Abstract
Context:
Dental caries, a ubiquitous multifactorial infectious disease, is primarily caused by microorganisms like Streptococcus mutans and Lactobacillus acidophilus. Use of antimicrobials is an important strategy to curb cariogenic microorganisms.
Aim:
The aim was to evaluate the in vitro antimicrobial activity of C. sinensis extract on S. mutans and L. acidophilus.
Study Setting and Design:
Experimental design, in vitro study, lab setting.
Materials and Methods:
Aqueous, acetone and ethanolic extracts of C. sinensis were subjected to antioxidant analysis. The ethanolic extract was used for assessment of antimicrobial properties. Ethanolic green tea extract at ten different concentrations and 0.2% chlorhexidine was used. Microbiological investigations were carried out to determine the minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC) and zone of Inhibition of the test and control agents against S. mutans and L. acidophilus.
Statistical Analysis:
Kruskall–Wallis and Mann–Whitney U-test.
Results:
MIC of green tea extract on S. mutans and L. acidophilus was found to be 0.2% and 0.3% respectively, MBC was found to be 0.8% and 0.9%, respectively. The mean zone of inhibition for 30 μl containing 300 μg of ethanolic extract of green tea and control against S. mutans were 18.33 mm and 14.67 mm, respectively. The mean zone of inhibition for 30 μl containing 300 μg of ethanolic extract of green tea and control against L. acidophilus were 12.67 mm and 7.33 mm, respectively.
Conclusion:
Green tea has antibacterial activity against predominant cariogenic bacteria namely S. mutans and L. acidophilus.
Keywords: Camellia sinensis, dental caries, green tea, phytochemical analysis
Introduction
Dental caries is one of the most common oral infectious disease in man.[1,2] Oral microorganisms play a significant role in the etiology of dental caries. Over 400 species of microbes inhabit as commensals in the oral cavity of a healthy adult.[3] An aberration to this ecology due to dietary habits, improper oral hygiene or systemic factors lead to an increase in cariogenic microorganisms.[4]
Cariogenic microorganisms like Streptococcus mutans and Lactobacillus acidophilus encourage the accumulation and adherence of plaque biofilm by metabolizing sucrose into sticky glucan. Besides, the microorganisms in dental plaque degrade the dietary carbohydrates producing lactic acid leading to localized demineralization and the eventual formation of dental caries.[5,6] Although mechanical plaque control methods like brushing and flossing help to limit the growth of oral microbes, removal of plaque from inaccessible areas of the oral cavity remains a challenge. Hence, the use of the antimicrobial agent is warranted to limit the growth of cariogenic microorganisms and prevent dental caries.[3] Chlorhexidine, triclosan, cetyl pyridinium chloride, essential oils, fluoride based solution are some of the antimicrobial agents tested against oral microbes.[7,8,9] Chlorhexidine is the gold standard chemical plaque control agent. Its ability to bind with soft and hard tissues in the oral cavity enables it to act for a long period after application. However, brown discoloration of dentition and restorative material, dorsum of the tongue, taste perturbation, oral mucosal ulceration, unilateral/bilateral parotid swelling and enhanced supra gingival calculus formation, have been reported as side effects of long term chlorhexidine use.[10,11]
Drug resistance and side effects encountered with the use of synthetic drugs has led to the surge for novel and safe alternatives.[4] Since ancient times, plants have proved to be an archetypal source of medicine. Camellia sinensis is a shrub of the Theaceae family usually clipped to a height of 2–5 feet in cultivation, grown in semi-tropical environment[12]. Leaves are usually handpicked and based on the processing of the leaves three different types of tea are produced, namely Green tea (non-fermented), Oolong tea (semi-fermented) and Black tea (fermented).[13] Green tea being the non-fermented type possess more Catechin than the other two types.[4] Green tea has antioxidant, antiviral and antitumoral properties.[4,5] Despite abundant literature on the general health benefits of drinking green tea exists, its effect on cariogenic microbes is limited. With this background, the present study was carried out to determine the in vitro antibacterial effect of C. sinensis extract on S. mutans and L. acidophilus.
Materials and Methods
Materials, chemicals, and reagents
Green tea leaves (Gtee Botanicals, Chennai). Acetone; ethanol; n-butanol; methanol (Fischer Scientific, Mumbai), Folin and Ciocalteaus reagent (Loba Chemie, Mumbai), Muller Hinton media (Hi media, Mumbai), Paper discs (Hi media, Mumbai), 2-Diphenyl-picrylhydrazyl (DPPH); Butylated Hydroxy Toluene (BHT) (Sigma Aldrich, USA), Ultraviolet (UV) double beam spectra scan (Chemito, India).
Preparation of green tea extracts
This study was conducted after obtaining clearance from Institutional review board of Ragas Dental College, Chennai. C. sinensis leaves were procured from Gtee Botanical Extract Private Limited, Chennai. The physical and botanical characteristics of the leaves were authenticated by an expert botanist. 15 g of dried green tea leaves were mixed with 150 ml of acetone or ethanol (75%) or aqueous for 1 min using an Ultra Turax mixer (13,000 rpm) and soaked overnight at room temperature and filtered using Whatman No. 1 paper. The filtered solution was evaporated under vacuum at 40°C to a constant weight and then dissolved in respective solvents.[14,15]
Phytochemical screening of green leaf extracts
Phytochemical screening of green tea extracts with acetone, ethanol and aqueous as solvents was done using standard methods.[16,17,18] The presence of major natural chemical groups such as tannins, saponins, flavonoids, phenols, terpenoids, alkaloids, glycosides, cardiac glycosides, coumarins and steroids were analyzed.
Qualitative analysis of antioxidant activity of green tea
The qualitative antioxidant activity of the green tea extracts using all the three solvents were determined using DPPH.[19] 50 μl of extracts of green tea was taken in the microtiter plate. 100 μl of 0.1% methanolic DPPH was added over the samples and incubated for 30 min in dark condition. The samples were then observed for discoloration. The antioxidant positive samples were subjected for further quantitative analysis.
Quantitative analysis of free radical scavenging activity of green tea
The antioxidant activities were determined using DPPH (Sigma-Aldrich, USA) as a free radical.[20] A volume of 100 μl of green tea extract was mixed with 2.7 ml of methanol and 200 μl of 0.1% of methanolic DPPH and incubated for 30 min. Subsequently, at every 5 min interval, the absorption maxima of the solution were measured using a UV double beam spectra scan (Chemito, India) at 517 nm. Absorption of the blank sample containing the earlier mentioned amount of methanol and DPPH solution was prepared and measured as a control. The antioxidant activity of the sample was compared with known synthetic standard of (0.16%) of BHT. Free radical scavenging activity was calculated by the following formula:

Antioxidant activity of green tea extract using all the three solvents was determined. Heretofore, the extract that possessed greater antioxidant activity was used for further procedures.
Estimation of total phenol, flavonoid, tannin contents in Green tea
Total phenolic content in the ethanolic green leaf extracts of C. sinensis was determined by the Folin-Ciocalteau colorimetric method.[21] 0.5 ml of green tea extract was added to 0.1 ml of Folin-Ciocalteau reagent (0.5 N) and mixed thoroughly. Later 2.5 ml of Sodium carbonate was added, and the mixture was allowed to stand for 30 min in dark at room temperature. Gallic acid was used as a standard for the calibration curve and plotted at 0.2, 0.4, 0.6, 0.8 and 0.1 mg/ml, respectively. The absorbance was measured at 765 nm in a UV-visible spectrophotometer. Total phenolic content was expressed as milligram of gallic acid equivalents (GAE)/100 g of samples.
Total flavonoid content in the ethanolic green tea extract was determined by the aluminum chloride colorimetric method.[22] 0.5 ml of green tea extract, 0.1 ml aluminum chloride (10%), 0.1 ml of potassium acetate and 2.8 ml distilled water were added sequentially. The test solution was vigorously shaken. Quercetin was used as standard for the calibration curve, and plotted at 0.08, 0.16, 0.24, 0.32 and 0.40 mg/ml respectively. Absorbance was recorded at 415 nm in a UV-visible spectrophotometer. The concentrations of flavonoid in the test samples were calculated from the calibration plot and expressed as mg quercetin equivalent per gram of sample.
Tannin content of green tea leaf extract was estimated using Folin-Ciocalteau's reagent.[23] 1 ml of green tea extract was mixed with Folin-Ciocalteau's reagent (0.5 ml), then 1 ml of sodium carbonate solution and 8 ml of distilled water was added and allowed to stand for 30 min at room temperature. Tannin acid was used as standard for the calibration curve, and plotted at 0.08, 0.16, 0.24, 0.32 and 0.40 mg/ml respectively. The supernatant was obtained by centrifugation and absorbance was recorded at 725 nm using UV-visible spectrophotometer. The tannin content was expressed as mg tannic acid equivalent (TAE)/gram of the sample.
Antibacterial activity of green leaf extracts
Extraction of active compound
The fine powder of the green tea leaf was macerated with 75% of ethanol and evaporated. The residue was mixed with n-butanol and water (2:1). The upper layer of n-butanol and lower layer of water were separated and evaporated under vacuum. The upper layer of n-butanol residues was washed with petroleum ether to remove fatty components and then extracted with ethanol.[24]
Culturing of freeze dried Streptococcus mutans
The freeze dried S. mutans strains (MTCC 890) obtained from IMTECH, Chandigarh were made viable by transferring to Brain heart Infusion Broth (Hi media Laboratory Pvt. Ltd, Mumbai, India) and incubating at 37°C for 24 h. L. acidophilus strains were obtained from those isolated and maintained by Poonga Biotech Research Center, Chennai.
Minimum inhibitory concentration
Minimum inhibitory concentration (MIC) of green tea against both the bacteria was assessed by serial dilution method. Ten different concentrations of the green tea ethanolic extract (0.1%, 0.2%, 0.3%, 0.4%, 0.5%, 0.6%, 0.7% 0.8%, 0.9%, 1.0%) were incorporated into nutrient broth in different test tubes. In each test tube, 5 ml of green tea extract was added to 4.9 ml of nutrient broth and 0.1 ml of bacterial culture. A control tube containing the growth medium and the bacteria was set-up. The mixtures were incubated at 37°C for 24 h and analyzed for turbidity. The minimum concentration of green tea extract that will inhibit the growth of the microorganism was determined as MIC.
Minimum bactericidal concentration
Minimum bactericidal concentration (MBC) was determined from MIC range using Spread plate method. Mueller Hinton agar in Petri dishes were sub-cultured from tubes without growth and incubated at 37°C for 24 h. The petri dishes were observed macroscopically. The highest dilution that yielded no bacterial colony on a solid medium was taken as MBC.
Zone of inhibition
Antibacterial activity was measured using the standard method of diffusion disc plates on agar.[25] 0.1 ml of each culture of bacteria was spread on agar plate surfaces. For antibacterial assay, all bacterial strains were grown in Muller Hinton Broth Medium (Hi media) for 24 h at 37°C and plated on Muller Hinton Agar (Hi media). Paper disc (6 mm in diameter) were placed on the agar medium to load 10 μl containing 100 μg, 20 μl containing 200 μg and 30 μl containing 300 μg of ethanolic extract of green tea. Inhibition diameters were measured after incubation for 24–48 h at 37°C. Control disks were prepared by infusing with 0.2% chlorhexidine. The plates were incubated at 37°C for 48 h. After 48 h, antibacterial activity of the extract against both the test bacteria was observed by growth free zone of inhibition near the respective disc.[25]
Statistical analysis
The data obtained was subjected to statistical analysis. The mean zone of inhibition was compared using Kruskall–Wallis followed by Mann–Whitney U test for pairwise comparison. Data analysis was performed using Statistical package for Social Sciences (SPSS) (SPSS Version 19, IBM Corp, New York). For all comparison P < 0.05 was considered as significant.
Results
Phytochemical analysis revealed that tannins, phenols, alkaloids, flavonoids, quinones, cardiac glycosides, terphenoids were present in C. sinensis extract irrespective of the solvent used [Table 1]. Glycosides were absent in all the three extracts. Qualitative analysis showed that ethanolic and aqueous extracts had stronger antioxidant property which was substantiated by quantitative analysis presenting 93.90% antioxidant activity for ethanolic extract. Whereas aqueous and acetone extracts had 89.02% and 79.26%, respectively [Figure 1]. Henceforth, all analysis were carriedout only with ethanolic extract of green tea. The total phenol content was found to be 96 mg GAE/g. The total flavonoid and tannin content was found to be 7.5 mg QE/g and 243.75 mg TAE/g, respectively [Table 2].
Table 1.
Phytochemical screening of green tea extracts
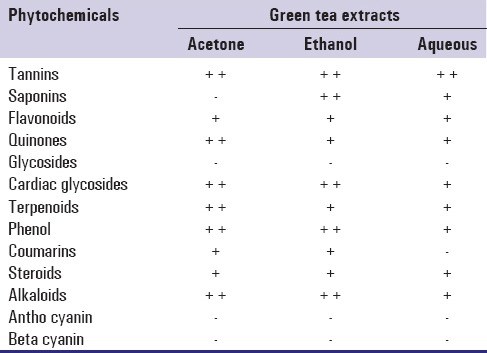
Figure 1.
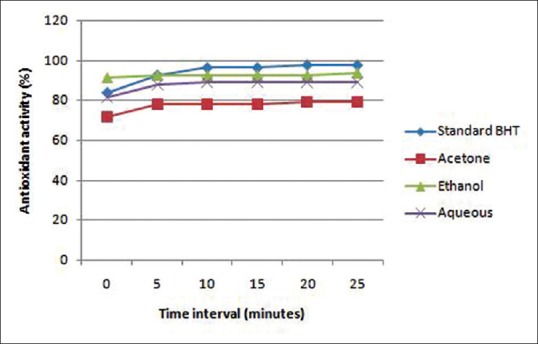
Antioxidant activity of acetone, ethanol and aqueous extracts of green tea
Table 2.
Total phenol, flavanoid and tannin content in green tea extracts

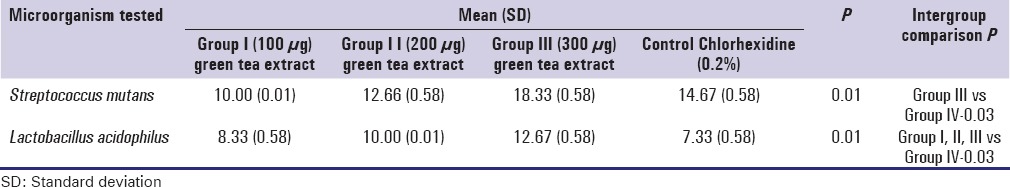
Camellia sinensis extract exhibited antibacterial effect on S. mutans and L. acidophilus. The MIC for S. mutans and L. acidophilus was 0.2% and 0.3% respectively [Table 3]. The MBC for S. mutans and L. acidophilus is 0.8% and 0.9% respectively. Zone of inhibition was assessed in triplets [Figure 2]. The mean zone of inhibition for 30 μl containing 300 μg of ethanolic extract of green tea and control against S. mutans were 18.33 mm and 14.67 mm respectively, and the difference was found to be statistically significant. The mean zone of inhibition for 30 μl containing 300 μg of ethanolic extract of green tea and control against L. acidophilus were 12.66 mm and 7.67 mm respectively and this difference was found to be significant [Table 4].
Table 3.
Minimum inhibitory concentration of streptococcus mutans and lactobacillus acidophilus
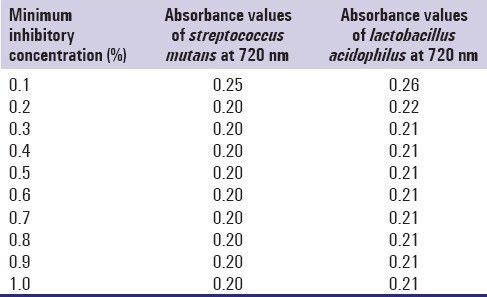
Figure 2.
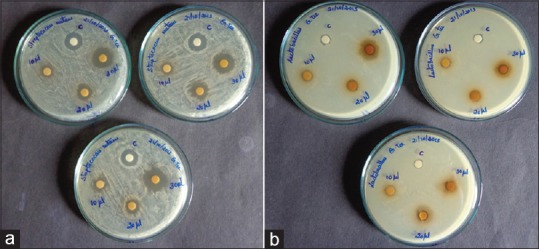
(a) Zone of inhibition of Streptococcus mutans against green tea extract at three different concentrations. (b) Zone of inhibition of S. mutans and Lactobacillus acidophilus against green tea extract
Table 4.
Zone of Inhibition of Streptococcus mutans and Lactobacillus acidophilus against green tea extract
Discussion
“Better to be deprived of food for 3 days, than tea for one” an ancient Chinese proverb elucidates the significance of tea. Although native of China, tea is widely used in the world for over 2000 years and is the second largely consumed drink in the world after water.[4,26] In recent days, there is an increased recognition on the versatile health benefits of green tea. Hence, this study was done to assess the anticariogenic, oral health benefits of green tea.
Microorganisms like S. mutans, Lactobacillus species, Actinomyces naeslundii, Actinomyces viscosus, Rothia dentocariosa, Propionibacterium, Prevotella, Veillonella, Bifidobacterium, Scardovia and Enterococcus faecalis are associated with the etiology of dental caries.[27] Despite the complexity of oral flora, there is convincing evidence that S. mutans and L. acidophilus, being acidogenic and aciduric are the main culprit microorganisms in the etiology of dental caries.[28] Hence in this study the antimicrobial activity of these two microorganisms were tested.
Qualitative analysis revealed that various phytochemicals were present. Tannins, phenols, alkaloids, flavonoids were present in C. sinensis extract irrespective of the solvent used. The flavonoids possess anti glycosyl activity and can inhibit adherence of microbes. Tannins can inhibit both glucosyl transferace (GFT) activity and bacterial growth by their strong iron-binding capacity. Alkaloids interfere with the division of cells thus inhibiting their growth.
In this study tannin, phenol and flavonoids content was found to be 243.75 mg/g, 96 mg/g and 7.5 mg/g, respectively. In a study done by Subramaniam et al. in 2013 tannin, phenol and flavonoid content of green tea was found to be 0.308 mg/g, 0.0105 mg/g and 350 mg/g respectively.[4] This variation in the chemical constituents in green tea may be attributed to the variation in geographic location of plant specimen like soil and climate.
Among the 4000 bioactive compounds present in Green tea, the active constituents are polyphenols.[4] The major catechins present in green tea polyphenols are epicatechin EG, epigallocatechin (EGC), EGC gallate (EGCG), EGCG.[5] EGCG can cause cell membrane disruption and prevent DNA super coiling eventually leading to bacterial destruction. EGC interacts with proteins and distorts their tertiary structure. Since cariogenic bacteria have the ability to produce lactic acid that erode the tooth surface and can encourage the plaque adherence by producing sticky dextran from sucrose. GFT is a key enzyme, which metabolizes dietary sucrose.[12]
The study done by Tahir et al. in 2011 obtained MIC and MBC value of 0.7%, whereas in this study MIC of 0.2% and MBC of 0.3% was obtained.[2] These reduced values can be attributed to the higher tannin content in the sample studied. The ethanolic extract had greater antioxidant activity compared to acetone and aqueous extracts. This finding is in agreement with that of Subramaniam et al., 2013 in which ethanolic extract had greater antioxidant activity than aqueous extract.[4]
The inhibitory zones increased with increasing volumes of green tea ethanol extracts. It is remarkable to note that greater zone of inhibition was obtained for 300 μg/ml of green tea than Chlorhexidine against both S. mutans and L. acidophilus. As with any in vitro studies this study is not without limitations. This study has been performed in planktonic cultures of S. mutans and L. acidophilus. In vivo studies in this area are further required as limited data are available regarding their biofilm counterpart. Hence, further studies are required for understanding antimicrobial effect of green tea extract in biofilm.
Conclusion
Green tea has antibacterial activity against predominant cariogenic bacteria namely S. mutans and L. acidophilus.
Acknowledgment
The authors would like to acknowledge Poonga Biotech Research Center, Chennai for carrying out this study in their laboratory.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Naderi NJ, Niakan M, Kharazi Fard MJ, Zardi S. Antibacterial activity of Iranian green and black tea on Streptococcus mutans: An in vitro study. J Dent (Tehran) 2011;8:55–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Tahir A, Moeen R. Comparison of antibacterial activity of water and ethanol extracts of Camellia sinensis (L.) Kuntze against dental caries and detection of antibacterial components. J Med Sci Res. 2011;5:4504–10. [Google Scholar]
- 3.Chatterjee A, Saluja M, Agarwal G, Alam M. Green tea: A boon for periodontal and general health. J Indian Soc Periodontol. 2012;16:161–7. doi: 10.4103/0972-124X.99256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Subramaniam P, Eswara U, Maheshwar Reddy KR. Effect of different types of tea on Streptococcus mutans: An in vitro study. Indian J Dent Res. 2012;23:43–8. doi: 10.4103/0970-9290.99037. [DOI] [PubMed] [Google Scholar]
- 5.Namita P, Mukesh R, Vijay K. Camellia sinensis (Green tea): A review. Glob J Pharmacol. 2012;6:52–9. [Google Scholar]
- 6.Taylor PW, Hamilton-Miller JM, Stapleton PD. Antimicrobial properties of green tea catechins. Food Sci Technol Bull. 2005;2:71–81. doi: 10.1616/1476-2137.14184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Subramaniam P, Nandan N. Effect of xylitol, sodium fluoride and triclosan containing mouth rinse on Streptococcus mutans. Contemp Clin Dent. 2011;2:287–90. doi: 10.4103/0976-237X.91790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kulkarni VV, Damle SG. Comparative evaluation of efficacy of sodium fluoride, chlorhexidine and triclosan mouth rinses in reducing the mutans streptococci count in saliva: An in vivo study. J Indian Soc Pedod Prev Dent. 2003;21:98–104. [PubMed] [Google Scholar]
- 9.Parikh-Das AM, Sharma NC, Du Q, Charles CA. Superiority of essential oils versus 0.075% CPC-containing mouthrinse: A two-week randomized clinical trial. J Clin Dent. 2013;24:94–9. [PubMed] [Google Scholar]
- 10.Parwani SR, Parwani RN, Chitnis PJ, Dadlani HP, Prasad SV. Comparative evaluation of anti-plaque efficacy of herbal and 0.2% Chlorhexidine gluconate mouthwash in a 4-day plaque re-growth study. J Indian Soc Periodontol. 2013;17:72–7. doi: 10.4103/0972-124X.107478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ernst CP, Prockl K, Willershausen B. The effectiveness and side effects of 0.1% and 0.2% chlorhexidine mouthrinses: A clinical study. Quintessence Int. 1998;29:443–8. [PubMed] [Google Scholar]
- 12.Goenka P, Sarawgi A, Karun V, Nigam AG, Dutta S, Marwah N. Camellia sinensis (Tea): Implications and role in preventing dental decay. Phcog Rev. 2013;7:152–6. doi: 10.4103/0973-7847.120515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Venkateswara B, Sirisha K, Chava VK. Green tea extract for periodontal health. J Indian Soc Periodontol. 2011;15:18–22. doi: 10.4103/0972-124X.82258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lu Y, Foo Y. Antioxidant activities of polyphenols from sage (Salvia officinalis) Food Chem. 2001;75:197–202. [Google Scholar]
- 15.Pizzale L, Bortolomeazzi R, Vichi S, Conte LS. Antioxidant activity of sage and oregano extracts related to their phenolic compound content. J Sci Food Agric. 2002;82:1645–51. [Google Scholar]
- 16.Savithramma N, Linga RM, Bhumi G. Phytochemical screening of Thespesia populnea (L.) Soland and Tridax procumbens L. J Chem Pharm Res. 2011;3:2834. [Google Scholar]
- 17.Siddiqui AA, Ali M. 1st ed. New Delhi: CBS Publisher and Distributors; 1997. Practical Pharmaceutical Chemistry; pp. 126–31. [Google Scholar]
- 18.Brinda P, Sasikala P, Purushothaman KK. Pharmacognostic studies of Merugan kizhangu. Bull Med Ethics Bot Res. 1981;3:84–96. [Google Scholar]
- 19.Hsiao G, Teng CM, Wu CL, Ko FN. Marchantin H as a natural antioxidant and free radical scavenger. Arch Biochem Biophys. 1996;334:18–26. doi: 10.1006/abbi.1996.0424. [DOI] [PubMed] [Google Scholar]
- 20.Lee SE, Hwang HJ, Ha JS, Jeong HS, Kim JH. Screening of medicinal plant extracts for antioxidant activity. Life Sci. 2003;73:167–79. doi: 10.1016/s0024-3205(03)00259-5. [DOI] [PubMed] [Google Scholar]
- 21.McDonald S, Prenzler PD, Antolovich M, Robards K. Phenolic content and antioxidant activity of olive extracts. Food Chem. 2001;73:73–84. [Google Scholar]
- 22.Mervat MM, Far EL, Hanan A, Taie A. Antioxidant activities, total Anthocyanins, phenolics and flavonoids contents of some Sweet potato genotypes under stress of different concentrations of sucrose and sorbitol. Aust J Basic Appl Sci. 2009;3:3609–16. [Google Scholar]
- 23.Siddiqua A, Premakumari KB, Sultana R, Vithya, Savitha Antioxidant activity and estimation of total phenolic content of muntingia calabura by colorimetry. Int J Chem Tech Res. 2010;2:205–8. [Google Scholar]
- 24.Degenhardt A, Knapp H, Winterhalter P. Separation and purification of anthocyanins by high-speed countercurrent chromatography and screening for antioxidant activity. J Agric Food Chem. 2000;48:338–43. doi: 10.1021/jf990876t. [DOI] [PubMed] [Google Scholar]
- 25.Erturk O, Kati H, Yayli N, Demürbaú Z. Antimicrobial properties of Silene multifida (Adams) Rohrb. Plant extracts. Turk J Biol. 2006;30:17–21. [Google Scholar]
- 26.Tariq AL, Reyaz AL. Phytochemical analysis of Camellia sinensis leaves. Int J Drug Dev Res. 2012;4:311–6. [Google Scholar]
- 27.Karpinski T, Szkaradkiewicz A. Microbiology of dental caries. J Biol Earth Sci. 2013;3:M21–4. [Google Scholar]
- 28.Tehrani MH, Asghari G, Hajiahmadi M. Comparing Streptococcus mutans and Lactobacillus colony count changes following green tea mouth rinse or sodium fluoride mouth rinse use in children (randomized double-blind controlled clinical trial) Dent Res J (Isfahan) 2011;8:S58–63. [PMC free article] [PubMed] [Google Scholar]


