Abstract
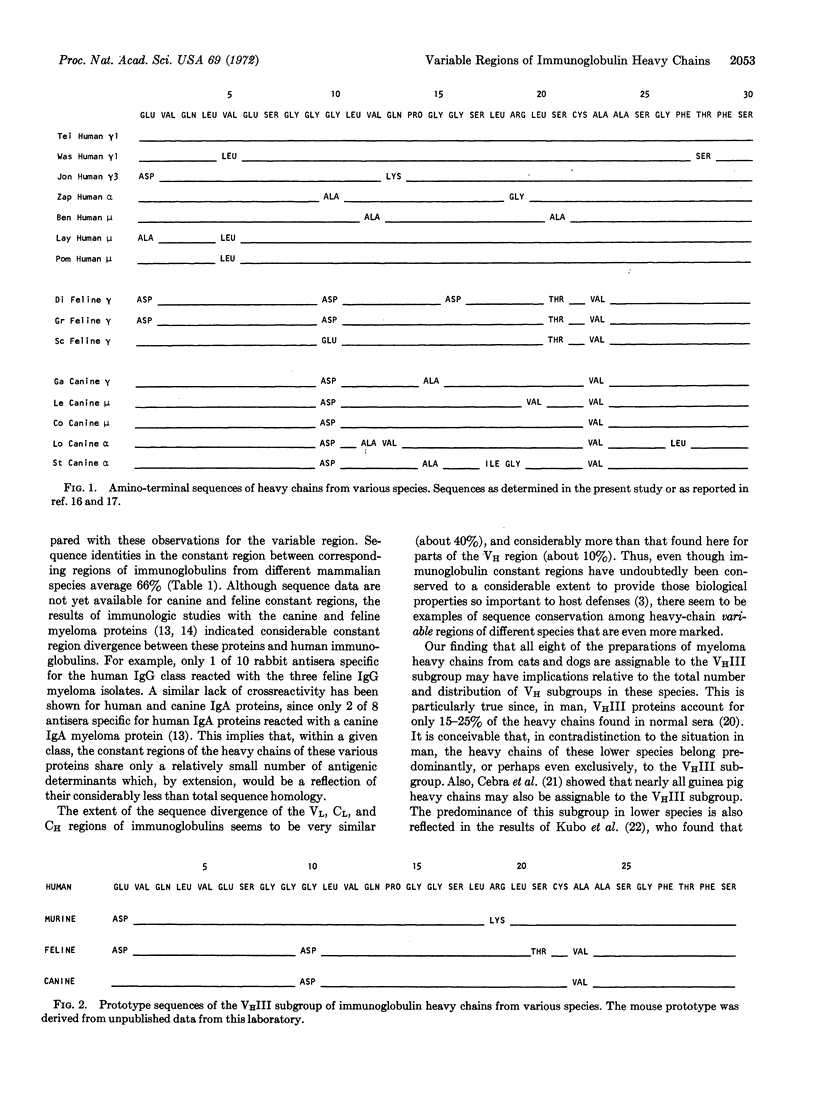
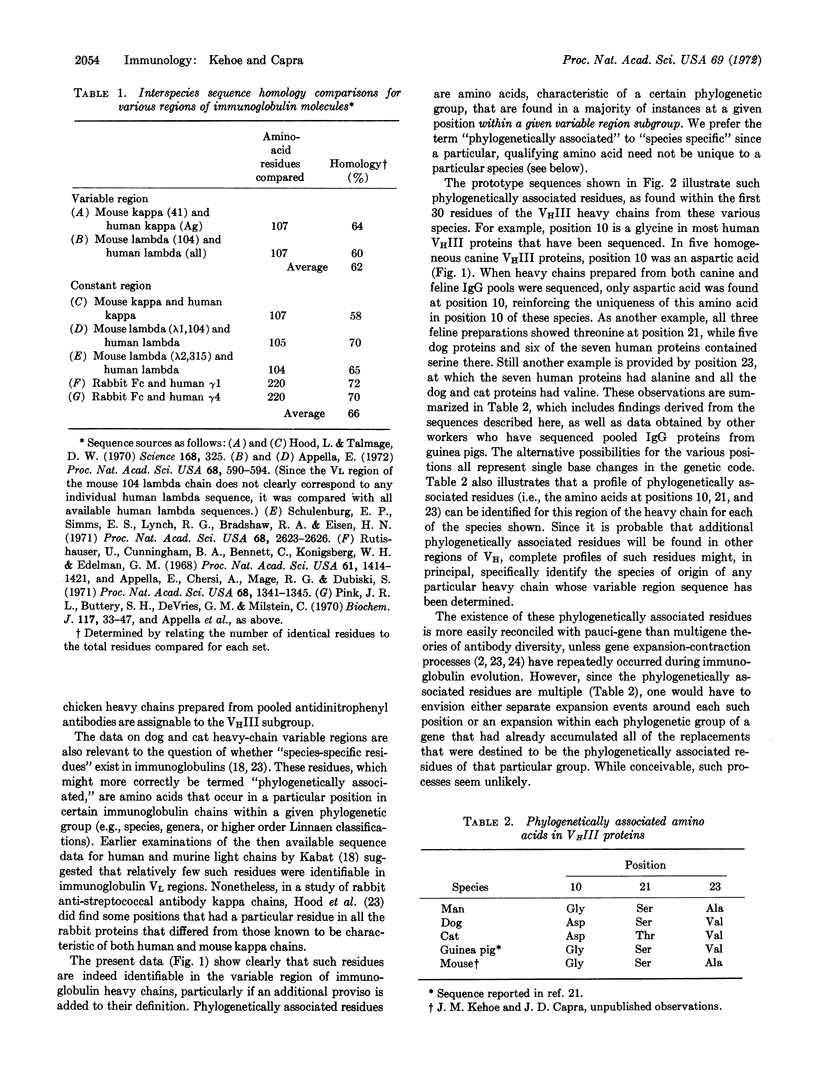
Immunoglobulin heavy chains of myeloma proteins from dogs and cata have been subjected to automated sequence analysis. When the results were compared with human heavy-chain sequences, all the dog and cat proteins could be unequivocally assigned to the VHIII subgroup. This pattern contrasts with that in human proteins in which only 25% of all heavy chains sequenced belong to this subgroup. The 30 residues at the NH2 termini of dog, cat, and human heavy chains had sequence identities near or exceeding 90%, in contrast to established interspecies sequence homologies of constant regions of about 60%. Some genes of the immunoglobulin heavy-chain variable region thus appear to have been conserved through a considerable period of evolutionary time.
The analyses also showed that the presence of certain amino acids at certain positions in these heavy chains could be correlated with the species of origin. The occurrence of such “phylogenetically associated” residues is most consistent with the presence of a restricted number of genes in the heavy-chain variable region pool.
Keywords: VHIII subgroup, dog, cat, man, theories of antibody diversity
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bourgois Alain, Fougereau Michel. Partial amino acid sequence of the variable region of a mouse gammaG2a immunoglobulin heavy chain. Evidence for the existence of a third sub-group of variability for the heavy chain pool. FEBS Lett. 1970 Jun 27;8(5):265–268. doi: 10.1016/0014-5793(70)80283-6. [DOI] [PubMed] [Google Scholar]
- Capra J. D. Hypervariable region of human immunoglobulin heavy chains. Nat New Biol. 1971 Mar 10;230(10):61–63. doi: 10.1038/newbio230061a0. [DOI] [PubMed] [Google Scholar]
- Capra J. D., Kehoe J. M., Williams R. C., Jr, Feizi T., Kunkel H. G. Light chain sequences of human IgM cold agglutinins (variable-region subgroups amino-acid sequence-kappa light chain-N-terminal). Proc Natl Acad Sci U S A. 1972 Jan;69(1):40–43. doi: 10.1073/pnas.69.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capra J. D., Kehoe J. M., Winchester R. J., Kunkel H. G. Structure-function relationships among anti-gamma globulin antibodies. Ann N Y Acad Sci. 1971 Dec 31;190:371–381. doi: 10.1111/j.1749-6632.1971.tb13549.x. [DOI] [PubMed] [Google Scholar]
- Cunningham B. A., Pflumm M. N., Rutishauser U., Edelman G. M. Subgroups of amino acid sequences in the variable regions of immunoglobulin heavy chains. Proc Natl Acad Sci U S A. 1969 Nov;64(3):997–1003. doi: 10.1073/pnas.64.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M., Cunningham B. A., Gall W. E., Gottlieb P. D., Rutishauser U., Waxdal M. J. The covalent structure of an entire gammaG immunoglobulin molecule. Proc Natl Acad Sci U S A. 1969 May;63(1):78–85. doi: 10.1073/pnas.63.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gally J. A., Edelman G. M. Somatic translocation of antibody genes. Nature. 1970 Jul 25;227(5256):341–348. doi: 10.1038/227341a0. [DOI] [PubMed] [Google Scholar]
- Hilschmann N., Craig L. C. Amino acid sequence studies with Bence-Jones proteins. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1403–1409. doi: 10.1073/pnas.53.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood L., Eichmann K., Lackland H., Krause R. M., Ohms J. J. Rabbit antibody light chains and gene evolution. Nature. 1970 Dec 12;228(5276):1040–1044. doi: 10.1038/2281040a0. [DOI] [PubMed] [Google Scholar]
- Hood L., Ein D. Immunologlobulin lambda chain structure: two genes, one polypeptide chain. Nature. 1968 Nov 23;220(5169):764–767. doi: 10.1038/220764a0. [DOI] [PubMed] [Google Scholar]
- Hood L., Talmage D. W. Mechanism of antibody diversity: germ line basis for variability. Science. 1970 Apr 17;168(3929):325–334. doi: 10.1126/science.168.3929.325. [DOI] [PubMed] [Google Scholar]
- Hurvitz A. I., Kehoe J. M., Capra J. D. Characterization of three homogeneous canine immunoglobulins. J Immunol. 1971 Sep;107(3):648–654. [PubMed] [Google Scholar]
- Kabat E. A. The paucity of species-specific amino acid residues in the variable regions of human and mouse Bence-Jones proteins and its evolutionary and genetic implications. Proc Natl Acad Sci U S A. 1967 May;57(5):1345–1349. doi: 10.1073/pnas.57.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehoe J. M., Capra J. D. Localization of two additional hypervariable regions in immunoglobulin heavy chains. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2019–2021. doi: 10.1073/pnas.68.9.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo R. T., Rosenblum I. Y., Benedict A. A. Amino terminal sequences of heavy and light chains of chicken anti-dinitrophenyl antibody. J Immunol. 1971 Dec;107(6):1781–1784. [PubMed] [Google Scholar]
- Köhler H., Shimizu A., Paul C., Moore V., Putnam F. W. Three variable-gene pools common to IgM, IgG and IgA immunoglobulins. Nature. 1970 Sep 26;227(5265):1318–1320. doi: 10.1038/2271318a0. [DOI] [PubMed] [Google Scholar]
- Milstein C. Linked groups of residues in immunoglobulin k chains. Nature. 1967 Oct 28;216(5113):330–332. doi: 10.1038/216330a0. [DOI] [PubMed] [Google Scholar]
- Milstein C., Pink J. R. Structure and evolution of immunoglobulins. Prog Biophys Mol Biol. 1970;21:209–263. doi: 10.1016/0079-6107(70)90026-x. [DOI] [PubMed] [Google Scholar]
- Niall H. D., Edman P. Two structurally distinct classes of kappa-chains in human immunoglobulins. Nature. 1967 Oct 21;216(5112):262–263. doi: 10.1038/216262a0. [DOI] [PubMed] [Google Scholar]
- Press E. M., Hogg N. M. Comparative study of two immunoglobulin G Fd-fragments. Nature. 1969 Aug 23;223(5208):808–810. doi: 10.1038/223807a0. [DOI] [PubMed] [Google Scholar]
- Wang A. C., Fudenberg H. H., Pink J. R. Heavy-chain variable regions in normal and pathological immunolobulins. Proc Natl Acad Sci U S A. 1971 Jun;68(6):1143–1146. doi: 10.1073/pnas.68.6.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu T. T., Kabat E. A. An analysis of the sequences of the variable regions of Bence Jones proteins and myeloma light chains and their implications for antibody complementarity. J Exp Med. 1970 Aug 1;132(2):211–250. doi: 10.1084/jem.132.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]


