Abstract
Background:
Tobacco abuse is a well-recognized scourge on health and healthcare costs. Attempts to facilitate tobacco cessation are rarely better than marginally effective.
Primary Objective:
To describe an observational trial of an existing and highly successful tobacco cessation program featuring health coaching as the primary intervention. Core components of program design and data are presented and may serve as a model for other public health settings.
Methods:
Health coaching and three complementary program components (auriculotherapy, alpha-electrical stimulation, and relaxation techniques) are presented. Quit rates at 6 months for 161 patients over 3 years are provided featuring 30-day point prevalence smoke free and intent-to-treat values. Comparisons for telephonic vs in-clinic health coaching, free choice vs mandated participation, and program costs are provided.
Results:
Point prevalence quit rate was 88.7% while the more conservative intent-to-treat quit rate was 51.6%. Telephonic and in-clinic health coaching were not significantly different at any time point. Smoke-free rates at 6 and 12 months were 76.9% and 63.2%, respectively.
Conclusions:
Two cost-effective smoking cessation models featuring health coaching are presented. Point prevalence (30-day) above 80% and an enduring effect was seen. Personal and societal burdens (health and financial) of tobacco use might be greatly impacted if such programs were successfully implemented on a larger scale.
Key Words: Smoking cessation, wellness coaching, quit rates, quit line, auriculotherapy, cranial electrical stimulation
摘要
背景:大家公认滥用烟草会造成健 康和医疗费用的祸害。但是,促进 戒烟的意图很少有实效。
主要目的:描述一个现有的非常 成功的戒烟计划,其特点是将健 康指导作为主要干预措施。本文 给 出 程 序 设 计 和 数 据 的 核 心 内 容,这两方面内容可用作其他公 共卫生设置的模型。
方法:采用健康指导和辅助计划内 容(耳针疗法、阿尔法-电刺激以及 放松技巧)。提供了在3年多时间 161例患者在6个月时的戒烟率,维 持3年多时间,其指标为30天时点不 吸烟和意向性治疗值。 还比较了通 过电话与诊所内指导、自由选择与 要求参与、以及方案费用的情况。
结果:时点戒烟率为88.7%,而 较 保 守 的 意 向 性 治 疗 戒 烟 率 为 51.6%。在任何时间点,通过电 话和诊所训练的戒烟效果无显著 差异。在6和12个月的无吸烟率分 别为76.9%和63.2%。
结论:提供了两种成本高效、提 供健康指导的戒烟模式。时点戒 烟率(30天)达80%以上,而且 效果持久。如果这样的戒烟项目 大规模实施,将对烟草消费所造 成的个人和社会的负担(健康和 经济方面)产生极大的影响。
SINOPSIS
Antecedentes:
El abuso del tabaco está reconocido como perjudicial para la salud e implica gastos para la asistencia sanitaria. Los intentos a la hora de ayudar a dejar de fumar rara vez resultan mejores que simplemente efectivos parcialmente.
Objetivo principal:
Describir un estudio observacional de un programa ya existente y de gran éxito para dejar de fumar, ofrecido por un consejero de salud como intervención primaria. Los componentes básicos del diseño del programa y la información se presentan y pueden servir como modelo en otros centros de salud públicos.
Métodos:
Se presentan tres componentes complementarios del programa y un consejero de salud (auriculoterapia, estimulación eléctrica de ondas alfa y técnicas de relajación). Las tasas de abandono a los 6 meses de 161 pacientes a lo largo de 3 años se presentan por cada 30 días de abstinencia y en valores de intención de tratamiento. Se proporcionan comparaciones de las consultas telefónicas frente a consultas médicas en la clínica, de libre elección frente a la participación obligatoria y se facilitan los costes del programa.
Resultados:
La prevalencia puntual de la tasa de abandono fue de un 88,7 % mientras que la tasa de abandono más conservadora del grupo con intención de tratamiento fue de un 51,6 %. La asistencia sanitaria telefónica y la asistencia en la clínica no fueron significativamente diferentes en ningún momento. Las tasas de abstinencia total al tabaco a los 6 y 12 meses fueron de 76,9 % y 63,2 %, respectivamente.
Conclusiones:
Se presentan dos modelos rentables para dejar de fumar como asesoramiento de salud. Se observa una prevalencia puntual (30 días) por encima de un 80 % y un efecto duradero en el tiempo. Las cargas personales y sociales (de salud y económicas) implícitas en el tabaco podrían verse considerablemente afectadas si dichos programas se pusiesen en práctica a mayor escala.
Tobacco use, and particularly cigarette smoking, is a primary risk factor for the leading causes of mortality in the developed world—namely, vascular diseases and cancers. Population-level interventions to encourage cessation of tobacco use were markedly advanced in 1998 after a landmark (ie, Master Settlement Agreement) ruling that held tobacco companies liable for causing fatal diseases.1 Subsequently, government programs emphasized media campaigns and programming to diminish the impact of tobacco on public health. These efforts have been somewhat successful in reducing tobacco use start-up rates and less effective at improving tobacco quit rates.2 A strategy frequently employed to encourage smoking cessation is the quit line. Quit lines are usually government-sponsored, toll-free phone numbers that are easily accessed and often involve advice and possibly a “coach” conversation. More than 500,000 potential quitters called US state quit lines in 2009,3 and the service is widely recognized as useful in helping people quit smoking.
The effectiveness of quit lines for the purpose of smoking cessation has recently been called into question.2 It appears that the quit-attempt rate has increased, but so has the quit-failure rate. Furthermore, the quit line–implemented process of telephonic health coaching apparently is failing as an aid to smoking cessation by association with quit lines. However, the coaching process is poorly described in most quit line studies. Accordingly, one must question whether the coaching process is truly being delivered in an effective manner via large, government-sponsored quit lines. Recently, Wolever et al4 called for broad-based acceptance of a working definition of health coaching (HC) to allow a common understanding of the process. They described HC as a patient-centered process based on behavior change theory delivered by health professionals and including patient-determined goals, self-discovery, and mechanisms for behavioral accountability. With this definition, the process of HC can be properly examined as an intervention process. Investigations that clearly define and examine HC as a smoking cessation intervention are needed.
The Mercy-Springfield Road to Freedom Tobacco Cessation Program (MTCP) was initiated in 2009 and offers HC as a primary intervention along with several complementary therapies. The coaching process delivered by the MTCP staff is a client-centered approach meeting the aforementioned HC definition.4 The purpose of this article is to describe MTCP efficacy and program components and does not describe a randomized or controlled trial. This is an institutional case report, an observational study of an existing practice applying STROBE guidelines5 to improve consistency and strength of presenting clinically relevant information. This article also provides a comparison of face-to-face vs telephonic coaching and free-choice vs employer-coerced participation in a tobacco cessation program. The intention is to provide program details including cost information so others might consider an HC-based model to enhance tobacco quit rates in other clinical settings.
METHODS
Patients
Between 2009 and 2011, 161 patients (87 male and 74 females, mean [SD] age: 44.4 ± 11.8 y) entered the MTCP. Patients were included in a treatment cohort if they had smoked at least five cigarettes (or chewed tobacco 30 min) daily for at least 6 months. These patients, largely employees of (n=82) or dependents of employees (n=41) of the Mercy Hospital system, were supported by an outside employer (n = 26) or came through word-of-mouth referral (n = 9). Some MTCP patients participated because of an offer by their employer to achieve a $600 insurance premium reduction. Participants reported average smoking of 18.9 (±11.5) cigarettes/day at the outset of treatment and had smoked for an average of 23.8 years (±12.9). Nine patients were exclusively tobacco chewers. Most (88.1%) had tried to quit smoking previously, averaging 2.9 (±2.8) quit attempts. On enrolling, each patient initially received a quit plan to consider and a questionnaire asking about tobacco use and motivation for behavior change. No other written or educational materials were systematically supplied to patients. Data are presented anonymously and were collected as part of routine clinical practice. For these reasons, informed consent was not collected and the Mercy Springfield Hospital Institutional Review Board did not believe retrospective project review was necessary before dissemination.
Basic Program Design
This was an institutional case study. The project design was a single-site, prospective, non-randomized, observational study of an existing practice (ie, MTCP) with multiple follow-ups examining the effects of an HC tobacco cessation intervention. There was no control group, but the ineffectiveness of non-coaching tobacco cessation efforts are well known and described later. It made little sense to turn away patients who wanted to quit smoking for the establishment of a control group.
The flow of patient enrollment and progress through the MTCP is illustrated in Figure 1. The MTCP required patient participation in HC and offered several complementary therapies. In addition to HC, in-clinic patients received alpha-electrical stimulation (AES), relaxation techniques, and auriculotherapy. Of 161 patients, 93 (57.8%) utilized in-clinic services while 68 used only telephonic HC. HC involved establishing an individualized quit plan while acknowledging the participant as the final decision maker in when to quit.
Figure 1.

Patient Flow Options for Mercy Tobacco Cessation Program. Mandated patients were co-opted to participate by an insurance premium reduction. After an in-clinic session, each patient continued with in-clinic visits or chose remote treatment using only telephonic health coaching.
Initial Visit. This first session was with an experienced tobacco cessation RN educator and planned to last 45 minutes. Patients returned a completed questionnaire on smoking habits, worked on a co-created quit plan, and determined if they would use complementary therapies. Those opting for only telephonic HC remotely completed all future treatment.
Health Coaching. HC was delivered by two registered nurses with Wellcoaches training (Wellcoaches Corp, Wellesley, Massachusetts). This approach emphasizes relationship development, is patient-centered, and encourages a self-discovery process while using mindfulness and motivational interviewing techniques. The initial HC visit was followed by subsequent visits planned to last 20 to 25 minutes. HC could be delivered telephonically or patients could elect for in-clinic, face-to-face appointments. Telephonic HC patients were given information about and often participated in relaxation techniques (see below). The amount of HC was patient determined; however, 6 to 8 sessions over 3 months were recommended. In following a true health coaching paradigm, patients were supported when considering any additional or complementary therapies and were permitted to use them as desired.
Complementary Therapies. All patients had the option of using AES, relaxation techniques, and auriculotherapy as complementary treatment to HC (unless travel distance precluded it). Before HC, in-clinic patients typically completed 20 minutes of relaxation techniques while receiving AES prior to engaging in a 15-minute session of auriculotherapy. Cranial electrotherapy via AES enhances alpha-wave activity with the intention of inducing a calmer and more relaxed state.6,7 AES is delivered in 10 s waves of 0.5 Hz in a pattern range of 100 to 300 micro-amps via earlobe-located electrodes. Alpha-Stim SCS (Electromedical Products International, Mineral Wells, Texas) is a class IIa, Type B medical device with federal law restricting its use. A physician's orders were required for including AES in a patient's quit plan. Relaxation techniques involving deep breathing and progressive muscle relaxation [PMR] were initially taught to patients by staff. Several minutes of deep breathing were followed by several minutes of PMR using standard Jacobsen techniques. By the third visit, most patients practiced self-guided relaxation techniques for 20 minutes.
Auriculotherapy is a micro-current stimulation to reflex and acupuncture points on the ear delivered using the Electro Medical Stim Flex (Electro Medical Inc, Tulsa, Oklahoma). Site selection followed the National Acupuncture Detoxification Association (NADA) 5-point protocol,8 and these sites are related to treatment for addiction, relaxation, and anxiety. Auriculotherapy was administered in 15 to 30–minute sessions, and as an acupuncture-like process, is reported to assist with diminishing nicotine addiction.9,10 Therapists were certified by the Auriculotherapy Certification Institute (Los Angeles, California).
Medications (particularly those acting at the nicotinic receptor) were a prevalent health coaching topic, but such treatment was a personal choice reached in consultation with a primary care physician. A large minority of MTCP patients (43.7%) used some form of nicotinic receptor therapy (Bupropion Hcl [Wellbutrin, 3%]; varenicline [Chantix, 17%]; and nicotine replacement therapy [NRT, 24%]) during the program. Exercise was another patient activity sometimes employed with therapeutic intent. Patients were encouraged to begin or continue a program of regular exercise. No literature or exercise advice was systematically offered. Additional treatments, as well as monetary incentive to participate provided by an employer, are potential confounders of the HC effect. Confounding variables such as NRT, employer mandate, and the prevalent use of complementary therapies are addressed statistically in the results section. The goal of the MTCP was to follow a true coaching model, employing maximum therapeutic flexibility to empower cessation of tobacco use.
Data Management and Statistical Analysis
Participation and Motivational Variables. Participants were defined as those completing at least one HC session and grouped as free choice or mandated (employer required five visits for insurance premium reduction). Patients indicated importance of change of tobacco use on a simple 10-point Likert scale along with readiness to change. Readiness to change is based on the transtheoretical model, which allowed classification of patients into one of five stages (ie, precontemplation, contemplation, preparation, action, maintenance).11
Quit Rate Measures. Smoking quit rates were calculated as the number of patients who reported smoking cigarettes at baseline but not at follow-up. Self-reported cessation (7 mo, 30-d point prevalence) is currently the standard used by the North American Quitline provided a 50% response rate can be achieved. Smoke-free rates examined follow-up on those who initially quit and were determined at 1, 3, 6, 12, 18, and 24 months after quit date. Intent-to-treat was calculated as all patients who quit smoking at 6 months divided by all participants who reported smoking at baseline. Patients lost to follow-up were presumed to have relapsed for the conservative intent-to-treat calculation. For 30-day point prevalence calculation, patients reporting that they were smoke-free for 30 days at 7 months determined the numerator while all patients reporting smoking at baseline was the denominator, excluding those not completing at least four HC sessions, those whose participation was mandated, or those who were lost to follow-up.
Statistical Analysis. Program impact on tobacco use (quit and smoke-free rates) was compared between coaching groups using chi-square. The influence of coaching on quit rates and smoke-free rates was examined in conjunction with motivational variables and covariates (smoking history and NRT use). Due to the number of logistic analyses conducted (one for each time point), the criterion for statistical significance was set at P<.007 based on a Bonforroni correction (.05/7). Two additional tests were conducted to examine reduction in tobacco use: a t-test was used to examine pre-to-post program changes in tobacco use, and a regression examined predictors (coaching, motivational variables, history of smoking behavior, and NRT) on reduced tobacco use. Data analyses were conducted in SPSS v21 (IBM Corp, Armonk, New York).
RESULTS
Participation
For the 161 participants in this study, an average of 6.20 (±2.71, range=2-15) HC sessions were recorded. The initial consult was 42.1 min (±9.80) with 35.8 min (±13.37) per subsequent session. A total of 119 patients (73.9%) demonstrated program adherence of four or more coaching sessions. Patients were motivated to change tobacco habits (7.86±2.17) and most (88.8%) were at least contemplating change (precontemplation 11.2%; contemplation 26.7%; planning 28%; action 34.1%). There were 65 participants (40.4%) whose participation was considered employer-mandated (ie, motivated to take part by an insurance premium reduction).
Quit Rates
Overall, 72.7% (117 of 161) of participants quit smoking. Intent-to-treat quit rate at 6 months was 51.6% (83 of 161). Thirty-day point prevalence quit rate at 7 months was 88.7% (47 of 53). Overall smoke-free rates at 1, 3, 6, 12, 18, and 24 months were 95.3% (102 of 107), 89.9% (98 of 109), 76.9% (83 of 108), 63.2% (67 of 106), 51.8% (44 of 85), and 40.5% (30 of 74), respectively. Figure 2 shows no difference in smoke-free rates between patients receiving in-clinic care (HC plus complementary therapies) vs telephonic HC.
Figure 2.
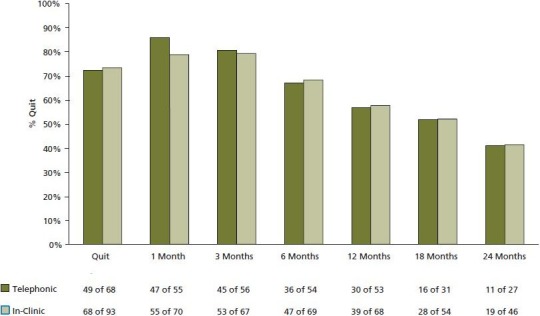
Treatment/Coaching Style. In-clinic patients received health coaching face-to-face in combination with complementary therapies while Telephonic patients remotely received health coaching and no complementary therapies. Note that the zero (0) time point reflects the average smoke-free rate for the subgroups. Values below the x-axis reflect the number of patients responding at each time point.
The predictors, coaching subgroup (in-clinic/telephonic), motivational factors (premium reduction– mandated participation, importance, and stage of change) and covariates, smoking history (average amount smoked per day and years of use), and NRT, reliably distinguished between quitters and non-quitters as seen in the Table. These variables explained a significant, small-moderate effect in smoking cessation (χ2(8)=48.81, P<.001; Nagelkerke's R2=.39; 79% prediction success). Insurance mandate and stage of change significantly contributed to prediction. Specifically, when patients participated freely (ie, without a premium reduction mandate), they were 6.26 times more likely to quit smoking (P<.001). A unit increase in stage of change (eg, from precontemplation to contemplation) saw patients 3.18 times more likely to quit smoking (P<.001). The covariates did not contribute to quit rates above and beyond these factors. The Table also details the predictive ability of this model for smoke-free rates at 1, 3, 6, 12, 18, and 24 months. It appears that the farther removed participants are from coaching, the more their initial rates of smoking influence remaining smoke free, with those who smoked the most at baseline having the most difficulty remaining smoke-free.
Table.
Logistic Regression Predictors of Smoking Cessation and Smoke-free Rates
| OR | 95% CI | B | SE | χ2(8) | R2 | |||
|---|---|---|---|---|---|---|---|---|
| Quit | 48.81a | 0.39 | ||||||
| NRT | 3.07 | 1.19 | 7.94 | 1.12 | 0.49 | |||
| Amount smoked | 0.96 | 0.92 | 1.00 | –0.04 | 0.02 | |||
| Years of use | 1.00 | 0.97 | 1.04 | 0.00 | 0.02 | |||
| Quit attempts | 0.89 | 0.77 | 1.04 | –0.12 | 0.08 | |||
| Importance | 0.91 | 0.72 | 1.15 | –0.09 | 0.12 | |||
| Mandate | 6.26a | 2.49 | 15.71 | 1.83 | 0.47 | |||
| Stage of change | 3.18a | 1.78 | 5.66 | 1.16 | 0.30 | |||
| Coaching | 0.46 | 0.18 | 1.14 | –0.79 | 0.47 | |||
| 1 Mo | 18.50 | 0.45 | ||||||
| NRT | 0.25 | 0.03 | 2.34 | –1.37 | 1.13 | |||
| Amount smoked | 0.87 | 0.76 | 1.01 | –0.14 | 0.07 | |||
| Years of use | 0.91 | 0.78 | 1.07 | –0.09 | 0.08 | |||
| Quit attempts | 1.30 | 0.80 | 2.12 | 0.26 | 0.25 | |||
| Importance | 1.20 | 0.74 | 1.94 | 0.18 | 0.25 | |||
| Mandate | 1.90 | 0.20 | 18.39 | 0.64 | 1.16 | |||
| Stage of change | 8.48 | 1.29 | 55.95 | 2.14 | 0.96 | |||
| Coaching | 5.40 | 0.22 | 131.25 | 1.69 | 1.63 | |||
| 3 Mo | 15.63 | 0.29 | ||||||
| NRT | 0.34 | 0.07 | 1.62 | –1.08 | 0.80 | |||
| Amount smoked | 0.90 | 0.83 | 0.99 | –0.10 | 0.05 | |||
| Years of use | 0.97 | 0.91 | 1.05 | –0.03 | 0.04 | |||
| Quit attempts | 0.93 | 0.72 | 1.19 | –0.08 | 0.13 | |||
| Importance | 1.09 | 0.78 | 1.52 | 0.09 | 0.17 | |||
| Mandate | 2.19 | 0.46 | 10.45 | 0.79 | 0.80 | |||
| Stage of change | 2.10 | 0.84 | 5.25 | 0.74 | 0.47 | |||
| Coaching | 1.43 | 0.28 | 7.20 | 0.36 | 0.83 | |||
| 6 Mo | 33.05a | 0.41 | ||||||
| NRT | 0.31 | 0.10 | 0.99 | –1.18 | 0.60 | |||
| Amount smoked | 0.91 | 0.86 | 0.98 | –0.09 | 0.03 | |||
| Years of use | 1.00 | 0.95 | 1.05 | 0.00 | 0.03 | |||
| Quit attempts | 0.91 | 0.74 | 1.12 | –0.10 | 0.11 | |||
| Importance | 0.84 | 0.61 | 1.15 | –0.18 | 0.16 | |||
| Mandate | 6.54a | 1.88 | 22.78 | 1.88 | 0.64 | |||
| Stage of change | 2.76a | 1.32 | 5.77 | 1.01 | 0.38 | |||
| Coaching | 0.75 | 0.22 | 2.53 | –0.29 | 0.62 | |||
| 12 Mo | 32.84a | 0.37 | ||||||
| NRT | 0.26 | 0.09 | 0.70 | –1.36 | 0.52 | |||
| Amount smoked | 0.91a | 0.86 | 0.96 | –0.09 | 0.03 | |||
| Years of use | 1.00 | 0.96 | 1.04 | 0.00 | 0.02 | |||
| Quit attempts | 0.87 | 0.72 | 1.05 | –0.14 | 0.10 | |||
| Importance | 0.92 | 0.71 | 1.18 | –0.09 | 0.13 | |||
| Mandate | 3.40 | 1.20 | 9.69 | 1.23 | 0.53 | |||
| Stage of change | 1.83 | 1.04 | 3.22 | 0.60 | 0.29 | |||
| Coaching | 0.90 | 0.32 | 2.51 | –0.10 | 0.52 | |||
| 18 Mo | 32.82a | 0.44 | ||||||
| NRT | 0.59 | 0.19 | 1.83 | –0.53 | 0.58 | |||
| Amount smoked | 0.90a | 0.84 | 0.96 | –0.11 | 0.03 | |||
| Years of use | 0.99 | 0.94 | 1.03 | –0.02 | 0.02 | |||
| Quit attempts | 0.75 | 0.56 | 1.00 | –0.29 | 0.15 | |||
| Importance | 0.87 | 0.65 | 1.16 | –0.14 | 0.15 | |||
| Mandate | 2.33 | 0.75 | 7.29 | 0.85 | 0.58 | |||
| Stage of change | 2.26 | 1.13 | 4.54 | 0.82 | 0.36 | |||
| Coaching | 0.49 | 0.14 | 1.69 | –0.72 | 0.64 | |||
| 24 Mo | 26.57a | 0.42 | ||||||
| NRT | 0.46 | 0.13 | 1.70 | –0.77 | 0.66 | |||
| Amount smoked | 0.92 | 0.86 | 0.98 | –0.09 | 0.03 | |||
| Years of use | 0.98 | 0.93 | 1.03 | –0.02 | 0.03 | |||
| Quit attempts | 0.79 | 0.58 | 1.07 | –0.24 | 0.16 | |||
| Importance | 0.85 | 0.62 | 1.15 | –0.17 | 0.16 | |||
| Mandate | 3.97 | 1.11 | 14.26 | 1.38 | 0.65 | |||
| Stage of change | 1.75 | 0.86 | 3.56 | 0.56 | 0.36 | |||
| Coaching | 0.82 | 0.22 | 3.11 | –0.20 | 0.68 | |||
Abbreviation: NRT, nicotine receptor therapy.
P<.007
Tobacco Reduction
Participants significantly reduced tobacco use rates to an average of 3.52 (±7.45) cigarettes per day (t(134) = 17.94, P<.001). Tobacco reduction was predicted by one's history of smoking (quantity smoked per day b[SE] = 0.27 (0.05), P<.001; years smoked b[SE] = .09[0.04], P=.04; number of previous quit attempts b[SE] = –0.01[.18], P=.97; NRT (b[SE] = –0.33[1.00], P=.74; motivational variables (importance b[SE] = –0.38[0.25], P=.13; stage of change b[SE] = –2.26(0.55), P<.001; insurance mandate b[SE] = 1.64[.97], P=.10; and their coaching group (in clinic/telephonic Β[SE]=2.73[1.04], P<.01), F(8,155) = 11.82, P<.001; R2 = .39). Every additional cigarette smoked at baseline related to .42 more cigarettes smoked at follow-up; every additional year patients had smoked led to an additional .15 cigarettes smoked; with every increase in stage of change, .31 fewer cigarettes were smoked; and patients in the telephonic coaching group smoked .18 cigarettes fewer than those receiving in-clinic coaching.
Discussion
Quitting tobacco dependency is an arduous and typically unsuccessful process. Successful quit rates are at a meager 4.4% when examining programs over a 20-year period (1991-2010).12 This poor rate of success exists despite well-funded and widely accessible smoking cessation interventions implemented after the 1998 Master Settlement Agreement.1 “Cold turkey” and unassisted quitting reveal a success rate of about 5%13 while some programs using behavioral therapy with NRT claim quit rates exceeding 40%.14 Accordingly, the MTCP 30-day point prevalence (88.7%) and intent-to-treat (51.6%) success rates are remarkable. According to longitudinal (5-8 y) data,15 the overall quit rate (57%) achieved by MTCP patients at 12 months is a very good predictor of long-term success.
Telephonic coaching (or counseling) is a process widely used for tobacco cessation programs, particularly by accessible government-supported quit lines (eg, 1-866 NY-QUITS or 1-800-QUITNOW). Previous studies of coaching reported quit rates above 30%,16 but sometimes below 15%.17 However, the training and background of coaches in previous studies is poorly described and unlikely to meet the HC paradigm recently forwarded by Wolever et al.4 MTCP health coaches were registered nurses with extensive coach training who delivered HC compatible with the Wolever et al definition. Others have called for further study of such coaching methods because of preliminary success in smoking cessation.18 We speculate the care and techniques provided by MTCP coaches accounted for the outstanding rate of successfully achieving tobacco independence in the present report.
Adding complementary therapies did little to enhance MTCP quit rates. Telephonic-only coaching quit rates were not significantly different than in-clinic treatment that included AES and auriculotherapy. Cranial stimulation and acupuncture are accepted clinical procedures with effectiveness in addiction/smoking cessation.7,9,19,20 In the present study, however, providing these procedures before each HC session did not improve effects of HC alone on quit rates. While these procedures may be effective, combining them with HC likely removed their influence on the quitting process. It seems that whether HC was administered with or without complementary therapies, it was the primary treatment explaining smoking cessation in these patients.
The question of coaching effectiveness when delivered telephonically vs face-to-face is of great interest with ease and cost-effectiveness of remote coaching, making it preferred assuming it is equally effective. The absence of facial expressions and body language are considered shortcomings of the telephonic method. Our results, however, demonstrated telephonic coaching to be equally effective as in-clinic, face-to-face sessions. It may be speculated that many patients are less inhibited speaking on the phone than in person, making them inclined to more fully tell their stories, contributing to HC success. Our tobacco cessation results are not surprising given that telephonic coaching sessions apparently are also effective for weight loss21 and treatment of depression.22 Our data lend further credence to remote, telephonic delivery of coaching processes as an effective behavior change intervention.
The use of readiness-to-change scores appears extremely valuable for health coaches working with patients attempting to quit smoking. Moving a patient one behavior-change category (eg, from contemplation to preparation) more than doubled chances of quitting and equally affected chances of remaining smoke-free. With such a large influence on outcome, monitoring, and influencing, stage of behavior change seems to be a critical HC strategy.
This report is not without limitations. This is an account of an existing practice; therefore, these data are not from a prospectively designed, randomized, controlled trial (RCT). These data were systematically collected; however, conclusive assessment of the MTCP coaching process may require an experimentally designed study. While ideal, designing such a project may not be ethically pragmatic given it requires withholding an apparently effective treatment from a (control) group of patients who need to be as motivated to quit smoking as those assigned to treatment. As a description of an existing practice and not an RCT, this project did not control for other factors that may impact smoking cessation and might have augmented the HC effect. For example, NRT23,24 is a controversial intervention sometimes described as effectively assisting with quitting a tobacco habit. Our analysis found that HC patients using NRT were no more likely to quit tobacco than patients without NRT. Many MTCP patients employed NRT (44% at one point or another) though HC was the only treatment consistently applied for all patients. In standard practice, a health coach using a patient-centered approach encourages patients to seek other strategies, aids, or social supports to optimize positive behavior change. The goal of the MTCP was to follow a true coaching paradigm allowing maximum therapeutic flexibility. Undoubtedly, a high level of treatment flexibility was achieved; however, HC was the common and primary intervention for all patients in this highly successful, tobacco cessation program.
Smoking has huge economic implications with a $96 billion CDC estimated annual US health cost burden25 shared by smokers and tax-payers in general. This number has recently been translated to a corporate cost of greater than $5000 per smoking employee26 with additional costs for human pain and suffering immeasurable. Finding a strategy to incentivize smoking cessation is economically imperative and many employers attempt to provide such motivation. Our observations revealed coercing smoking cessation through an insurance premium reduction mandate is not a good strategy. Patients required to participate to avoid an insurance-premium penalty were about six times less likely to quit smoking than those participating freely. Program compliance (>4 visits) improved success for these mandated participators, but they were still about twice as likely to continue smoking as free-choice patients. Nearly twice as many mandated participants were in precontemplation (ie, not considering quitting) compared to those freely entering MTCP. Providing an insurance premium upcharge apparently may not be a good strategy to augment smoking cessation. However, a recent study indicates other employer-organized programs, using financial incentives, are viable to motivate behavior change.27
Programs for smoking cessation can be expensive while claiming efficacy rates of 25% to 30%. The MTCP in-clinic program had quitting success rates exceeding 70% and smoke-free rate above 60% at 1 year with patient cost of $38 per visit ($266 for seven visits) and total staff labor costs of $204 for the average patient who had about seven visits. More impressively, patient cost for the equally effective telephonic-only HC was $32 per session with labor costs of only $112 for (on average) seven sessions.
In practice, implementing a hospital-based, HC smoking cessation program is highly feasible. Allied healthcare personnel (existing staff, eg, nurses) building on their knowledge base, with as little as 50 to 200 hours of training in relational and communication skills, may become qualified as an HC. Once an HC staff exists then adding the program into existing departments (eg, cardiac rehabilitation or employee/ community wellness) becomes a matter of best logistical fit. Given the potential economic and societal benefit, adding an HC-grounded smoking cessation program to the menu of patient treatment options should be a primary consideration for many public healthcare settings. Furthermore, government sponsorship of such programs deserves careful investigation and deliberation given the prospects for reducing national healthcare costs.
SUMMARY AND CONCLUSIONS
The MTCP features a carefully defined program of HC, and reports a very high quit rate (72.7%) and excellent smoke free rates at 6 mo (76.9%) and 12 mo (63.2%). Habitual tobacco use is a very difficult addiction to overcome, is widely recognized as primary risk factor for chronic diseases, and is a scourge on public health. Most smoking cessation programs report quit rates that rarely exceed 30% at 6 mo and unassisted quitting efforts have success rates well below 10%. For scientific purposes, greater experimental controls on our data would be ideal, but these results are highly encouraging and the success of MTCP practices for many individuals that were struggling with tobacco addiction is clear. Within the limitations of this study, health coaching (when defined by strict patient-centered standards) appears to be an effective tobacco cessation intervention. Other clinical and public healthcare settings should consider adapting and implementing this cost efficient model to assist their patients with tobacco abstention.
Disclosures The authors completed the ICMJE Disclosure Form for Potential Conflicts of Interest, and no conflicts were disclosed.
Contributor Information
Gary A. Sforzo, Department of Exercise & Sport Sciences, Ithaca College Ithaca, New York (Dr Sforzo), United States.
Miranda Kaye, Department of Exercise & Sport Sciences, Ithaca College Ithaca, New York (Dr Kaye), United States.
Gale D. Ayers, Mercy Corporate Health & Wellness, Springfield, Missouri (Ms Ayers), United States.
Betina Talbert, Mercy Corporate Health & Wellness, Springfield, Missouri (Ms Talbert), United States.
Marilyn Hill, Mercy Corporate Health & Wellness, Springfield, Missouri (Ms Hill), United States.
REFERENCES
- 1.Redhead CS.CRS Report for Congress: Tobacco Master Settlement Agreement (1998): overview, implementation by states, and Congressional issues. http://www.law.umaryland.edu/marshall/crsreports/crsdocuments/RL30058.pdf AccessedJuly21, 2014.
- 2.Pierce JP, Cummins SE, White MM, Humphrey A, Messer K.Quitlines and nicotine replacement for smoking cessation: Do we need a policy change? Annu Rev Public Health. 2012;33:341–56. [DOI] [PubMed] [Google Scholar]
- 3.Barry MB, Saul J, Bailey LA.US quitlines at crossroads: utilization, budget, and service trends 2005-2010. http://c.ymcdn.com/sites/www.naquit-line.org/resource/resmgr/reports_2010/100407_special-report.pdf AccessedJuly21, 2014.
- 4.Wolever R, Simmons L, Sforzo Get al. A systematic review of the literature on health and wellness coaching: Defining a key behavioral intervention in health care. Global Adv Health Med J. 2013;2:34–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61:344–9. [DOI] [PubMed] [Google Scholar]
- 6.Kirsch DL, Nichols F.Cranial electrotherapy stimulation for treatment of anxiety, depression, and insomnia. Psychiatr Clin North Am. 2013;36(1):169–76. [DOI] [PubMed] [Google Scholar]
- 7.Schmitt R, Capo T, Boyd E.Cranial electrotherapy stimulation as a treatment for anxiety in chemically dependent persons. Alcohol Clin Exp Res. 1986;10(2):158–60. [DOI] [PubMed] [Google Scholar]
- 8.Oleson TD, Kroening RJ, Bresler DE.An experimental evaluation of auricular diagnosis: the somatotopic mapping or musculoskeletal pain at ear acupuncture points. Pain. 1980;8(2):217–29. [DOI] [PubMed] [Google Scholar]
- 9.Tahiri M1, Mottillo S, Joseph L, Pilote L, Eisenberg MJ.Alternative smoking cessation aids: a meta-analysis of randomized controlled trials. Am J Med. 2012;125(6):575–84. [DOI] [PubMed] [Google Scholar]
- 10.He D, Berg JE, Hastmark AT.Effects of acupuncture on smoking cessation or reduction for motivated smokers. Prev Med. 1997;26(2):208–14. [DOI] [PubMed] [Google Scholar]
- 11.Prochaska JO, DiClemente CC.Stages of change in the modification of problem behaviors. Prog Behav Modif. 1992;28:183–218. [PubMed] [Google Scholar]
- 12.Zhu SH, Lee M, Zhuang YL, Gamst A, Wolfson T.Interventions to increase smoking cessation at the population level: how much progress has been made in the last two decades? Tob Control. 2012;21(2):110–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Cancer Society. A word about success rates for quitting smoking. http://www.cancer.org/healthy/stayawayfromtobacco/guidetoquittingsmoking/guide-to-quitting-smoking-success-rates AccessedJuly21, 2014.
- 14.Quit For Life. A collaboration for a healthier workplace. http://www.cancer.org/healthy/stayawayfromtobacco/quit-for-life AccessedJuly21, 2014.
- 15.Nohlert E, Ohrvik J, Tegelberg A, Tillgren P, Helgason AR.Long-term follow-up of a high- and a low-intensity smoking cessation intervention in a dental setting—a randomized trial. BMC Public Health 2013;13:592–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zbikowski S Jack L McClure J, et al. Utilization of services in a randomized trial testing phone- and web-based interventions for smoking cessation. Nicotine Tob Res. 2011;13(5):319–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D'Silva J, Boyle R, Lien R, Rode P, Okuyemi KS.Cessation outcomes among treatment-seeking menthol and nonmenthol smokers. Am J Prev Med. 2012;43(5 Suppl 3):S242–8. [DOI] [PubMed] [Google Scholar]
- 18.Mantler T, Irwin JD, Morrow D.Assessing motivational interviewing through co-active life coaching tools as a smoking cessation intervention: a demonstration study. Intern J Evidence Based Coaching Mentoring. 2010;8:49–63. [Google Scholar]
- 19.Ausfeld-Hafter B, Marti F, Hoffmann S.Smoking cessation with ear acupuncture. Descriptive study on patients after a smoking cessation treatment with ear acupuncture. Forsch Komplementarmed Klass Naturheilkd. 2004;11(1):8–13. [DOI] [PubMed] [Google Scholar]
- 20.Klawansky S1, Yeung A, Berkey C, Shah N, Phan H, Chalmers TC.Meta-analysis of randomized controlled trials of cranial electrostimulation. Efficacy in treating selected psychological and physiological conditions. J Nerv Ment Dis. 1995;183(7):478–84. [DOI] [PubMed] [Google Scholar]
- 21.Perri MG, Limacher MC, Durning PEet al. Extended-care programs for weight management in rural communities. Arch Intern Med. 2008;168(21):2347–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohr D, Ho J, Dunnecy Jet al. Effect of telephone-administered vs face-to-face cognitive behavioral therapy on adherence to therapy and depression outcomes among primary care patients: a randomized trial. JAMA. 2012;307(21):2278–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemmens V, Oenema A, Knut IK, Brug J.Effectiveness of smoking cessation interventions among adults: a systematic review of reviews. Eur J Cancer Prev. 2008;17(6):535–44. [DOI] [PubMed] [Google Scholar]
- 24.Alpert H, Connolly G, Biener L.A prospective cohort study challenging the effectiveness of population-based medical intervention for smoking cessation. Tob Control. 2013;22:32–7. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses—United States, 2000-2004. MMWR. 2008;57:1226–8 TobaccoControltobaccocontrol.bmj.com [PubMed] [Google Scholar]
- 26.Berman M, Crane R, Seiber E, Munur M.Estimating the cost of a smoking employee. Estimating the cost of a smoking employee. Tob Control. Published Online First June3, 2013. doi:10.1136/tobaccocontrol-2012-050888. [DOI] [PubMed] [Google Scholar]
- 27.Cawley J, Price JA.A case study of a workplace wellness program that offers financial incentives for weight loss. J Health Econ. 2013;32(5):794–803. [DOI] [PubMed] [Google Scholar]


