Abstract
In this work, we present a novel ascorbic acid (AA) sensor applied to the detection of AA in human sera and pharmaceuticals. A series of Au nanoparticles (NPs) and graphene oxide sheets (Au NP/GO) composites were successfully synthesized by reduction of gold (III) using sodium citrate. Then the Au NP/GO composites were used to construct nonenzymatic electrodes in practical AA measurement. The electrode that has the best performance presents attractive analytical features, such as a low working potential of +0.15 V, a high sensitivity of 101.86 μA mM−1 cm−2 to AA, a low detection limit of 100 nM, good reproducibility and excellent selectivity. And more,it was also employed to accurately and practically detect AA in human serum and clinical vitamin C tablet with the existence of some food additive. The enhanced AA electrochemical properties of the Au NP/GO modified electrode in our work can be attributed to the improvement of electroactive surface area of Au NPs and the synergistic effect from the combination of Au NPs and GO sheets. This work shows that the Au NP/GO/GCEs hold the prospect for sensitive and selective determination of AA in practical clinical application.
Ascorbic acid (AA), commonly called vitamin C, is one of the most important nutrients and effective antioxidant in protecting human from oxidative stress1,2,3. Today, AA is widely used in biomedical chemistry, diagnostics and the identification of food ingredients. The concentration of AA is usually kept in a low level, so accurate detection of AA content is of great importance to guarantee health conditions and food security4,5. However, some other accompanied electroactive compounds of similar electrochemical properties with AA, such as dopamine (DA) and uric acid (UA) glucose, and other oxidizable species, complicate their electrochemical identification, especially in biological environment6,7. Hence, the clear separation of the electrochemical signals of AA from these compounds is still a great challenge.
In the past few decades, electrochemical techniques have been used to detect small biomolecules owing to their high sensitivity, rapid response, flexibility, and low expense8,9. In an electrochemical system, the AA voltammetric peak can be separated from that of interfering species using functional material modified electrodes10,11. Compounding two different reasonable selected materials is an effective way to further improve the electrochemical properties12,13,14. Specifically, GO sheets have been considered as a suitable substrate for assembling nanomaterials because of their large surface area, excellent thermal and mechanical properties and chemical modification capability. In addition, they exhibit specific properties such as hydrophilicity, multiple oxygen moieties, and controllable electronic properties15,16,17,18. Meanwhile, in recent years, Au NPs have been widely investigated and applied to molecular catalysis, biosensors, and mutifunctional reagents due to their quantum size effect19,20,21. Therefore, there is a potential development of a non-enzymatic electrochemical biosensor for AA involving the complementary use of GO sheets and Au NPs. For example, Tian et al. used a Au NP-β-cyclodextrin-graphene modified electrode to detect AA, DA and UA22. The detection limit for AA (based on S/N = 3) is 10 μM and the linear response range is 30–2000 μM. Zhang et al. used a facile one-step method to fabricate nanoflower-like dendritic Au NPs and polyaniline composite nanosheets and applied them on glass carbon electrode as an AA sensor14. A linear relation between the current response and the AA concentration was obtained between 10 μM and 12 mM, with a detection limit of 8.3 μM (S/N = 3) and the sensitivity of 25.69 μA mM−1 cm−2. However, there have been very few reports on the detection of AA in real-life human sera or pharmaceuticals to demonstrate the practical applications of AA biosensors.
In this work, we have chemically reduced Au(III) using sodium citrate to form Au NPs of approximately 13–16 nm average diameter on GO sheets23. These Au NP/GO composite nanomaterials were then immobilized on the glass carbon electrodes (GCE) for AA detection. These modified electrodes were used to monitor AA concentration in real-life human sera and pharmaceuticals.
Results and Discussion
Characterization of the Au NP/GO composites
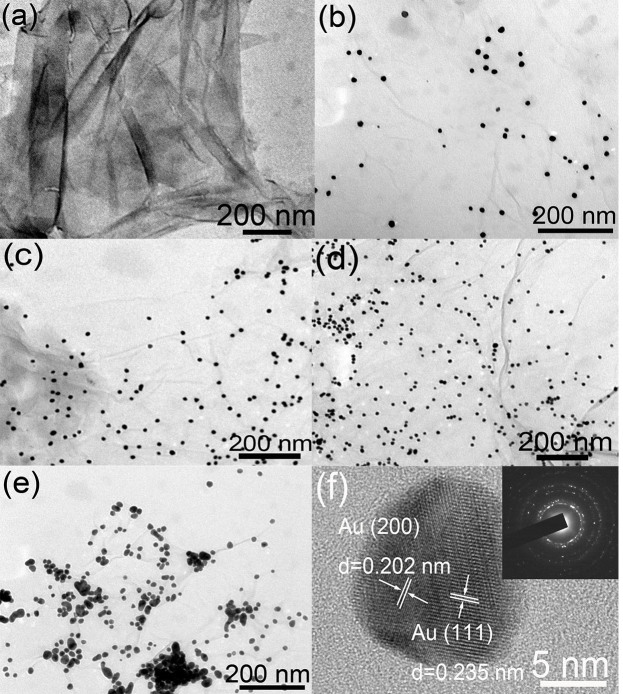
The morphology of the GO sheets used in this work was first examined by transmission electron microscope (TEM) technique, and the results are shown in Fig. 1a. The GO sheets exhibit flake-like shape and is few-layer flexible wrinkled. The smooth and planar surface indicates that GO sheets can provide a high surface to volume ratio and a 2D structure, which is necessary for loading Au NPs. Then the GO sheets were surface loaded with different amount of Au NPs (Fig. 1b–1e). As can be seen, the loaded Au demonstrates regular small NP morphology and can grow on the GO sheet independently. The size histograms of Au NPs in each sample were carefully measured and shown in Fig. S2. The size distribution of Au NPs shows a slightly growing trend from S1 to S4 and the average size of S1, S2, S3 and S4 are determined to be 13.3, 14.2, 15.3 and 16.0 nm, respectively. Besides, as the amount of initial HAuCl4 gradually increased, the amount of Au NPs loaded on the GO sheets also increased. Note that when the amount of initial HAuCl4 reached 20 mg, the Au NPs loaded on the GO sheet tended to aggregate together (Fig. 1e). Fig. 1f shows the high resolution TEM (HRTEM) image, in which the interplanar distances can be clearly seen. The as marked interplanar distances are 0.235 nm and 0.202 nm, corresponding to the (111) and (200) face of face-centered cubic Au, respectively. The inset of Fig. 1f is the selected area electron diffraction (SAED) pattern of Au NP/GO composites (S3). The diffraction patterns irregularly distribute and the diffraction rings can be observed, which indicates this sample yields a polycrystalline structure24. The conditions of the other samples are the same.
Figure 1.
TEM images of (a) GO sheets. (b–e) Au NP/GO composites based on S1, S2, S3 and S4. (f) HRTEM image of Au/GO composite based on S3, the inset is the corresponding SEAD pattern.
The Energy-dispersive X-ray spectra (EDX) were measured to characterize the elemental heterogeneity of Au NP/GO composites (S1, S2, S3 and S4), as shown in Fig. S1 (see in supporting information). The representation of Au, C and O elements further indicates the existence of GO and Au. The strong Cu and Si peaks are attributed to the substrates. The crystal structure of Au NP/GO composites (S1, S2, S3 and S4) was characterized by X-ray diffraction (XRD) patterns, and the results obtained are shown in Fig. 2a. All the four Au NP/GO composites show similar XRD peaks, which well match to the face-centered cubic crystalline Au (JCPDS 04-0784) and no any trace of other phases is detected. This indicate that the increasing concentration of HAuCl4 bring no influence to the crystal structure. Since the GO powder does not have a crystal structure, no obvious characteristic peak of GO can be distinguished.
Figure 2.
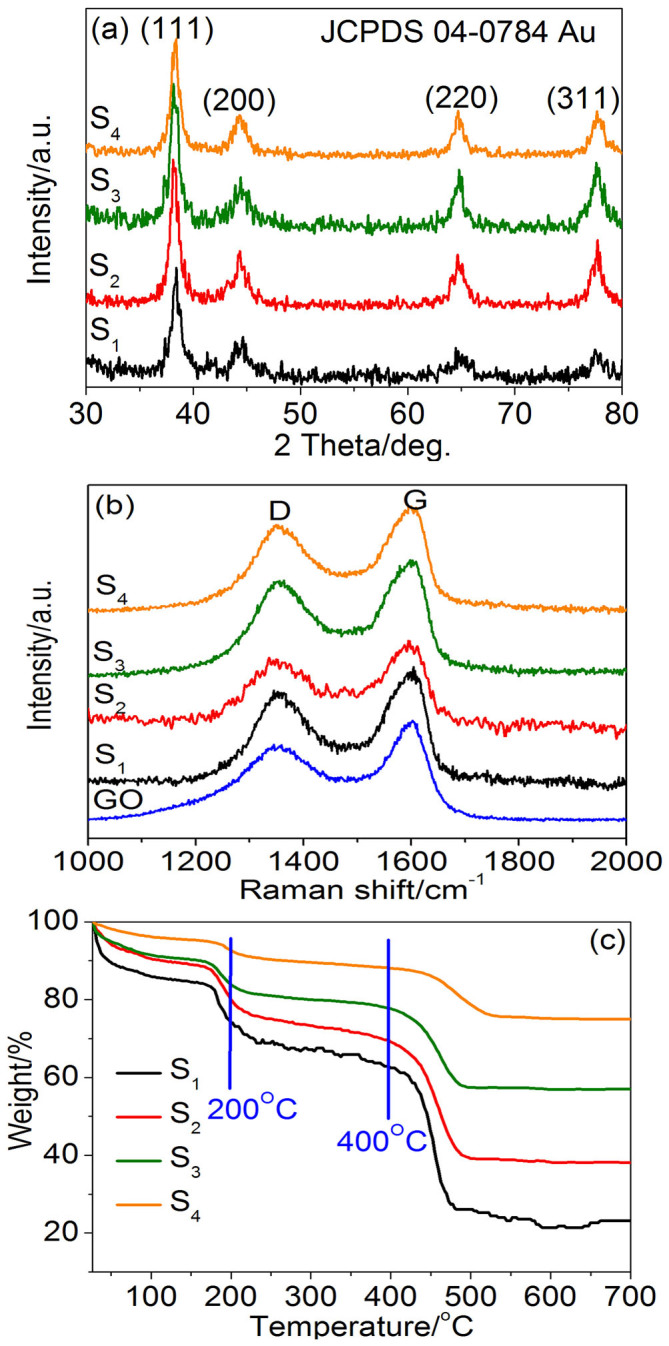
(a) XRD patterns of Au/GO composites S1, S2, S3 and S4. (b) Raman spectra of GO and Au/GO composites. (c) TGA curves of S1, S2, S3 and S4 samples.
The Raman spectra of Au NP/GO composites compared with pure GO are shown in Fig. 2b. In the studied range, all the samples have two similar characteristic Raman peaks, which belong to the D and G bands of GO sheets. The disorder-induced D bands at 1351 cm−1 arising from sp3-hybridized carbon were observed25. The tangential stretch G band represents the E2g zone center mode of the crystalline graphite. Generally, the relative intensity ratio of the D and G bands (ID/IG) depends strongly on the degree of disorder in the graphitic material26. Generally, the ID/IG ratio increases when more defects are brought into GO. According to Fig. 2b, the ID/IG ratios of the four Au NP/GO composites are calculated to be 0.80, 0.82, 0.81 and 0.82, respectively, which are a little higher than that of pure GO sheet (0.77). This shows that the loading amount of Au NPs has only a little influence on the ID/IG ratio, implying that the nucleation of Au NPs at GO surfaces introduces very few defects into the GO structure.
To further confirm the concentration of Au and GO in each composite sample, thermogravimetric analysis (TGA) curves were investigated. As shown in Fig. 2c, the mass loss below 200°C (25.7%, 19.8%, 16.2% and 8.2% for S1, S2, S3, and S4, respectively) can be attributed to the evaporation of adsorbed water. The slight mass loss between 200°C and 400°C (12.4%, 10.3%, 6.0% and 4.4% for S1, S2, S3, and S4, respectively) arose from the decomposition of some residual oxygen-containing groups27. Then the finial significant mass loss occurred when the Au NP/GO composites were heated above 400°C (38.6%, 30.8%, 20.5 and 12.9% for S1, S2, S3, and S4, respectively). This was most likely caused by the decomposition of carbon skeleton from GO27. Accordingly, the mass percentage of Au in S1, S2, S3 and S4 are calculated to be 24.0%, 39.0%, 57.4% and 75.5%, respectively, which also implies the successful loading of Au NPs on GO sheets.
Enhanced electrochemistry behavior of Au NP/GO/GCEs toward AA detection
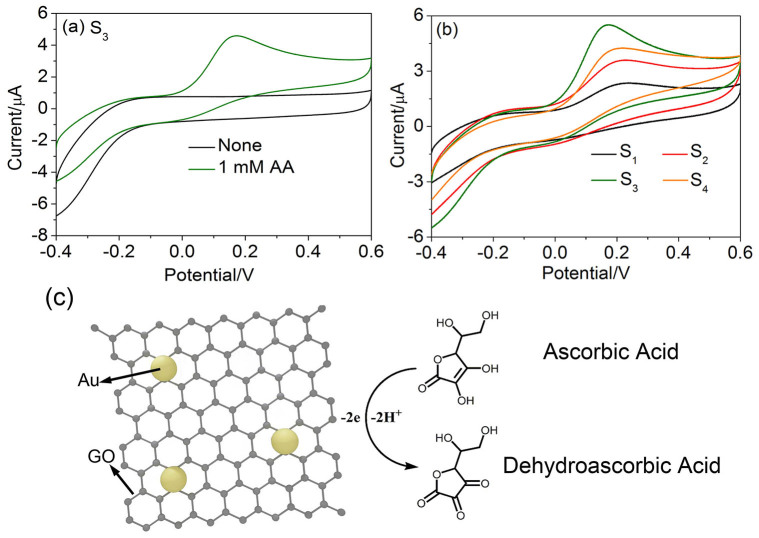
To determine the working condition of effective detecting AA, the Au/GO/GCEs were first characterized by cyclic voltammetry (CVs) between the potentials of −0.4 and +0.6 V in 0.1 M phosphate-buffered solution (PBS) at a scan rate of 100 mV/s. First the CVs in the absence (shown in black trace) and presence (shown in green trace) of 1 mM AA of different electrodes were measured and shown in Fig. 3a. To compare, the CV curves at a bare electrode and a GO/GCE was conducted and the results obtained are shown in Fig. S3 (supporting information). As can be observed, the position of oxidation peak of AA for bare electrode is about +0.3 V, corresponding to the irreversible oxidation of AA (see Fig. S3a). After surface modified by pure GO sheets (Fig. S3b), no clear response current change can be found after addition of AA. The reason can be summarized as the electrical conductivity of GO is not as good as that of graphene and other metal materials which contain much free electrons. However, after loading Au NPs on GO sheets (take S3 for example, Fig. 3a), a broad and clear oxidation peak with a peak potential of about +0.15 V was observed again after the addition of 1 mM AA and the potential value was one time lower than that of bare GCE. The appearance and shift of the oxidation peak in Au NP/GO/GCE can be attributed to the synergistic effects between Au NPs and GO sheets. Since the interfering substances like UA, DA, and NADH are always coexisted with AA and the oxidation peak position of them are usually around +0.2 V to +0.5 V6,22,28. The decrease of the peak position of Au/GO/GCE (from +0.3 V to +0.15 V) is important for avoiding the influence. Particularly, the oxidation peak might correspond to the oxidation reaction of AA occurring on the electrode surface. The electro-oxidation mechanism of AA is illustrated in Fig. 3c. The AA molecules in the solution were adsorbed onto the surface of Au NP/GO/GCE. Then the AA molecules hydrolyzed with water were oxidized to dehydroascorbic acid29. The oxidation process of AA can release electrons and the current can be detected at a constant working voltage.
Figure 3.
CV curves of (a) Au/GO composite (S3) in 0.1 M PBS solution with the absence (black trace) and presence (green trace) of 1 mM AA. (b) Au/GO composites (S1, S2, S3 and S4) in 0.1 M PBS solution with the present of 1 mM AA. (c) Schematic illustration of AA sensing mechanism by using Au/GO composite.
In addition, the Au NP/GO/GCEs based on different samples (S1, S2, S3, and S4) were further investigated and compared, which can give more information about the difference of the electron transfer kinetics on the electrode surface. As shown in Fig. 3b, all the Au/GO/GCEs appear similar broad reduction peak round +0.15 V. With increasing of loading amount of Au NPs, the corresponding peak currents of the Au NP/GO/GCEs also show a rising trend at first and reach the maximum value at Au NP/GO/GCE based on S3. After that, when further increasing the loading amount of Au NPs (S4) the peak current shows a decreased trend, the reason can be ascribed to the aggregation of Au NPs on GO sheets as shown in Fig. 1d, which may cause the decline of surface area. The peak current values of Au NP/GO/GCEs based on S1, S2, S3 and S4 samples were measured to be 2.4, 3.6, 5.5 and 4.2 μA, respectively. That is to say, Au/GO/GCE based on S3 exhibits the best performance for AA detection, which can be attributed to the enough loading amount and well dispersion of Au NPs on GO sheets.
The electrochemical controlled process is further studied through the CVs of 0.1 M PBS solution at a S3 Au NP/GO/GCE at different scan rates after injection of 1 mM AA, as depicted in Fig. S4. The redox peak currents increase linearly with the scan rate in the range of 50–250 mV/s, indicating a surface-controlled electrochemical process30.
Amperometric detection of AA of Au NP/GO/GCEs
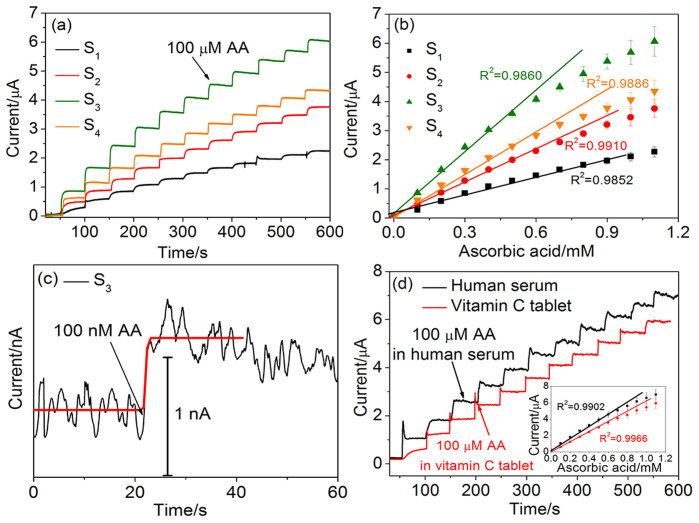
To determine the amperometric sensing application of Au/GO/GCEs, current-time (I-t) curves were performed at +0.15 V (vs. Ag/AgCl) in 0.1 M PBS solution. Fig. 4a shows the typical amperometric responses of S1, S2, S3 and S4 by successive addition of certain amount of AA. Well-defined, stable and fast amperometric response could be observed. Similar with the CVs behavior, the Au NP/GO/GCE based on S3 shows the best sensitive response with the change of AA concentration. The calibration curves corresponding to four Au/GO/GCEs are shown in Fig. 4b, and all these electrodes display good linearity. According to the calibration curves, the sensitivity,linearity range and response time of each electrodes were calculated and listed in Table 1. The linear regression equations were carefully calculated and shown in Table 2. As is listed, with the increasing amount of loaded Au NPs on GO sheets (from S1 to S3), the corresponding sensitivity gradually increases and the detection limit gradually reduces. The Au NP/GO/GCE based on S4 tend to decrease on sensitivity since the Au NPs on the GO sheets started to aggregate together. The response times of Au/GO/GCEs based on S1, S2, S3 and S4 are determined after injected in 100 μM AA, respectively. As can be clearly seen in Table 1, the increasing amount of Au NPs can significantly improve the response speed. Fig. 4c shows the obvious amperometric response of Au NP/GO/GCE based on S3 after the addition of 100 nM AA with a signal-to-noise ratio (S/N) of ~3 in order to obtain the accurate detection limit. This result is much lower than the previously reported graphene-based electrochemical sensors and electrical sensors assisted by enzymes as listed in Table 3. Besides, as compared, the Au NP/GO/GCE based on S3 in this work also shows high sensitivity, low detection limit and large liner range. According to the sensitivity improvement, two factors need to be considered: On the one hand, the amount of Au NPs influences dominantly on the performance when they are gradually increased. The reason can be attributed to the good electrical conductivity and large surface area introduced by Au NPs. The Au NPs on S4 tend to aggregate together so the surface area decreases and the sensitivity is negatively influenced. On the other hand, the GO sheets can provide a large area for the Au NPs loading, so they can grow independently without aggregating in a certain amount range. So the GO sheets also make a great contribution to the improvement of the sensitivity.
Figure 4.
Amperometric response of (a) S1, S2, S3 and S4 to successive additions of 0.1 mM AA at an applied potential of 0.15 V. (b) The corresponding calibration curves of (a). (c) S3 to the additions of 100 nM AA at an applied potential of +0.15 V. (d) S3 to successive additions of human serum (black trace) and Vitamin C tablet (red trace) contained 100 μM AA. Inset is the corresponding calibration curves of (d).
Table 1. A list of AA sensing properties of different electrodes at an applied potential of 0.15 V.
| Sample | Sensitivity/μA mM−1 cm−2 | R2 | Linear range | Response time/s |
|---|---|---|---|---|
| S1 | 29.05 | 0.9852 | 1.21 μM to 1 mM | 12.5 |
| S2 | 53.39 | 0.9910 | 0.86 μM to 0.8 mM | 7.1 |
| S3 | 101.86 | 0.9860 | 0.11 μM to 0.6 mM | 4.2 |
| S4 | 68.53 | 0.9886 | 0.39 μM to 0.7 mM | 4.6 |
Table 2. Particle size, Au to carbon ratio and linear regression equations for S1, S2, S3 and S4.
| Particle Size/nm | Concentration of Au | Linear Regression Equations | |
|---|---|---|---|
| S1 | 13.3 | 24.0% | I (μA) = 2.03431c (mM) + 0.16937 |
| S2 | 14.2 | 39.0% | I (μA) = 3.73879c (mM) + 0.13651 |
| S3 | 15.8 | 57.4% | I (μA) = 7.13305c (mM) + 0.11692 |
| S4 | 16.0 | 75.5% | I (μA) = 4.79901c (mM) + 0.09919 |
Table 3. Comparison of the Au/GO/GCEs with other AA sensors.
| Electrodes | Working potential/V | Sensitivity/μA mM−1 cm−2 | Detection limit/μM | Ref. |
|---|---|---|---|---|
| Au/GO/GCE | 0.15 | 101.86 | 0.11 | This work |
| SiW12-CNTs-PAn | 0.4 | 22.11(0–10 μM) 266.5(0.01–9 mM) | 0.51 | 31 |
| Au-PANI | 0.2 | 25.69 | 8.2 | 14 |
| P-APTT | 0.255 | 140.8 ± 14.08 | 0.14 | 32 |
| EMGON5-1 | 0.38 | 78.63 | 1.54 | 33 |
| 3DGF/CuO nanoflowers | 0.2 | 2060 | 0.43 | 7 |
| IrOx nanofibers | −0.01 | 194.4 ± 6.8 | 0.4 | 10 |
Moreover, the practical AA sensing ability of Au NP/GO/GCE based on S3 was further investigated in real human serum with Au NP/GO/GCE based on S3 and in clinical vitamin C tablet. As shown in Fig. 4d, the I–t curve corresponds to human serum was obtained by adding human serum contained 100 μM AA, and the I–t curve corresponds to vitamin C tablet was gotten by addition of aqueous solution of vitamin C tablet which contained 100 μM AA. As can be seen, the amperometric response of the vitamin C tablet is slightly lower than that in human serum. It may caused by the food additives in vitamin C tablet which may attach on and then fouling the electrode surface. The inset of Fig. 4d shows the calibration curves and both of them display good linearity in the range of 0.1–0.6 mM. The sensitivities of the Au NP/GO/GCE based on S3 are calculated to be 108.82 μAmM−1cm−2 and 89.76 μAmM−1cm−2 corresponding to the human serum and vitamin C tablet environments. The error ranges are 106.87% and 88.12% for the human serum and vitamin C tablet environments after calculated.
Reproducibility, stability, anti-interference property of the Au NP/GO/GCEs
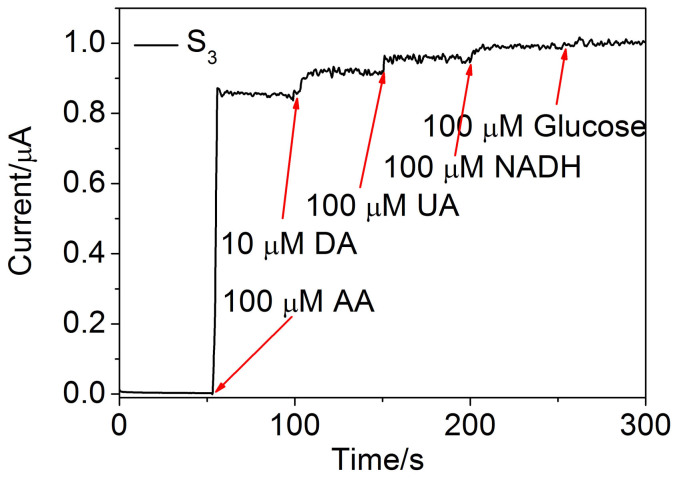
The reproducibility, stability and anti-interference property of Au NP/GO/GCE based on S3 were further evaluated. For reproducibility, five same Au NP/GO/GCEs based on S3 were investigated at +0.15 V to compare their amperometric current responses. The relative standard deviation (R.S.D.) was 8.4%, confirming that the preparation method was highly reproducible. The stability of Au NP/GO/GCE based on S3 was tested once per week in 0.1 M PBS solution at a scan rate of 100 mV/s and lasted for a month (data not shown). The sensitivity decrease of the electrode was no more than 10%, indicating a good stability of Au NP/GO/GCE. Since there are many interfering substances coexist with AA in the reported normal physiological concentration, the selectivity will be a big restrictive factor for the measurement34,35,36,37. Here, anti-interference property were carried out by successive injection of 100 μM AA, UA, NADH, glucose and 10 μM DA depending on the normal physiological concentration, which are the most common coexistence material of AA. The normal physiological concentration for DA is about 100 nM34, which is 100 times lower than the concentration we injected. The CV curves obtained by detecting UA, DA and NADH independently or simultaneously were first shown in Fig. S5 in supporting information. As seen in Fig. S5a, the oxidation peak positions of UA, DA, and NADH are +0.3 V, +0.4 V, and +0.55 V, respectively, which are much higher than that of AA (+0.15 V). When the 1 mM UA, 0.1 mM DA, and 1 mM NADH coexist with 1 mM AA, the CV curve shows an obvious oxidation peak at +0.15 V, corresponding to oxide peak of AA. This indicates that the other interfering substances have a little influence on that of AA. In order to further prove the fact, amperometric response of S3 modified electrode at +0.15 V with successive additions of different analytes are shown in Fig. 5, and the current responses of interfering species are also calculated accurately. Compared to AA, the current responses of interfering species are determined to be 4.7% (100 μM UA), 3.5% (100 μM NADH) and 8.2% (10 μM DA) at +0.15 V. It can be concluded that similar amount of these interfering substances can be neglected. The excellent sensitivity indicates the potential practical application of the as-prepared AA sensor.
Figure 5. Amperometric response of S3 at +0.15 V with successive additions of different analytes.
Conclusions
In conclusion, we have successfully synthesized a series of Au NP/GO composites. The loading amount of Au NPs on GO sheets control of Au NP/GO composite has been investigated and the as–prepared samples are used to detect AA. The optimal sample of these Au NP/GO composites sheets is S3 which presents high sensitivity and low detection limit compared to the other samples. The sensor also shows good reproducibility, excellent selectivity and accurate measurement in real serum sample and vitamin C tablet. The reason can be attributed to the improvement of electroactive surface area of Au NPs on GO sheets and the synergistic effect from the combination of Au NPs and GO. It is anticipated that the Au NP/GO composite material holds great potential for developing novel AA sensors.
Methods
Materials
All chemicals used were analytical grade and without further purification. GO sheets were purchased from Nanjing XFNANO Materials Tech Co., Ltd. KH2PO4 (99.5%), KCl (99%), Na2HPO4 (99%), NaCl (99.5%) and sodium citrate (C6H5Na3O7·2H2O, 99%) were purchased from Beijing Chemical Works. HAuCl4·3H2O (99.9%), Chitosan (93%), AA (99%), DA (99%), UA (99%) and NADH (97%) were supplied by Sigma-Aldrich. PBS (0.1 M, pH 7.4) was prepared by dispersing 0.2 g KCl, 8 g NaCl, 0.2 g KH2PO4 and 1.54 g Na2HPO4 in 1 L deionized water.
Preparation of Au NP/GO composite
To synthesize Au NP/GO composites, 4 mg GO was dispersed in 50 mL deionized water under magnetic stirring at room temperature for 1 h, before 5, 10, 15 and 20 mg HAuCl4 was added, respectively. These corresponding composite suspensions were then labeled as S1, S2, S3 and S4. The composite suspensions were left for 30 min to promote the interaction of gold ions with the graphene surface. Next, 5 mL sodium citrate solution (with a sodium citrate to deionized water mass ratio of 0.02) was added under magnetic stirring for 30 min. Then the solution was heated to 80°C and kept for 1 h. Finally, the nanocomposites were obtained by washing the corresponding solution for 3 times with distilled water and by centrifugating (7000 rpm) to remove the free Au NPs formed in solution. The final Au NP/GO modified composites were collected after placed in a 60°C oven for 12 h. Moreover, the Au NP/GO was synthesized in 100°C for comparison and examined by TEM technique shown in Fig. S6.
Preparation of Au NP/GO modified electrodes
Before surface modification, a GCE (dia. 3 mm) was polished with 1 μm and 0.05 μm alumina slurries sequentially, and was rinsed with deionized water. Finally, the electrode was sonicated in nitric acid (0.2 M), acetone, ethanol, and deionized water, respectively. Then the GCE was placed in 0.5 M H2SO4 and 100 cyclic voltammetry scans were conducted between −1.0 and +1.0 V to introduce some active functional groups (e.g. hydroxy and carboxyl) on the electrode surface22. This GCE was then washed and dried at room temperature and was ready for modification. In the solution used for electrode modification, a mixture of 5 mg Au NP/GO and 1 mL ethanol was sonicated for 1 h. Then 5 μL different suspensions were applied to the GCE, respectively. After that, 5 μL of 0.2 wt% chitosan solution was applied to the electrode surface to immobilize the Au NP/GO. The as-prepared electrodes (denote as Au NP/GO/GCE) were air-dried at room temperature before usage.
Apparatus
The TEM and HRTEM images of the samples were inspected using JEM-2010 (JEOL, Japan) under a working voltage of 200 kV. XRD patterns were conducted on a Rigaku D/max 2550 X-ray diffractometer, using a mono-chromatized Cu target radiation source (λ = 1.54 Å) (Japan). TGA data were acquired on a SDT 2960 differential thermal analyzer (TA Instruments, New Castle, DE) at a heating rate of 10°C/min in air. Resonance Raman spectra were measured on an inVia H30434 Raman spectrophotometer (Renishaw, England). Electrochemical measurements were performed on a model CHI630D electrochemical analyzer (ChenHua Instruments Co. Ltd., Shanghai, China). All experiments were conducted using a three-electrode electrochemical cell consisting of a glassy carbon working electrode, a Ag/AgCl reference electrode and a platinum wire counter electrode.
Author Contributions
J.S. conducted the most of investigation for the samples and wrote the main paper. L.X. supervised the project, had given valuable advices on the proceeding of this work, and revised the manuscript. D.L.L. and H.W.S. had provided precious suggestions on the selection of test. R.Q.X., Q.L.L. and C.Y.Z. supported the characterization of the samples. All authors discussed the results and commented on the manuscript at all stages.
Supplementary Material
Supporting information
Acknowledgments
This work was supported by NSFC (Grant no. 61204015, 81301289, 61177042), Program for Chang Jiang Scholars and Innovative Research Team in University (No. IRT13018). The China Postdoctoral Science Foundation Funded Project (2012M511337 and 2013T60327), the State Key Laboratory of Bioelectronics of Southeast University, the Jilin Province Natural Science Foundation of China (No. 20140101171JC), and Graduate Innovation Fund of Jilin University (No. 2014060).
References
- Agius F. et al. Engineering increased vitamin C levels in plants by overexpression of a D-galacturonic acid reductase. Nat. Biotechnol. 21, 177–181 (2003). [DOI] [PubMed] [Google Scholar]
- Green M. & Fry S. Vitamin C degradation in plant cells via enzymatic hydrolysis of 4-O-oxalyl-L-threonate. Nature 433, 83–87 (2005). [DOI] [PubMed] [Google Scholar]
- Padayatty S. J. et al. Vitamin C as an antioxidant: Evaluation of its role in disease prevention. J. Am. Coll. Nutr. 22, 18–35 (2003). [DOI] [PubMed] [Google Scholar]
- Yang J. J., Wu J. Z. & Qiao C. H. Expedient Synthesis of Epigoitrin from L-Ascorbic Acid. Synthetic Commun. 44, 1240–1244 (2014). [Google Scholar]
- Bautista-Ortega J., Cortes-Cuevas A., Ellis E. A. & Ruiz-Feria C. A. Supplemental (L)-arginine and vitamins E and C preserve xanthine oxidase activity in the lung of broiler chickens grown under hypobaric hypoxia. Poultry Sci. 93, 979–988 (2014). [DOI] [PubMed] [Google Scholar]
- Sun C. L., Lee H. H., Yang J. M. & Wu C. C. The simultaneous electrochemical detection of ascorbic acid, dopamine, and uric acid using graphene/size-selected Pt nanocomposites. Biosens. Bioelectron. 26, 3450–3455 (2011). [DOI] [PubMed] [Google Scholar]
- Ma Y. et al. 3D graphene foams decorated by CuO nanoflowers for ultrasensitive ascorbic acid detection. Biosens. Bioelectron. 59, 384–388 (2014). [DOI] [PubMed] [Google Scholar]
- Ammam M. Electrochemical and electrophoretic deposition of enzymes: Principles, differences and application in miniaturized biosensor and biofuel cell electrodes. Biosens. Bioelectron. 58, 121–131 (2014). [DOI] [PubMed] [Google Scholar]
- Wang X. et al. Direct, reagentless electrochemical detection of the BIR3 domain of X-linked inhibitor of apoptosis protein using a peptide-based conducting polymer sensor. Biosens. Bioelectron. 61, 57–62 (2014). [DOI] [PubMed] [Google Scholar]
- Kim S. J. et al. Electrospun iridium oxide nanofibers for direct selective electrochemical detection of ascorbic acid. Sens. Actuat. B-Chem. 196, 480–488 (2014). [Google Scholar]
- Sun C. L. et al. Microwave-Assisted Synthesis of a Core-Shell MWCNT/GONR Heterostructure for the Electrochemical Detection of Ascorbic Acid, Dopamine, and Uric Acid. Acs Nano 5, 7788–7795 (2011). [DOI] [PubMed] [Google Scholar]
- Zhuang Z. B., Sheng W. C. & Yan Y. S. Synthesis of Monodispere Au@Co3O4 Core-Shell Nanocrystals and Their Enhanced Catalytic Activity for Oxygen Evolution Reaction. Adv. Mater. 26, 3950–3955 (2014). [DOI] [PubMed] [Google Scholar]
- Shearer C. J., Cherevan A. & Eder D. Application and Future Challenges of Functional Nanocarbon Hybrids. Adv. Mater. 26, 2295–2318 (2014). [DOI] [PubMed] [Google Scholar]
- Zhang H. et al. Fabrication of nanoflower-like dendritic Au and polyaniline composite nanosheets at gas/liquid interface for electrocatalytic oxidation and sensing of ascorbic acid. Electrochem. Commun. 30, 46–50 (2013). [Google Scholar]
- Novoselov K. S. et al. Electric field effect in atomically thin carbon films. Science 306, 666–669 (2004). [DOI] [PubMed] [Google Scholar]
- Mohanty N. & Berry V. Graphene-Based Single-Bacterium Resolution Biodevice and DNA Transistor: Interfacing Graphene Derivatives with Nanoscale and Microscale Biocomponents. Nano Lett. 8, 4469–4476 (2008). [DOI] [PubMed] [Google Scholar]
- Henriksen E. A. et al. Cyclotron resonance in bilayer graphene. Phys. Rev. Lett. 100, 087403 (2008). [DOI] [PubMed] [Google Scholar]
- Zhu Y. W. et al. Carbon-Based Supercapacitors Produced by Activation of Graphene. Science 332, 1537–1541 (2011). [DOI] [PubMed] [Google Scholar]
- Zope B. N., Hibbit D. D., Neurock M. & Davis R. J. Reactivity of the Gold/Water Interface During Selective Oxidation Catalysis. Science 330, 74–78 (2010). [DOI] [PubMed] [Google Scholar]
- Yan Y. M. et al. Biocatalytic growth of Au nanoparticles immobilized on glucose oxidase enhances the ferrocene-mediated bioelectrocatalytic oxidation of glucose. Adv. Mater. 20, 2365–2370 (2008). [Google Scholar]
- Cecchini M. P. et al. Self-assembled nanoparticle arrays for multiphase trace analyte detection. Nat. Mater. 12, 165–171 (2013). [DOI] [PubMed] [Google Scholar]
- Tian X. et al. Simultaneous determination of L-ascorbic acid, dopamine and uric acid with gold nanoparticles-beta-cyclodextrin-graphene-modified electrode by square wave voltammetry. Talanta 93, 79–85 (2012). [DOI] [PubMed] [Google Scholar]
- Goncalves G. et al. Surface Modification of Graphene Nanosheets with Gold Nanoparticles: The Role of Oxygen Moieties at Graphene Surface on Gold Nucleation and Growth. Chem. Mater. 21, 4796–4802 (2009). [Google Scholar]
- Tesler Alexander B. et al. Solid-State Thermal Dewetting of Just-Percolated Gold Films Evaporated on Glass: Development of the Morphology and Optical Properties. J. Phys. Chem. C 117, 11337–11346 (2013). [Google Scholar]
- Zhao Y., Song X., Song Q. & Yin Z. A facile route to the synthesis copper oxide/reduced graphene oxide nanocomposites and electrochemical detection of catechol organic pollutant. CrystEngComm. 14, 6710–6719 (2012). [Google Scholar]
- Zhou M., Zhai Y. & Dong S. Electrochemical Sensing and Biosensing Platform Based on Chemically Reduced Graphene Oxide. Anal. Chem. 81, 5603–5613 (2009). [DOI] [PubMed] [Google Scholar]
- Chen H. et al. Mechanically strong, electrically conductive, and biocompatible graphene paper. Adv. Mater. 20, 3557–3561 (2008). [Google Scholar]
- Shang N. G. et al. Catalyst-Free Efficient Growth, Orientation and Biosensing Properties of Multilayer Graphene Nanoflake Films with Sharp Edge Planes. Adv. Funct. Mater. 18, 3506–3514 (2008). [Google Scholar]
- Hu G., Ma Y., Guo Y. & Shao S. Electrocatalytic oxidation and simultaneous determination of uric acid and ascorbic acid on the gold nanoparticles-modified glassy carbon electrode. Electrochim. Acta 53, 6610–6615 (2008). [Google Scholar]
- Dong X. C. et al. 3D Graphene-Cobalt Oxide Electrode for High-Performance Supercapacitor and Enzymeless Glucose Detection. ACS Nano 6, 3206–3213 (2012). [DOI] [PubMed] [Google Scholar]
- Zhang X., Lai G., Yu A. & Zhang H. A glassy carbon electrode modified with a polyaniline doped with silicotungstic acid and carbon nanotubes for the sensitive amperometric determination of ascorbic acid. Microchim. Acta 180, 437–443 (2013). [Google Scholar]
- Abdelwahab A. A., Kim D., Halappa N. & Shim Y. A Selective Catalytic Oxidation of Ascorbic Acid at the Aminopyrimidyl Functionalized-Conductive Polymer Electrode. Electroanal. 25, 1178–1184 (2013). [Google Scholar]
- Weng C. J. et al. Synthesis of electroactive mesoporous gold-organosilica nanocomposite materials via a sol-gel process with non-surfactant templates and the electroanalysis of ascorbic acid. J. Mater. Chem. B 1, 4983–4991 (2013). [DOI] [PubMed] [Google Scholar]
- Mo J. W. & Ogorevc B. Simultaneous measurement at dopamine and ascorbate at their physiological levels using voltammetric microprobe based on overoxidized poly(1,2-phenylenediamine)-coated carbon fiber. Anal. Chem. 73, 1196–1202 (2001). [DOI] [PubMed] [Google Scholar]
- Park S., Chun T. & Kim H. Nonenzymatic glucose detection using mesoporous platinum. Anal. Chem. 75, 3046–3049 (2003). [DOI] [PubMed] [Google Scholar]
- Yamada K. et al. The simultaneous measurement of nicotinamide adenine dinucleotide and related compounds by liquid chromatography/electrospray ionization tandem mass spectrometry. Anal. Biochem. 352, 282–285 (2006). [DOI] [PubMed] [Google Scholar]
- Cirillo P. et al. Uric acid, the metabolic syndrome, and renal disease. J. Am. Soc. Nephrol. 17, S165–8 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information