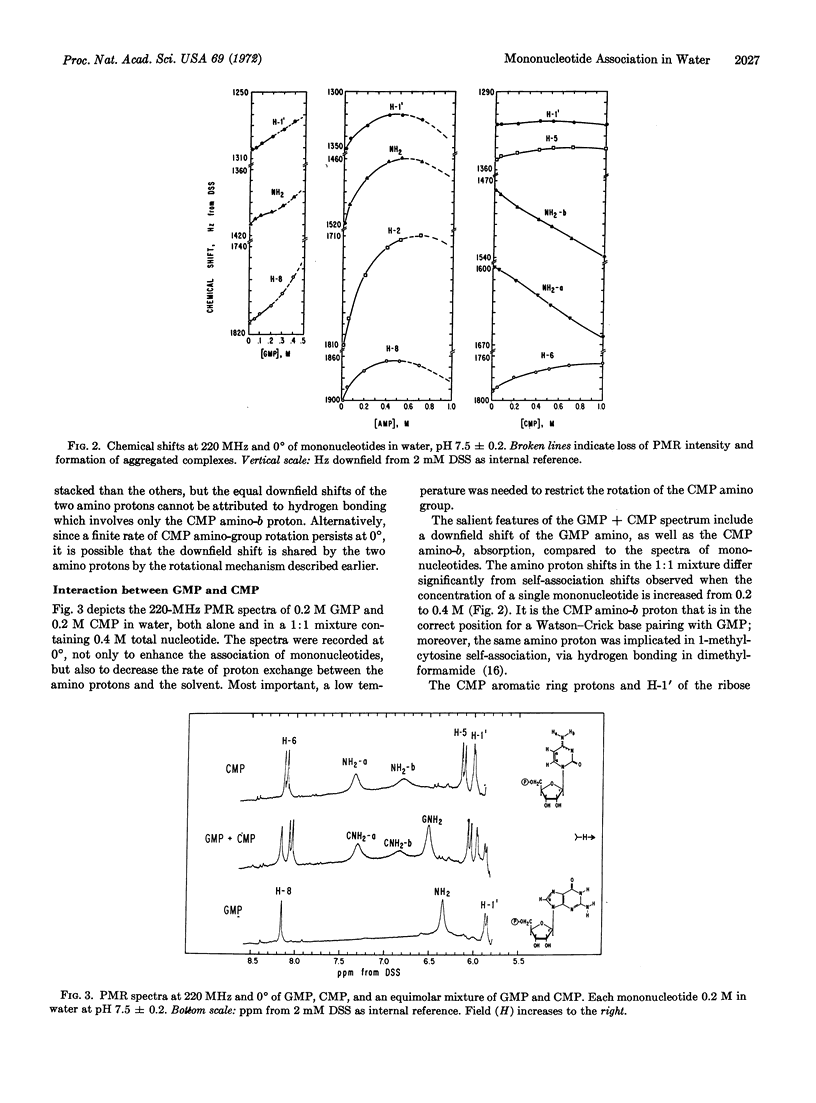
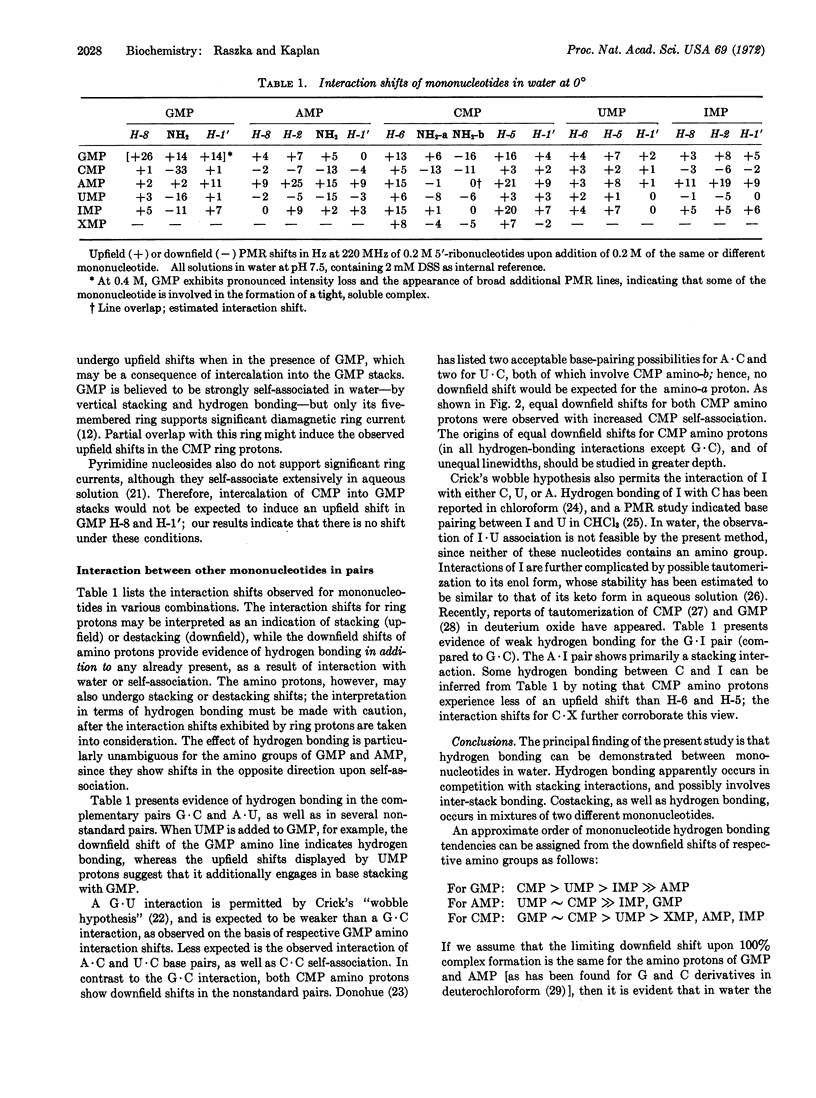
Evidence for hydrogen bonding between 5′-ribonucleotides in water has been obtained from a 220-MHz proton magnetic resonance study of nitrogenous protons. The amino groups of GMP, AMP, and CMP exhibit proton resonance lines which are somewhat broadened by proton exchange with the solvent at 0°; their downfield Shifts in mixtures of mononucleotides provide the basis for the following order of base-pairing tendencies: GMP·CMP > AMP·UMP. Hydrogen bonding is also observed in other pairs of mononucleotides, notably GMP·UMP, AMP·CMP, and CMP·UMP, to a lesser extent in GMP·IMP, CMP·XMP, and possibly in CMP·IMP. In agreement with previous reports, hydrophobic interactions of mononucleotides have also been observed; base pairing occurs in addition to vertical stacking of these bases, their hydrogen bonding to water, or self-association. Only CMP shows clear evidence of self-association via hydrogen bonding in water; the evidence for GMP is less direct, and that for AMP is negative. This lack of observable self-association may occur as a result of competition from strong stacking interactions. Only CMP shows restricted rotation of the amino group at 0° and neutral pH. As expected, higher temperatures increase the rate of rotation of the amino group for CMP, as well as accelerate the rate of proton exchange between water and the amino protons of mononucleotides.
High-resolution proton magnetic resonance spectroscopy could prove to be a valuable tool in mapping out the specificities conferred by hydrogen bonding between biomolecules in aqueous solution.
Keywords: base pairing, ribonucleotides, 220-MHz PMR, restricted amino group rotation, proton exchange
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Crick F. H. Codon--anticodon pairing: the wobble hypothesis. J Mol Biol. 1966 Aug;19(2):548–555. doi: 10.1016/s0022-2836(66)80022-0. [DOI] [PubMed] [Google Scholar]
- DONOHUE J., TRUEBLOOD K. N. Base pairing in DNA. J Mol Biol. 1960 Dec;2:363–371. doi: 10.1016/s0022-2836(60)80047-2. [DOI] [PubMed] [Google Scholar]
- Donohue J. HYDROGEN-BONDED HELICAL CONFIGURATIONS OF POLYNUCLEOTIDES. Proc Natl Acad Sci U S A. 1956 Feb;42(2):60–65. doi: 10.1073/pnas.42.2.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAMLIN R. M., Jr, LORD R. C., RICH A. HYDROGEN-BONDED DIMERS OF ADENINE AND URACIL DERIVATIVES. Science. 1965 Jun 25;148(3678):1734–1737. doi: 10.1126/science.148.3678.1734. [DOI] [PubMed] [Google Scholar]
- JARDETZKY O. PROTON MAGNETIC RESONANCE OF PURINE AND PYRIMIDINE DERIVATIVES. XI. PROTON MAGNETIC RESONANCE STUDIES OF NUCLEOTIDE INTERACTIONS. Biopolym Symp. 1964;13:501–514. [PubMed] [Google Scholar]
- Katz L., Penman S. Association by hydrogen bonding of free nucleosides in non-aqueous solution. J Mol Biol. 1966 Jan;15(1):220–231. doi: 10.1016/s0022-2836(66)80222-x. [DOI] [PubMed] [Google Scholar]
- Kyogoku Y., Lord R. C., Rich A. Hydrogen bonding specificity of nucleic acid purines and pyrimidines in solution. Science. 1966 Oct 28;154(3748):518–520. [PubMed] [Google Scholar]
- Lee G. C., Prestegard J. H., Chan S. I. Tautomerism of nucleic acid bases. I. Cytosine. J Am Chem Soc. 1972 Feb 9;94(3):951–959. doi: 10.1021/ja00758a038. [DOI] [PubMed] [Google Scholar]
- Lord R. C., Thomas G. J., Jr Raman studies of nucleic acids. II. Aqueous purine and pyrimidine mixtures. Biochim Biophys Acta. 1967 Jun 20;142(1):1–11. [PubMed] [Google Scholar]
- Newmark R. A., Cantor C. R. Nuclear magnetic resonance study of the interactions of guanosine and cytidine in dimethyl sulfoxide. J Am Chem Soc. 1968 Aug 28;90(18):5010–5017. doi: 10.1021/ja01020a041. [DOI] [PubMed] [Google Scholar]
- RANDERATH K., WEIMANN G. COMPLEX FORMATION BETWEEN DEOXYRIBOOLIGONUCLEOTIDES AND POLYRIBONUCLEOTIDES. ITS DETECTION BY A BARRIER TECHNIQUE BASED ON ANION EXCHANGE. Biochim Biophys Acta. 1963 Sep 17;76:129–131. [PubMed] [Google Scholar]
- Scheit K. H. Detection of hydrogen bridges between inosine and other nucleosides by NMR spectroscopy. Angew Chem Int Ed Engl. 1967 Feb;6(2):180–181. doi: 10.1002/anie.196701801. [DOI] [PubMed] [Google Scholar]
- Schweizer M. P., Broom A. D., Ts'o P. O., Hollis D. P. Studies of inter- and intramolecular interaction in mononucleotides by proton magnetic resonance. J Am Chem Soc. 1968 Feb 14;90(4):1042–1055. doi: 10.1021/ja01006a035. [DOI] [PubMed] [Google Scholar]
- Schweizer M. P., Chan S. I., Ts'o P. O. Interaction and association of bases and nucleosides in aqueous solutions. IV. Proton magnetic resonance studies of the association of pyrimidine nucleosides and their interactions with purine. J Am Chem Soc. 1965 Nov 20;87(22):5241–5247. doi: 10.1021/ja00950a045. [DOI] [PubMed] [Google Scholar]
- Shoup R. R., Becker E. D., Miles H. T. Restricted rotation of the amino group in 1-methylcytosine. Biochem Biophys Res Commun. 1971 Jun 18;43(6):1350–1353. doi: 10.1016/s0006-291x(71)80022-0. [DOI] [PubMed] [Google Scholar]
- Shoup R. R., Miles H. T., Becker E. D. NMR evidence of specific base-pairing between purines and pyrimidines. Biochem Biophys Res Commun. 1966 Apr 19;23(2):194–201. doi: 10.1016/0006-291x(66)90527-4. [DOI] [PubMed] [Google Scholar]
- Shoup R. R., Miles H. T., Becker E. D. Restricted rotation about the exocyclic carbon-nitrogen bond in cytosine derivatives. J Phys Chem. 1972 Jan 6;76(1):64–70. doi: 10.1021/j100645a012. [DOI] [PubMed] [Google Scholar]
- Solie T. N., Schellman J. A. The interaction of nucleosides in aqueous solution. J Mol Biol. 1968 Apr 14;33(1):61–77. doi: 10.1016/0022-2836(68)90281-7. [DOI] [PubMed] [Google Scholar]
- TUPPY H., KUECHLER E. EVIDENCE FOR SPECIFIC INTERACTIONS BETWEEN COMPLEMENTARY NUCLEOSIDES IN A CHROMATOGRAPHIC SYSTEM. Biochim Biophys Acta. 1964 Apr 27;80:669–671. doi: 10.1016/0926-6550(64)90312-3. [DOI] [PubMed] [Google Scholar]
- Thomas G. J., Jr, Kyogoku Y. Hypochromism accompanying purine-pyrimidine association interactions. J Am Chem Soc. 1967 Aug 2;89(16):4170–4175. doi: 10.1021/ja00992a600. [DOI] [PubMed] [Google Scholar]
- Ts'o P. O., Schweizer M. P., Hollis D. P. Contribution of nuclear magnetic resonance to the study of the structure and electronic aspects of nucleic acids. Ann N Y Acad Sci. 1969 May 16;158(1):256–297. doi: 10.1111/j.1749-6632.1969.tb56226.x. [DOI] [PubMed] [Google Scholar]
- Wolfenden R. V. Tautomeric equilibria in inosine and adenosine. J Mol Biol. 1969 Mar 14;40(2):307–310. doi: 10.1016/0022-2836(69)90479-3. [DOI] [PubMed] [Google Scholar]


