Abstract
Secreted protein acidic and rich in cysteine (SPARC) gene has been shown to be epigenetically silenced in several cancers. We investigated the loss of expression and promoter methylation of this tumor suppressor gene in gastric cancers and correlated the data with clinicopathological features. We observed the loss of SPARC mRNA and SPARC protein expression in 7 of 10 (70%) gastric cancer cell lines. Upon treatment of expression-negative cell lines with a demethylating agent, expression of mRNA and protein was restored in all cells. Methylation rate of SPARC gene was 80% in ten gastric cancer cell lines and 74% (163 of 220) in primary tumors, while it was 5% in normal gastric mucosa (n = 40). In intestinal gastric cancer, SPARC methylation correlated with a negative prognosis (P < 0.001; relative risk 2.754, 95% confidence interval 1.780–4.261). Immunostaining revealed that SPARC protein was overexpressed in stromal fibroblasts adjacent to neoplastic epithelium but rarely expressed in the primary gastric cancer cells. These results implicate SPARC promoter methylation as an important factor in the tumorigenesis of gastric carcinomas and provide new insights into the potential use of SPARC as a novel biomarker and the potential clinical importance in human gastric cancers.
Recent studies have shown that several tumor suppressor genes are methylated in gastric cancer1,2,3,4,5,6. Aberrant DNA methylation of tumor suppressor genes has emerged as a new focus of investigation in cancer research. Methylation most often occurs at cytosines that are 5′ to guanosines (known as the CpG dinucleotide)7. DNA methylation is a biochemical modification that, in human cells, primarily affects cytosines when they are part of the symmetrical CpG dinucleotide. DNA methylation is central to the aberrant epigenetics of cancer8. As described in recent reviews, cancer cells often have both a loss of methylation and a gain of methylation at the promoters of select CpG islands, resulting in the silencing of hundreds of genes per cancer cell, including tumor suppressor genes9.
Secreted protein acidic and rich in cysteine (SPARC), also known as osteonectin or BM-40, is a multifaceted secreted glycoprotein which is expressed in many types of cells and is associated with tissue remodeling, wound repair, morphogenesis, cellular differentiation, cell migration and angiogenesis. SPARC is differentially expressed in tumors and its surrounding stroma in various cancers. Higher levels of SPARC expression have been reported in breast cancer, melanomas, and glioblastomas. Lower levels of SPARC expression have been found in other types of cancers, such as ovarian, colorectal, and pancreatic cancers, and acute myelogenous leukemia10.
SPARC may promote vascularization of tumors, tumor progression and/or invasiveness by modulating the activity of cytokines and stimulating secretion of tissue remodeling metalloproteases. Owing to the correlation between expression and invasion, SPARC was thought to be a proinvasive protein11,12. However, SPARC was found to be significantly downregulated in ovarian cancer cells, and restoring its expression led to decreased tumor growth and apoptosis13,14. Our laboratory has previously shown that SPARC is a tumor suppressor gene, and it appears to mediate, through its suppressive effects on MMP-7 and VEGF, inhibition of gastric cancer growth15.
The purpose of our study was to investigate the mechanism by which gastric carcinoma cells downregulate SPARC expression. We hypothesized that epigenetic silencing of SPARC gene by aberrant methylation during gastric carcinogenesis was responsible for the downregulation of SPARC. Here, we examined mRNA and protein expression, and methylation of SPARC in gastric cancer cell lines, and examined the methylation and protein expression in primary tumors. We also correlated these findings with clinicopathological features.
Methods
Ethics statement
The Medical Ethics Committee of Peking University First Hospital approved the clinical study. The experiment was performed in accordance with approved guidelines. Informed written consent was obtained from the patients or their guardians and healthy control subjects.
Cell culture and tumor tissue samples
Human gastric cancer cell lines, BGC-823, MGC-803, SGC-7901 and the control gastric epithelial cell line (GES-1)16, MKN-4517, HGC-2718, KATO III, SUN-1, SUN-16, AGS, NCI-N87(ATCC), were grown in RPMI-1640 medium (Life Technologies Inc., Rockville, MD, USA) supplemented with 10% fetal bovine serum (FBS) and incubated in 5% CO2 at 37°C. From 2003–2007, a total of 220 surgically resected samples were obtained from patients with gastric cancer who had not received treatment prior to resection at the Peking University First Hospital, P.R. China. Samples were immediately frozen and stored at −80°C until use.
Reverse transcription-polymerase chain reaction (RT-PCR) assay
A RT-PCR assay was used to examine SPARC mRNA expression. Total RNA was extracted from cultured cells with Trizol (Life Technologies, Rockville, MD, USA) following the manufacturer's instructions. RNA was reverse transcribed using AMV reverse transcriptase (A3500, Promega, Madison, WI, USA), and aliquots of the reaction mixture were used for subsequent PCR amplification.
Primers for SPARC amplification were as follows: forward primer, 5′-GTGGGCAAAGGGAAGTAACA-3′; and reverse primer, 5′-GGGAGGGTGAAGAAAAGGAG-3′. The expected product size of the SPARC cDNA was 512 bp. PCR amplifications were performed in 25 μl reaction volumes containing 0.2 mM dNTPs, 20 pmol of each oligonucleotide primer and 0.2 U Taq polymerase in PCR buffer. PCR amplification was performed on a PCR thermal controller with an initial denaturation at 95°C for 5 min, followed by 27 cycles of 95°C for 1 min, 65°C for 1 min, and 72°C for 1 min, and a final extension step of 72°C for 10 min.
The housekeeping gene GAPDH (glyceraldehyde-3-phosphate dehydrogenase) was used as an internal control to confirm the success of RT reaction. Primers for GAPDH amplification were as follows: forward primer, 5′-CACTGGCGTCTTCACCACCATG-3′,and reverse primer, 5′-GCTTCACCACCTTCTTGATGTCA-3′. PCR amplification was carried out with an initial denaturation at 95°C for 5 min, followed by 25 cycles of 94°C for 30 s, 65°C for 45 s, and 72°C for 30 s. PCR products were analyzed on 2% agarose gels and visualized using ethidium bromide staining.
Western blotting
Protein lysates from cultured cells, tumors, and normal gastric tissues were extracted by RIPA buffer (Cell Signal Technology) containing protease inhibitors (Cell Signal Technology). Cells were washed in cold Dulbecco's phosphate-buffered saline (PBS; Sigma), lysed in RIPA buffer containing sodium orthovanadate and a cocktail of protease inhibitors, and sonicated as previously described19. Protein concentrations were determined using the BCA Protein Assay Kit (Pierce, Rockford, IL). Lysates were mixed with 1× SDS sample buffer, boiled for 5 min and analyzed using SDS-PAGE. Proteins were then transferred onto a nitrocellulose membrane and blocked with 5% BSA in PBS. Membranes were washed with PBS containing 0.2% Tween 20 (PBS-T) and incubated with a rabbit anti-human SPARC monoclonal antibody (Cell Signal Technology; 1:1000) overnight. After washing with PBS-T, the membranes were incubated with horseradish peroxidase-conjugated goat anti-rabbit IgG (Cell Signal Technology) for 1 h. Signals were visualized using the Immobilon Western Chemiluminescent HRP Substrate (Millipore). Measurements were performed using a Kodak image Station 4000 mm Pro System (Kodak, Rochester, NY, USA).
DNA extraction and sodium bisulfite conversion
DNA was obtained from gastric cancer cell lines, gastric tumor tissue sections (n = 220) and normal gastric mucosa samples (n = 40). DNA from peripheral blood lymphocytesof gastric cancer patients (n = 10) was also extracted. DNA from peripheral blood lymphocytesof healthy nonsmoking volunteers (n = 20) was used as a negative control for methylation specific assays. QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) was used according to manufacturer's recommended protocol. An EZ DNA Methylation-Gold Kit (Zymo Research, Orange, CA, USA) was used according to the manufacturer's instructions for sodium bisulfite treatment of genomic DNA. The bisulfite converted DNA was resuspended in 15 μl of elution buffer and stored at −20°C until use.
Methylation-specific polymerase chain reaction (MSP) and DNA sequencing
Methylation status of SPARC gene was determined using MSP as described previously20. Bisulfite-treated DNA (1 μg) was amplified using primers specific for either the methylated or the unmethylated DNA under the following conditions: 95°C for 5 min; 38 cycles of 95°C for 30 s, 62°C for 30 s, and 72°C for 30 s; and a final extension for 10 min at 72°C. Primer sequences were 5′-TTTTTTAGATTGTTTGGAGAGTG-3′ (forward) and 5′-AACTAACAACATAAACAAAAATATC-3′ (reverse) for unmethylated reactions (132 bp), and 5′-GAGAGCGCGTTTTGTTTGTC-3′ (forward) and 5′-AACGACGTAAACGAAAATATCG-3′ (reverse) for methylated reactions (112 bp)21. PCR product (8 μl) was loaded onto a 2% agarose gel and visualized using ethidium bromide staining. PCR products were then subjected to direct sequencing.
5-Aza-2′-deoxycytidine (5-Aza-Cdr) treatment
Tumor cell lines with SPARC hypermethylation and absent gene expression were incubated in culture medium with 5 μM 5-Aza-Cdr for 6 days, with medium changes at days 1, 3 and 5. Cells were harvested for RNA, DNA and protein on day 6, as described above.
Immunohistochemistry
Immunohistochemistry was performed as previously described21. Sections (4 μm) were cut onto coated slides and deparaffinized using routine techniques. Antigen retrieval was performed in 10 mM sodium citrate buffer (pH 6.0), heated at 95°C in a steamer for 20 min. After blocking endogenous peroxidase activity with a 3% aqueous H2O2 solution for 5 min, sections were incubated with an anti-SPARC monoclonal antibody at a final concentration of 4 μg/ml for 60 min. Immunolabeled signals were detected with the Envision Plus Detection Kit (DAKO, Carpentaria, CA, USA) following the manufacturer's protocol. Sections were counter-stained with hematoxylin. The extent of immunolabeling of SPARC was scored as follows: 0%, negative; ≤10%, focal; and >10%, positive. The intensity of immunolabeling was scored as weak (+), moderate (++) or strong (+++).
Cell proliferation, invasion and migration assay
To investigate the cell proliferation, four cell lines (BGC-823, SUN-1, MGC-803, HGC-27) exposed to 5 μM 5-Aza-Cdr for 3 days were seeded at 10,000 cells per well into a 48-well plate. Cells were counted from duplicate wells at 24, 48 and 72 h. Results were based on three independent experiments. The cells without 5-Aza-Cdr treatment were assayed at the same time as the controls.
Invasion assay was performed in a six-well Transwell chamber (Costar Corporation, Tewksbury, MA, USA) that contains an 8 μm pore size polycarbonate membrane precoated with 50 mg/L Matrigel (BD Biosciences, Bedford, MA, USA). Cells were re-suspended in a serum-free medium at a concentration of 5 × 104 cells/mL and seeded in the upper compartment of chamber and incubated in the presence of 5-Aza-Cdr for 24 h. A medium containing 10% fetal calf serum was added to the bottom chamber. After reculturing with 5% CO2 at 37°C for 24 hours, the Transwell chambers were inverted and stained with hematoxylin. The migration assay was done in a similar manner, but without Matrigel coated on the filters. The invasion and migration assays were done in triplicate. Five fields were randomly selected for cell counting on the membranes.
Statistical analysis
Continuous variables are shown as the mean ± SD, and differences between groups were evaluated using unpaired Student's t-test. The relationship between SPARC expression and clinicopathological parameters were analyzed using Fisher's exact test, chi-square test, or Pearson χ2 and Mann-Whitney test if necessary. Survival was calculated from the date of initial diagnosis until death or the date of the last follow-up. Survival was analyzed according to the Kaplan-Meier method, and differences in their distribution were evaluated by means of the log-rank test. A multivariate Cox proportional hazards model was developed to evaluate the joint effects of covariates. P values were two-sided, and a P value of less than 0.05 was defined as being statistically significant. Statistical analyses were conducted using the IBM SPSS Statistics software package (version 20, IBM-SPSS Statistics, Armonk, NY, USA).
Results
Aberrant methylation and expression of SPARC gene in cell lines
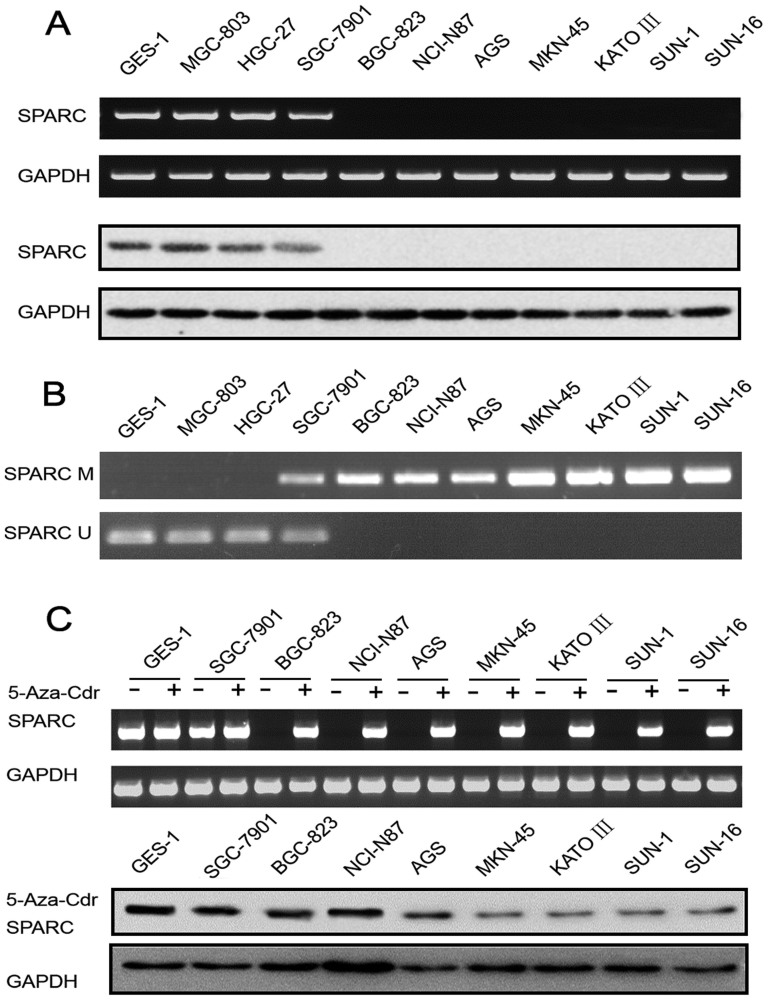
Expression of SPARC in cell lines was examined using RT-PCR, and the representative data are shown in the upper bands of Figure 1 A. Loss of SPARC expression was observed in 7 of 10 (70%) gastric cancer cell lines, while aberrant methylation was found in 8 of 10 (80%) of them (Figure 1 B). Concordance between loss of gene expression and aberrant methylation of SPARC was 70% (7 of 10) in gastric cancer cell lines. Both SPARC mRNA expression and aberrant methylation were detected in SGC-7901 cell lines.
Figure 1.
(A) Upper bands, representative data of RT-PCR for SPARC mRNA in 10 gastric cancer cell lines and a control gastric epithelial cell line GES-1. GAPDH was used as the internal reference for RNA integrity and reverse transcription. Lower bands, protein expression of SPARC in 10 gastric cancer cell lines and the GES-1 cell line. (B) Representative data of MSP assay for methylation in SPARC gene in cell lines. PCR products using methylated primers and unmethylated primers were separated and visualized in 2% agarose gels. M = methylated band; U = unmethylated band. (C) Upper bands, representative data of RT-PCR for SPARC mRNA in gastric cancer cell lines before (−) and after (+) 5-Aza-Cdr treatment. Lower bands, protein expression of SPARC in gastric cancer cell lines and GES-1 cell line after 5-Aza-Cdr treatment.
Western blotting
Western blotting showed that SPARC was undetectable in AGS, MKN-45, NCI-N87, BGC-823, KATO III, SUN-1 and SUN-16 cell lines, but detectable in GES-1, HGC-27, SGC-7901 and MGC-803 cell lines (lower bands of Figure 1 A).
5-Aza-2′-deoxycytidine (5-Aza-Cdr) treatment
To confirm that methylation of SPARC gene was responsible for the loss of SPARC protein expression, gastric cells lines were treated with the demethylating agent 5-Aza-Cdr. 5-Aza-Cdr treatment was able to restore SPARC mRNA expression in cell lines (BGC-823, AGS, NCI-N87, MKN-45, KATO III, SUN-1, SUN-16) that did not constitutionally express SPARC (upper bands, Figure 1C). Moreover, protein expression was restored in the seven cell lines (BGC-823, AGS, NCI-N87, MKN-45, KATO III, SUN-1, SUN-16) previously lacking SPARC expression (lower bands, Figure 1C).
Methylation analysis of SPARC gene in gastric cancer cell lines
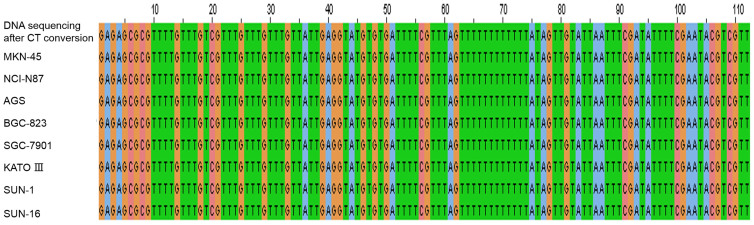
Bisulfite treated genomic DNA samples from SGC-7901, BGC-823, AGS, NCI-N87, MKN-45, KATO III, SUN-1 and SUN-16 cell lines were amplified using methylated primers, and the PCR products were sequenced. The results showed that all of the CpG sites in this region were methylated in the 8 gastric cancer cell lines (Figure 2).
Figure 2. SPARC DNA sequencing after CT conversion of DNA extracted from MKN-45, NCI-N87, AGS, BGC-823, SGC-7901, KATO III, SUN-1 and SUN-16 gastric cancer cell lines.
CpG dinucleotides “C, G” in the sequences are shown in orange.
Immunostaining of SPARC protein in gastric cancer tissues
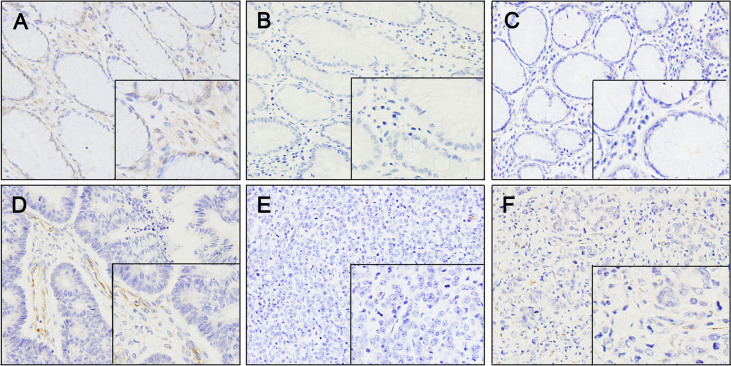
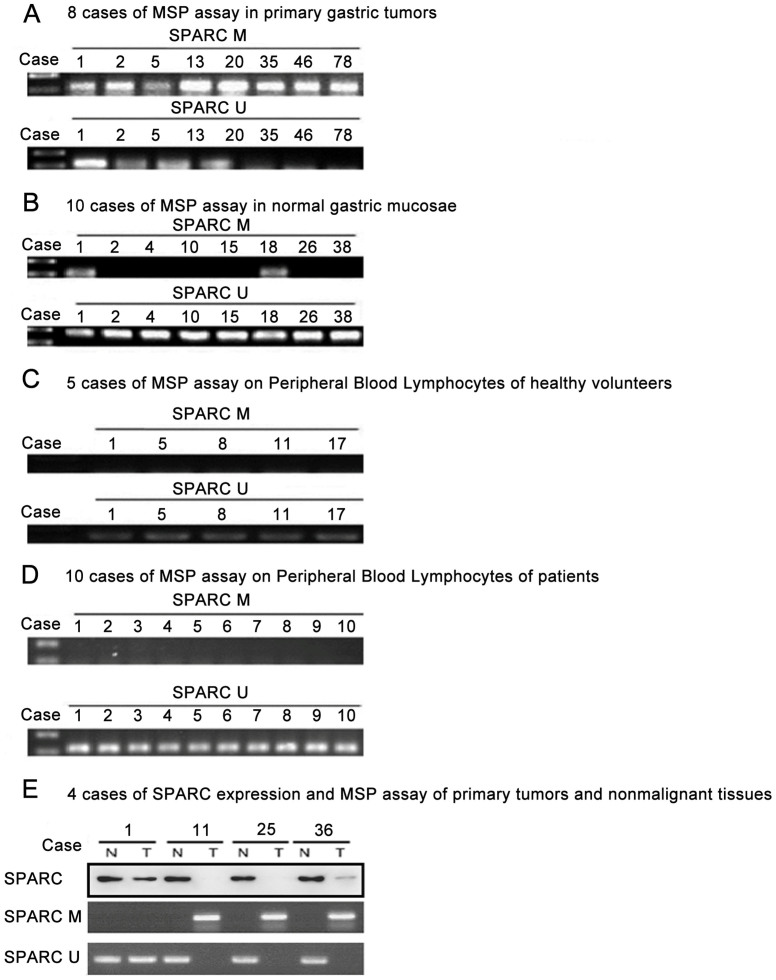
SPARC protein expression was examined in 172 primary gastric carcinoma tissues and 10 normal gastric tissues using immunohistochemistry and an anti-SPARC monoclonal antibody. In 134 (78%) of 172 cases, moderate (++) to strong (+++) SPARC expression was found in stromal cells, presumably fibroblasts (Figure 3). SPARC expression was observed in neoplastic epithelium in 58 (34%) of 172 cases, but 15 of 58 cases of the immunostaining was weak and focal. In the remaining 114 cases (66%), neoplastic cells did not express SPARC throughout the tumor. Of 172 cases, 116 cases showed negative (0) or weak (+) expression of SPARC protein as well as methylation of SPARC gene, and 43 cases showed moderate (++) to very strong (+++) expression without methylation of the gene (Figure 4E).
Figure 3. Immunostaining of SPARC in normal and malignant gastric tissues.
(A) Normal gastric tissue showing SPARC expression. The staining was present in stromal fibroblasts and normal epithelium. (B and C) Immunostaining of SPARC was weak in well-differentiated gastric cancer; however, stromal fibroblasts within and surrounding the tumor showed staining of variable intensity. (D) Immunostaining of SPARC was faint or absent in moderately differentiated gastric cancer. (E and F) Immunostaining of SPARC was absent in poorly differentiated gastric cancer and faint in stromal fibroblasts within the tumor. Positive staining of SPARC is indicated by a brown color. ×160 magnification.
Figure 4.
(A) Representative examples of the MSP assay using DNA from primary gastric tumors. PCR products were visualized in a 2% agarose gel stained with ethidium bromide. (B) Representative examples of the MSP assay using DNA from normal gastric mucosa samples. (C) Representative examples of the MSP assay using DNA from peripheral blood lymphocytes from healthy volunteers. (D) Representative examples of the MSP assay using DNA from peripheral blood lymphocytes from patients. M = methylated; U = unmethylated. (E) Four cases of SPARC expression and SPARC gene methylation in tumors and adjacent normal gastric tissues.
Aberrant methylation of SPARC gene in primary tumors
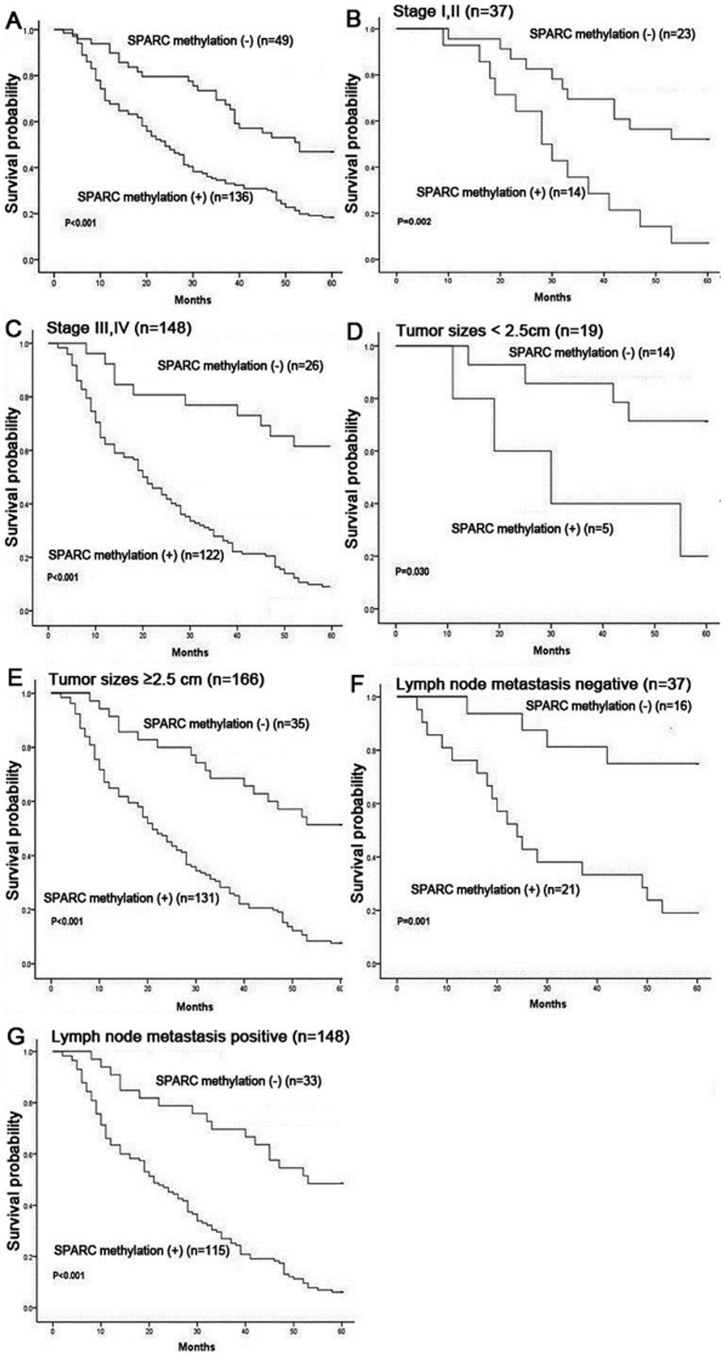
Results of SPARC methylation in primary tumors (n = 220), normal gastric mucosa samples (n = 40), peripheral blood lymphocytes from patients (n = 10) and healthy nonsmoking volunteers (n = 20) are detailed in Table 1 and Figure 4. SPARC methylation was a tumor-specific event in gastric cancers (P < 0.001) as compared with the corresponding adjacent non-malignant tissues. Of the 220 gastric cancers, methylation occurred in 163 (74%) samples. Of the 40 normal gastric mucosa tissues, methylation occurred in 2 (5%) samples. When comparison was made between SPARC methylation and clinicopathological data, we found that SPARC methylation was unrelated to gender and age. Remarkably, the overall survival was poorer in intestinal gastric cancer patients with SPARC methylation than in those without methylation (P < 0.001, log-rank test; Figure 5A). The relationship between overall survival and SPARC methylation in cancers was also found in the subgroups of patients at different stages (P = 0.002 for those at stages I and II; P < 0.001 for those at stages III and IV; Figure 5B and 5C), different tumor sizes (P = 0.03 for those with tumor size < 2.5 cm; P < 0.001 for those ≥ 2.5 cm; Figure 5D and 5E), and lymph node metastasis (P = 0.001 for those without lymph node metastasis; P < 0.001 for those with lymph node metastasis; Figure 5F and 5G). Using the multivariate Cox proportional hazards model, we found that SPARC methylation was an independent adverse prognostic factor (P < 0.001; RR 2.754, 95%, CI 1.780–4.261), similar to the clinically well-known prognostic factors of disease stage (P = 0.002; RR 2.334, 95% CI 1.351–4.034), tumor size (P = 0.012; RR 3.784, 95% CI 1.332–10.747) and lymph node metastasis (P = 0.020; RR 1.824, 95% CI 1.100–3.024) in adenocarcinoma cases (Table 2).
Table 1. SPARC methylation in gastric cancer cell lines, primary gastric cancers, and controls.
| Samples | Total no. | No. Methylated (%) |
|---|---|---|
| Gastric cancer cell line | 10 | 8(80) |
| Primary gastric cancer | 220 | 163(74) |
| Intestinal type | 185 | 136(74) |
| Diffuse type | 35 | 27(77) |
| Nonmalignant sample | 70 | |
| Normal gastric mucosa | 40 | 2(5) |
| Peripheral blood mononuclear cells* | 20 | 0(0) |
| Peripheral blood mononuclear cells† | 10 | 0(0) |
*From healthy nonsmoking volunteers.
†From patients.
Figure 5. Kaplan-Meier plots of overall survival in intestinal gastric cancer patients.
(A) Survival of patients with (n = 136) or without (n = 49) SPARC methylation. (B) Survival of patients with early stages I or II, with (n = 14) or without SPARC methylation (n = 23). (C) Survival of patients with advanced stages III or IV, with (n = 122) or without SPARC methylation (n = 26). (D) Survival of patients with tumor sizes < 2.5 cm, with (n = 5) or without SPARC methylation (n = 14). (E) Survival of patients with tumor sizes ≥ 2.5 cm, with (n = 131) or without SPARC methylation (n = 35). (F) Survival of patients lymph node metastasis negative, with (n = 21) or without SPARC methylation (n = 16). (G) Survival of patients lymph node metastasis positive, with (n = 115) or without SPARC methylation (n = 33). Probability of survival curves was calculated using the Kaplan-Meier product-limit method and compared via the log-rank test between groups.
Table 2. Univariate and multivariate statistics of the prognostic value of gender, age, size, lymph node metastasis, stage, and SPARC methylation for survival (5 years) in intestinal gastric cancer patients.
| No. (%) | Methylation (%) | Unmethylation (%) | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|---|---|
| Parameters | N = 185 | N = 136 | N = 49 | P-Value | P-Value | Risk ratio | 95% CI | P-value |
| Gender | bP = 0.613 | P = 0.942 | 1.268 | 0.892–1.803 | P = 0.185 | |||
| Male | 100 (54.1) | 72(72.0) | 28(28.0) | |||||
| Female | 85 (45.9) | 64(75.3) | 21(24.7) | |||||
| Age (years) | bP = 0.271 | P = 0.154 | 1.045 | 0.731–1.494 | P = 0.810 | |||
| ≥63a | 103 (55.7) | 79(76.7) | 24(23.3) | |||||
| <63 | 82 (44.3) | 57(69.5) | 25(30.5) | |||||
| Size (cm) | bP < 0.001 | P = 0.002 | 3.784 | 1.332–10.747 | P = 0.012 | |||
| <2.5 | 19 (10.3) | 5(26.3) | 14(73.7) | |||||
| ≥2.5 | 166 (89.7) | 131(78.9) | 35(21.1) | |||||
| TNM stage | bP < 0.001 | P = 0.007 | 2.334 | 1.351–4.034 | P = 0.002 | |||
| I, II | 37 (20.0) | 14(37.8) | 23(62.2) | |||||
| III, IV | 148 (80.0) | 122(82.4) | 26(17.6) | |||||
| Lymph node metastasis | bP = 0.010 | P = 0.001 | 1.824 | 1.100–3.024 | P = 0.020 | |||
| Negative | 37(20.0) | 21(56.8) | 16(43.2) | |||||
| Positive | 148 (80.0) | 115(77.7) | 33(22.3) | |||||
| SPARC methylation | P < 0.001 | 2.754 | 1.780–4.261 | P < 0.001 | ||||
| - | 49 (26.5) | |||||||
| + | 136 (73.5) | |||||||
Prognostic factors for overall survival was conducted by univariate analyses using the log-rank test and multivariate analyses using the Cox proportional hazards model. CI, confidence interval.
aDivided by median age of adenocarcinoma cases.
bPearson's χ2 test
Cell proliferation, invasion and migration assay
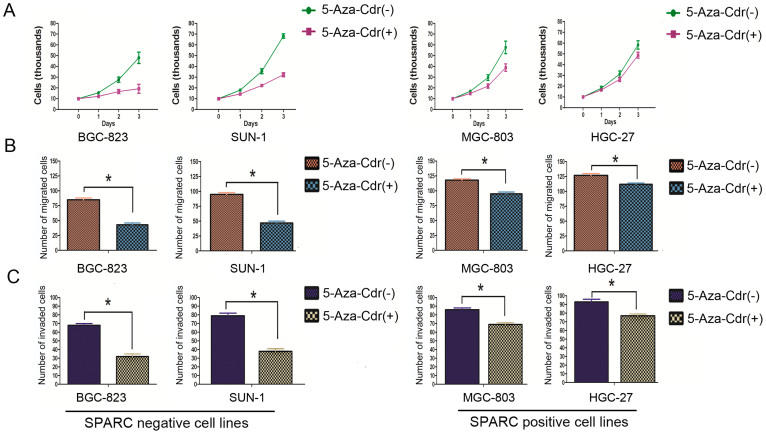
Treatment of 5-Aza-Cdr induced the expression of SPARC in gastric cancer cell lines (Figure 1C). We then examined the changes of cell proliferation, invasion and migration of the 4 gastric cancer cell lines (BGC-823, SUN-1, MGC-803 and HGC-27) after incubation with 5-Aza-Cdr. In BGC-823 and SUN-1 cell lines that had SPARC methylation (Figure 1B), cell proliferation decreased significantly after 5-Aza-Cdr treatment, beginning as early as 24 h of the treatment (Figure 6A). In contrast in MGC-803 and HGC-27 cell lines that had unmethylated SPARC and expressed SPARC (Figure 1A and 1B), decrease of cell proliferation was less prominent after 5-Aza-Cdr treatment, and was only noticeable after 2 days (Figure 6A). Similarly, cell migration (Figure 6B) and invasion (Figure 6C) were also decreased more in BGC-823 and SUN-1 cell lines and less in MGC-803 and HGC-27 cell lines after 5-Aza-Cdr treatment.
Figure 6. Changes of cell proliferation, invasion and migration in BGC-823, SUN-1, MGC-803, HGC-27 cell lines after 5-Aza-Cdr treatment.
Decrease of cell proliferation (A), cell migration (B) and cell invasion (C) were more in BGC-823 and SUN-1 cell lines than in MGC-803 and HGC-27 cell lines after 5-Aza-Cdr treatment. *: P < 0.01.
Discussion
In this study, we attempted to investigate the mechanism through which SPARC expression was reduced in human gastric carcinomas. Our results showed that the downregulation of SPARC was resulted from the hypermethylation of promoter region in SPARC gene. Previous studies from several groups have indicated methylation in SPARC promoter region resulting in the downregulation of SPARC expression in multiple neoplasms, including colon, pancreatic, ovarian and endometrial carcinomas13,21,22,23. In this study, SPARC expression was lost in association with the aberrant DNA methylation in SPRAC gene in most gastric cell lines and surgical gastric cancer samples, and the loss of SPARC expression could be rescued in gastric cancer cell lines upon treatment with the demethylating agent 5-Aza-Cdr. Methylation of specific CpG sites in SPARC gene was consistently detected in gastric cancer cell lines by MSP and sequencing. Although other mechanisms may also account for the downregulation of SPARC expression, the relationship between downregulation of SPARC expression and methylation in SPARC gene was confirmed by our excellent uniformity among mRNA expression by RT-PCR, protein expression by western-blotting, immunostaining, and DNA methylation in SPARC gene by MSP and sequencing in gastric cancer cell lines and primary tumors. 5-Aza-Cdr is a nucleoside anti-metabolite agent and a potent inhibitor of DNA methyltransferase 1 activity. 5-Aza-Cdr does not specifically inhibit the methylation in SPARC gene, but has a global effect on other methylated genes24. However in our findings, 5-Aza-Cdr obviously inhibited the cell proliferation, invasion and migration in the gastric cell lines with methylation in SPARC gene.
The downregulation of SPARC expression in gastric cancers was also found in pancreatic cancers, which usually exhibited the loss of SPARC and the higher expression level in normal epithelial cells21. Upregulation of SPARC was unusually present in stromal cells distant from the pancreatic cancers. These findings indicated that there was a complex pattern of simultaneous selected downregulation in a specific cell type (tumor cells) accompanied by selected upregulation in adjacent stromal cells. In our findings, SPARC was expressed in stromal cells and occasionally in tumor cells, similar to the findings in a previous report on gastric cancer study25; the loss of SPARC gene expression was associated with aberrant hypermethylation in SPARC gene and could be reversed by 5-Aza-Cdr treatment21, similar to the evidences previously described in pancreatic, ovarian and breast cancer cells14,21,26.
The phenomenon that hypermethylation of specific CpG sites in SPARC gene in most gastric cancer cell lines may suggest the usefulness of SPARC expression in cells as a diagnostic or predictive marker for gastric cancers. Complete or partial loss of SPARC expression in some stomach epithelia with morphologically normal appearance may in fact represent an early epigenetic event predisposing to become gastric cancer cells. This hypothesis requires prospective studies to be determined, but a similar pattern has been previously described for colorectal cancers24.
In our findings, higher stage (III or IV), larger tumor size (≥2.5 cm) and positive lymph node metastasis were apparently associated with a poor prognosis in gastric cancers as described in another report27. Additionally, patients with methylation in SPARC gene in gastric cancers were associated with a poorer prognosis than those without methylation. SPARC expression was downregulated in lung, pancreatic and ovarian cancers, but was upregulated in metastatic prostate, bladder and hepatocellular cancers. SPARC expression in normal and tumor cells were highly dependent on tumor type and culture conditions. SPARC expression in cancer tissues correlated with poor prognosis in malignant melanoma, bladder and esophageal cancer as reported by others, but some of these reports were solely based on the results of RT-PCR on whole specimens28,29,30. Therefore, aberrant expression of SPARC in primary tumors may be related to poor prognosis. However, some recent studies reported that SPARC were associated with poorer prognosis of the gastric cancer patients31,32,33,34. Our findings, however, demonstrated that patients without SPARC methylation was associated with a good disease outcome and a better long-term survival. Our study also showed that SPARC expression in stromal cells was significantly higher than that in cancer cells, and 5-Aza-Cdr inhibited the cell proliferation, invasion and migration in the gastric cell lines with methylation in SPARC gene. Our laboratory previous research also showed SPARC suppresses angiogenesis of gastric cancer by down-regulating the expression of VEGF and MMP-715. Others considered that this suppression might be related to the tumor growth, and SPARC had an antiproliferative function through modulating cell cycle regulatory proteins or growth factors22. Similar results have been reported in pancreatic cancer21.
Our results suggest the potential of clinical applications. Because that SPARC is frequently methylated in gastric cancers but not in normal gastric mucosa samples, a useful strategy may develop for gastric cancer diagnosis based on the detection of aberrantly methylation in SPARC gene by MSP in samples such as biopsies, serum, and gastric lavage. Several tumor suppressor genes such as p16 have already been shown to be useful for such a purpose35. The methylation of CpG sites in SPARC gene may be used as a specific diagnostic marker in gastric cancer, to which there is currently no perfect markers for the diagnosis of a noninvasive gastric cancer. Methylation in SPARC gene may also be useful to combine other markers such as circulating tumor cells in blood to predict the prognosis of patients, or to establish the epigenetic type of various tumors to evaluate their differences in sensitivity to chemotherapy, metastasis possibilities and/or overall prognosis36.
In summary, we have identified that SPARC was significantly downregulated in gastric cancer cells due to DNA methylation in SPARC gene. The DNA methylation correlated with the prognosis of gastric adenocarcinomas. We also provided evidence that DNA methylation in SPARC gene may play a role in the pathogenesis of gastric cancers.
Author Contributions
Z.Y.C., G.W.C. and Y.C.L. wrote the main manuscript text; Z.Y.C., J.L.Z., H.X.Y., P.Y.W., J.Z., W.W., X.W., Y.L.W. and S.W.C. prepared figures 1– 6. All authors reviewed the manuscript.
Acknowledgments
This study was supported by grants from the National Natural Science Foundation of China (no. 30901417/H1617). We thank Professor Yu Qi, Professor Ding-Fang Bu, Yu-Feng Xu of the Research Centre of Peking University First Hospital, for technical support.
References
- Kim J. G. et al. Comprehensive DNA methylation and extensive mutation analyses reveal an association between the CpG island methylator phenotype and oncogenic mutations in gastric cancers. Cancer Lett 330, 33–40 (2013). [DOI] [PubMed] [Google Scholar]
- Nomura T. et al. Influence of HRH2 promoter polymorphism on aberrant DNA methylation of DAPK and CDH1 in the gastric epithelium. BMC Gastroenterol 13, 1 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka T. et al. Loss of trefoil factor 1 is regulated by DNA methylation and is an independent predictive factor for poor survival in advanced gastric cancer. Int J Oncol 42, 894–902 (2013). [DOI] [PubMed] [Google Scholar]
- Hibi K. et al. Methylation of the WNT5A gene is frequently detected in early gastric carcinoma. Hepatogastroenterology 59, 2661–3 (2012). [DOI] [PubMed] [Google Scholar]
- Min S. Y. et al. Prognostic significance of glutathione peroxidase 1 (GPX1) down-regulation and correlation with aberrant promoter methylation in human gastric cancer. Anticancer Res 32, 3169–75 (2012). [PubMed] [Google Scholar]
- Takamaru H. et al. Aberrant methylation of RASGRF1 is associated with an epigenetic field defect and increased risk of gastric cancer. Cancer Prev Res (Phila) 5, 1203–12 (2012). [DOI] [PubMed] [Google Scholar]
- Ng H. H. & Bird A. DNA methylation and chromatin modification. Curr Opin Genet Dev 9, 158–63 (1999). [DOI] [PubMed] [Google Scholar]
- Issa J. P. CpG island methylator phenotype in cancer. Nat Rev Cancer 4, 988–93 (2004). [DOI] [PubMed] [Google Scholar]
- Herman J. G. & Baylin S. B. Gene silencing in cancer in association with promoter hypermethylation. N Engl J Med 349, 2042–54 (2003). [DOI] [PubMed] [Google Scholar]
- Tai I. T. & Tang M. J. SPARC in cancer biology: its role in cancer progression and potential for therapy. Drug Resist Updat 11, 231–46 (2008). [DOI] [PubMed] [Google Scholar]
- Ledda M. F. et al. Suppression of SPARC expression by antisense RNA abrogates the tumorigenicity of human melanoma cells. Nat Med 3, 171–6 (1997). [DOI] [PubMed] [Google Scholar]
- Lane T. F. & Sage E. H. The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J 8, 163–73 (1994). [PubMed] [Google Scholar]
- Socha M. J. et al. Aberrant promoter methylation of SPARC in ovarian cancer. Neoplasia 11, 126–35 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mok S. C. et al. SPARC, an extracellular matrix protein with tumor-suppressing activity in human ovarian epithelial cells. Oncogene 12, 1895–901 (1996). [PubMed] [Google Scholar]
- Zhang J. L. et al. Secreted protein acidic and rich in cysteine (SPARC) suppresses angiogenesis by down-regulating the expression of VEGF and MMP-7 in gastric cancer. PLoS One 7, e44618 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y. et al. MicroRNA-335 acts as a metastasis suppressor in gastric cancer by targeting Bcl-w and specificity protein 1. Oncogene 31, 1398–407 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinto O. et al. Inhibitory effect of a TGFbeta receptor type-I inhibitor, Ki26894, on invasiveness of scirrhous gastric cancer cells. Br J Cancer 102, 844–51 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y. et al. microRNA-503 inhibits gastric cancer cell growth and epithelial-to-mesenchymal transition. Oncol Lett 7, 1233–1238 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Said N. & Motamed K. Absence of host-secreted protein acidic and rich in cysteine (SPARC) augments peritoneal ovarian carcinomatosis. Am J Pathol 167, 1739–52 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J. G. et al. Methylation-specific PCR: a novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci U S A 93, 9821–6 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N. et al. SPARC/osteonectin is a frequent target for aberrant methylation in pancreatic adenocarcinoma and a mediator of tumor-stromal interactions. Oncogene 22, 5021–30 (2003). [DOI] [PubMed] [Google Scholar]
- Yang E. et al. Frequent inactivation of SPARC by promoter hypermethylation in colon cancers. Int J Cancer 121, 567–75 (2007). [DOI] [PubMed] [Google Scholar]
- Rodriguez-Jimenez F. J. et al. Overexpression of SPARC protein contrasts with its transcriptional silencing by aberrant hypermethylation of SPARC CpG-rich region in endometrial carcinoma. Oncol Rep 17, 1301–7 (2007). [PubMed] [Google Scholar]
- Cheetham S. et al. SPARC promoter hypermethylation in colorectal cancers can be reversed by 5-Aza-2'deoxycytidine to increase SPARC expression and improve therapy response. Br J Cancer 98, 1810–9 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke K. et al. Differential Expression of SPARC in Intestinal-type Gastric Cancer Correlates with Tumor Progression and Nodal Spread. Transl Oncol 2, 310–20 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhanesuan N. et al. Doxycycline-inducible expression of SPARC/Osteonectin/BM40 in MDA-MB-231 human breast cancer cells results in growth inhibition. Breast Cancer Res Treat 75, 73–85 (2002). [DOI] [PubMed] [Google Scholar]
- Medina-Franco H., Heslin M. J. & Cortes-Gonzalez R. Clinicopathological characteristics of gastric carcinoma in young and elderly patients: a comparative study. Ann Surg Oncol 7, 515–9 (2000). [DOI] [PubMed] [Google Scholar]
- Yamashita K. et al. Clinical significance of secreted protein acidic and rich in cystein in esophageal carcinoma and its relation to carcinoma progression. Cancer 97, 2412–9 (2003). [DOI] [PubMed] [Google Scholar]
- Yamanaka M., et al. Analysis of the gene expression of SPARC and its prognostic value for bladder cancer. J Urol 166, 2495–9 (2001). [PubMed] [Google Scholar]
- Massi D. et al. Osteonectin expression correlates with clinical outcome in thin cutaneous malignant melanomas. Hum Pathol 30, 339–44 (1999). [DOI] [PubMed] [Google Scholar]
- Sato T. et al. Clinical significance of SPARC gene expression in patients with gastric cancer. J Surg Oncol 108, 364–8 (2013). [DOI] [PubMed] [Google Scholar]
- Zhao Z. S. et al. SPARC is associated with gastric cancer progression and poor survival of patients. Clin Cancer Res 16, 260–8 (2010). [DOI] [PubMed] [Google Scholar]
- Jeung H. C. et al. A randomized phase 2 study of docetaxel and S-1 versus docetaxel and cisplatin in advanced gastric cancer with an evaluation of SPARC expression for personalized therapy. Cancer 117, 2050–7 (2011). [DOI] [PubMed] [Google Scholar]
- Oue N. et al. Characteristic gene expression in stromal cells of gastric cancers among atomic-bomb survivors. Int J Cancer 124, 1112–21 (2009). [DOI] [PubMed] [Google Scholar]
- Esteller M. et al. Detection of aberrant promoter hypermethylation of tumor suppressor genes in serum DNA from non-small cell lung cancer patients. Cancer Res 59, 67–70 (1999). [PubMed] [Google Scholar]
- Toyota M. et al. Aberrant methylation in gastric cancer associated with the CpG island methylator phenotype. Cancer Res 59, 5438–42 (1999). [PubMed] [Google Scholar]