Summary
The flow dynamics and pressure relationships in an ulcerated atherosclerotic carotid bulb obtained at post-mortem were studied and correlated with angiographic findings in a similar live patient.
Using the lost wax technique, we created replicas of an ulcerated atherosclerotic carotid bulb from a fresh cadaver; and placed those replicas in a circuit of pulsating non-Newtonian fluid. Flow profiles were adjusted to replicate human physiologic flows, and flow rates of400, 600, and 800 milliliters per minute were evaluated. In the replicas, the slipstreams were opacified with isobaric dyes, and images were recorded both on 35 mm film and on SuperVHS high speed video. Data were collected from needles placed radially in the common carotid artery, in the region of the maximal atherosclerotic narrowing, and in the internal carotid artery. Though pressure relationships could not be obtained in the live human for ethical reasons, angiography in a similar stenosis was evaluated for slipstream dynamics.
The post-mortem replica had a 55% diameter stenosis (88% area stenosis) of the carotid bulb with a shallow 3 mm ulcer.
Flow in the common carotid artery showed undisturbed slipstreams, but as these slipstreams entered the narrow bulb, they crowded together, accelerating dramatically, with a jet continuing distally beyond the maximal narrowing for at least 2 vessel diameters, where flow again became normal. As fluid entered the narrowed bulb, radial pressures decreased and within the ulcer a vortex circulation was found. Similar findings were observed on the angiographic images of the live patient.
This combination of events, the slowly swirling fluid in the ulcer, which would allow platelet aggregates to form, and the intermittent low pressure of the Bernoulli effect which could pull the aggregates into the adjacent rapidly flowing blood may help explain how ulcerated carotid plaques lead to embolic stroke.
Key words: flow dynamics, vascular pressure, carotid stenosis, ulcerated atherosclerotic plaque, angiography
Introduction
Distal embolisation of thrombus from narrowed carotid bulbs is a well recognized source of ischaemic infarction. To that end, carotid artery flow dynamics have been investigated but the studies have generally involved normal vessels, and direct pressure measurements from the changes caused by atherosclerosis have not been extensively studied1-8.
We have recently obtained a fresh cadaver containing an ulcerated stenotic bulb, have made accurate castings and reproductions of that carotid system, and have studied the flow dynamics and pressure relationships in it 9. Rapid serial angiography in a patient with a similar ulcerated narrowing was analyzed to verify the findings seen in the replica.
We would now like to report the results of our observations.
Material and Methods
Using a previously described technique, we created an accurate casting of an ulcerated atherosclerotic carotid bulb from a fresh human cadaver10,11.
Accuracy of the casting and the resultant replicas reproduced the original specimen to within 1%. The clear silicone replicas were placed in a circuit of pulsatile non-Newtonian fluid 12,13.
Fluid flow was provided by a heart pump (model 1421, Harvard apparatus, S Natick, MA) cycling at one pulse per second with fluid flows adjusted to replicate human physiologic flow profiles. Flow profiles were analyzed with a Square Wave Electromagnetic Flowmeter (Carolina Medical Electronics, Inc., King, NC), so that internal carotid artery flow during diastole was 40% that of peak systole. Diastolic flow in the external carotid artery fell to zero, replicating flow profiles found in normal humans by ultrasonography. Total common carotid artery flow rates at 400, 600, and 800 milliliters/minute (mL/min) were evaluated. Though in normal humans only about 30% of common carotid artery flow enters the external carotid artery, the stenosis resulted in about 50% of the total flow passing into the external carotid artery. We opacified the slipstreams with isobaric colored dyes, and recorded our images both on 35 mm film and on high speed super VHS video at shutter speeds of 1/1000 second. The pressure recording device (Dual Pressure Recorder, Opt. 21, Tektronix #414, Beaverton, OR) was calibrated in the non-Newtonian fluid and synchronized to the blood pump by an electrocardiograph monitor to record systole.
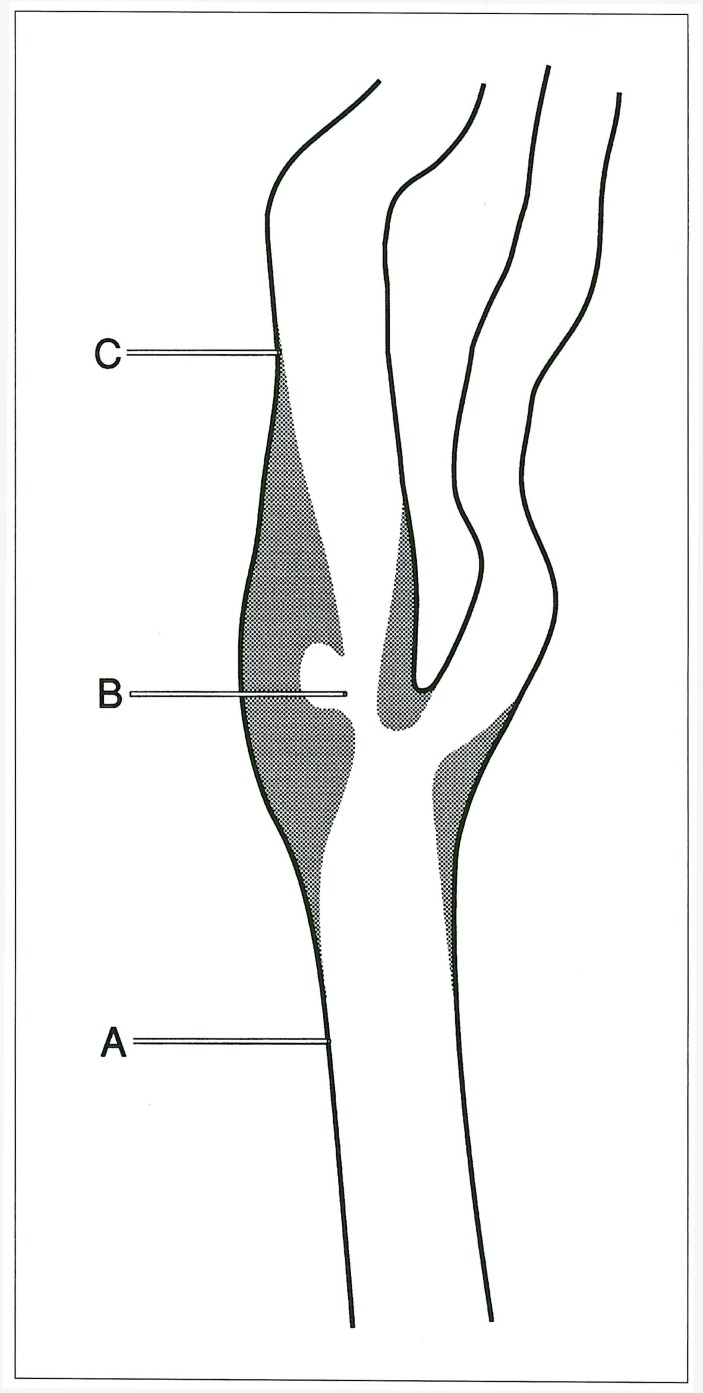
Blunt 23-gauge needles recorded radial pressures along the vessel wall. Radial pressure is the pressure perpendicular to the long axis of a vessel and is the pressure recorded by a blood pressure cuff. Radial recordings were made at three locations: the common carotid artery (2-3 vessel diameters proximal to the bulb), the internal carotid artery (2-3 vessels diameters distal to the bulb), and within the atherosclerotic stenosis (figure 1).
Figure 1.
Needle tip placements during radial pressure recordings: A) common carotid artery; B) stenotic ulcerated bulb; C) internal carotid artery.
A magnified cut film angiogram was performed in a live patient to evaluate transient cerebral ischaemia.
The patient had an ulcerated stenotic carotid bulb. Images were obtained at a rate of three films per second for a total of six seconds, and the resulting images were studied both from the plain film radiographs and with subtraction technique.
Results
For descriptive purposes, we will consider the internal carotid artery and the bulb a posterior structure, and the external carotid artery an anterior structure.
Measurements
The diameters of the vascular cast from the cadaver were measured in the regions where the pressure measurements were obtained. The common carotid artery measured 7.2 mm. In the narrowest portion of the atherosclerotic plaque, the internal carotid artery measured 2.5 mm.
Distal to the bulb, the internal carotid artery measured 5.5 mm.
The ulcer was shallow, measuring 5.0 mm long, 2.9 mm wide, and 3.3 mm deep. These values equate to a 55% diameter stenosis using NASCET criteria and an 88% area stenosis of the carotid bulb.
The diameters taken from the angiogram radiographic projections in a single plane were 6.3 mm at the narrowest portion of the carotid bulb, and 7.4 mm at the internal carotid artery measurement site. This equates to a 15% diameter stenosis of the carotid bulb by NASCET criteria.
Slipstream Flows
In the common carotid artery, the slipstreams were undisturbed and parallel to the vessel side wall. As in a human, these slipstreams were of generally equal velocity.
As the slipstreams entered the bulb though, they crowded together and accelerated significantly. Beyond the narrowing, a central high-velocity jet continued for a distance of two to three vessel diameters, passing mostly along the posterior wall of the internal carotid artery.
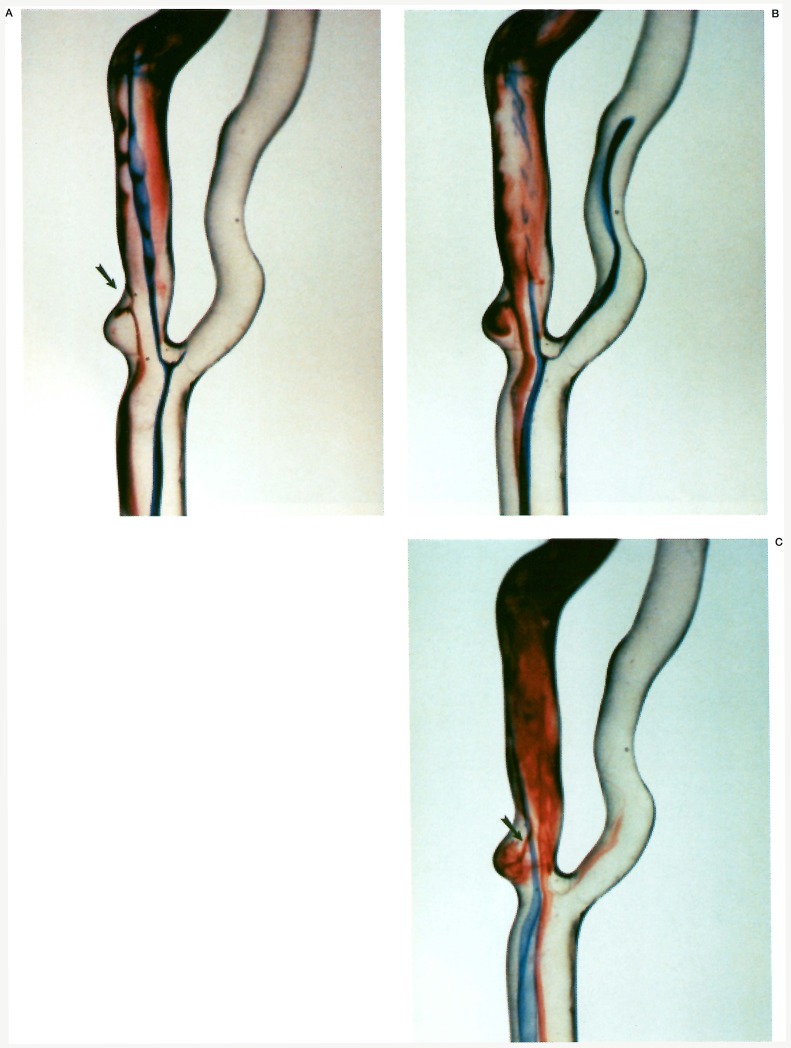
In the immediate post-stenotic segment, there was disturbed flow around the periphery of the jet, with slipstreams eddying circumferentially, but mostly anteriorly. As the slipstreams passed more distally into the internal carotid artery, 2-4 vessel diameters downstream, they regained a more normal profile, and assumed a helical flowing pattern especially in regions of vessel curvature. Some fluid slipstreams entered the distal portion of the ulcer, swirled along the posterior plaque opposite the direction of normal flow, and then exited via the proximal opening of the ulcer (figure 2).
Figure 2.
Ulcer entry and exit flow analysis. A) There is slipstream entry into the distal neck of the ulceration (arrow). B) Sluggish swirling slipstreams in a reverse vortex pattern are seen within the ulceration. C) Injections into different slipstreams show an opacified slipstream exiting the proximal neck of the ulcer (arrow) and being sucked into the central jet of the stenosis.
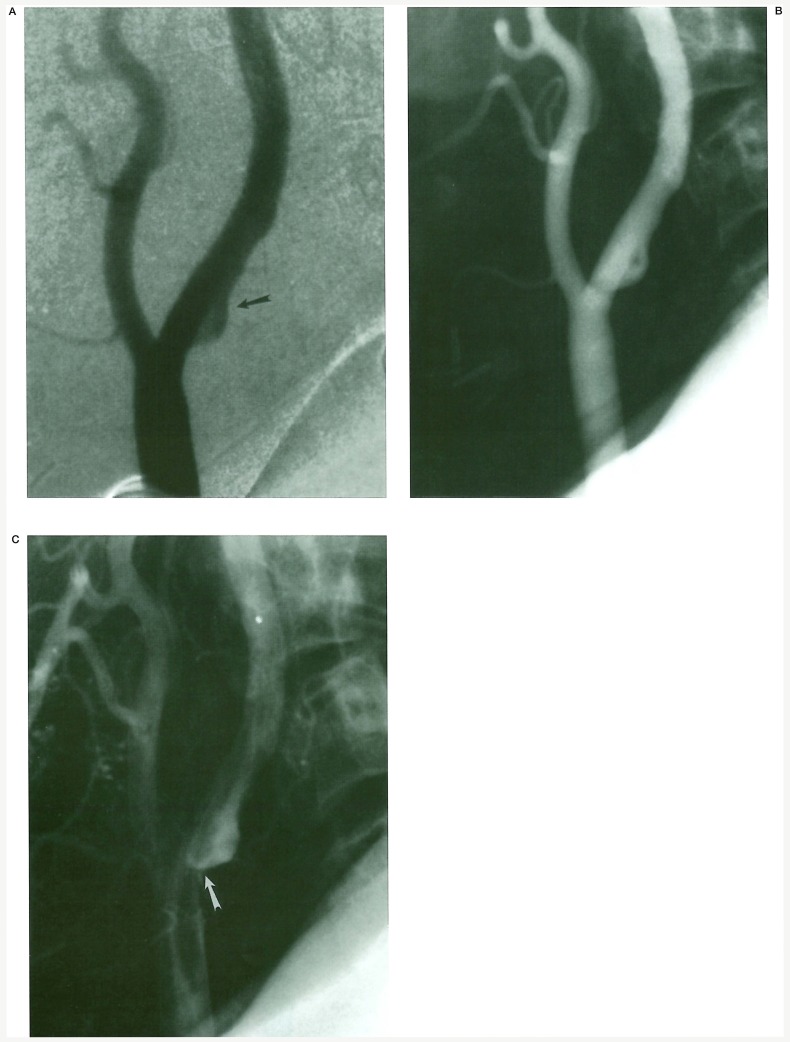
The angiographic images show a similar reverse vortex flow within the atherosclerotic ulcer of the live patient (figure 3).
Figure 3.
In vivo angiographic flow analysis. A) Slipstream entry into the distal neck of the carotid bulb ulcer (arrow). Film subtraction technique used for slipstream visualization. B) Vortex circulation is seen within the ulcer cavity. C) Slipstream exit from the proximal neck of the ulcer into the central jet of the stenosis (arrow).
Pressures
Radial pressure recordings throughout the cardiac cycle at flow rates of 400, 600, and 800 mL/min, which represent low, average, and high physiologic flow states, yielded the results in table 1.
Table 1.
Radial Pressures: peak systole / peak diastole
| CCA | Vessel segment Stenosis |
ICA | ||
|---|---|---|---|---|
| 400 | 26/14 | 21/10 | 21/11 | |
| Q | 600 | 43/22 | 27/16 | 34/17 |
| 800 | 52/23 | 39/16 | 44/22 | |
|
Q = Flow milliliters/minute (mL/min); CCA = Common carotid artery; Stenosis = Stenotic atherosclerotic bulb; ICA = Internal carotid artery; Pressures measured in centimeters of water (cm H2O) | ||||
As fluid entered the plaque narrowing and accellerated, radial pressures fell. The same inverse radial pressure to velocity relationships continued for about two vessel diameters downstream beyond the narrowing, but to a lesser degree. Most important, at peak systole, the lowest radial pressure with a sluggish vortex circulation was found at the ulceration.
These lowered readings were not apparent at a rate of 400 mL/min, but their relationships became obvious at the higher flow rates. We did not attempt invivo pressure correlation in the live patient because of ethical considerations.
Discussion
Ischaemic stroke remains a major problem in western countries, and it is likely that the majority of ischaemic strokes originate from an atherosclerotic carotid bulb. It is difficult to study what effect flow dynamics have on the origin and development of ischaemic stroke as flowing blood is opaque, and most of the methods used to study it are invasive, with the exception of duplex Doppler ultrasonography.
Angiography, for example, provides superb anatomic detail of both normal and stenotic arteries, but unfortunately gives essentially no physiologic data. Magnetic resonance angiography can provide physiologic information, but is relatively underutilized today for this. Individual slipstream flows and, more importantly, pressure changes cannot be measured by either of these modalities.
We have used clear elastic silicone vascular replicas which we have created from fresh human cadaver sources to allow a high resolution detailed recording of individual slipstream profiles in an attempt to understand carotid flow dynamics better.
Also, we are able to make in vitro measurements of physiologic forces such as pressures. Understanding these slipstream dynamics and those forces may provide important insight into pathologic processes. The angiographic series of images provides invivo confirmation of what was found in the laboratory and lends credence to the experimental observations and the subsequent conclusions.
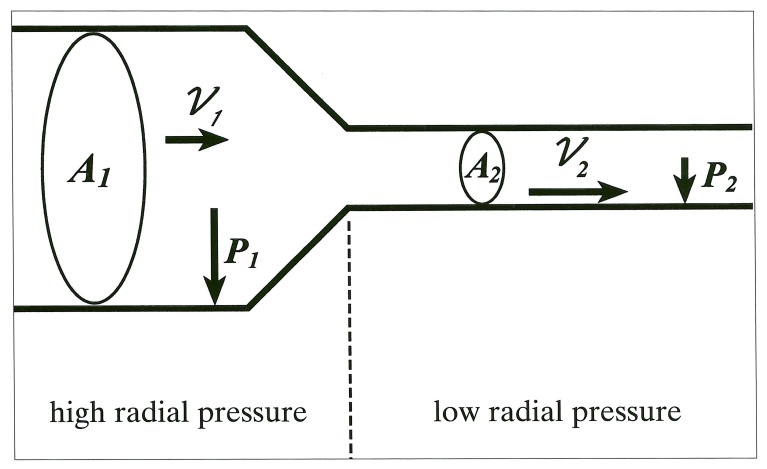
The underlying mathematics relating velocity to pressures is relatively simple. The radial pressure changes inversely and proportionally to the fluid velocity as described by the following equations14,15.
| A1ν1=A2ν2 | [equation (Eq)1] |
where A = cross-sectional area
and ν= fluid velocity
| [Eq 2] |
where = radial pressure
ρ = fluid viscosity, and
ν = fluid velocity
Thus in a Newtonian system (which is a simplification - blood being a non-Newtonian fluid) as arterial cross-sectional area decreases, the fluid velocity increases, and the radial pressure decreases (e.g. if A2 < A1, then ν2 > ν1 [Eq 1] and if ν2 > ν1, then P2 < P1 [Eq 2]) (figure 4). The physical principles thus favor a lower pressure in the stenosis during the highest velocities, e.g. during peak systole.
Figure 4.
Graphic depiction of area-velocity and velocity-pressure equations. Vector length is proportional to vector magnitude.
Conclusions
How does this help us understand distal embolisation? Following analysis of the fluid slipstream profiles, we found that in the common carotid artery, slipstreams in the replica were similar to those found by Doppler ultrasonography; that is all velocity vectors were nearly equal, except for those near the artery wall's boundary layer, where velocities fell rapidly toward zero16.
At the site of the stenosis, the opacified slipstreams crowded together and accelerated within the narrowing. Despite the narrowings caused by the plaques, fluid slipstreams in the poststenotic segment were non-turbulent, though disturbed.
As important, vortexes and reverse swirling flow were evident within the atherosclerotic ulcer cavity. If the ulcer cavity is denuded of endothelium, the combination of the slow swirling flow and the lack of endothelium would result in platelet activation and aggregation as well as thrombus formation. It was not possible to see the individual slipstreams in the human internal carotid artery because of complete mixing of the contrast agent.
Further, it must be remembered that contrast agent is not perfectly physiologic as it is denser than human blood. However, due to the slow swirling flow in the ulcer cavity, opacified individual slipstreams could be identified and confirm the laboratory observations.
In comparison to the radial pressures measured in the common carotid and internal carotid arteries, radial pressures in the high velocity stenotic segment decreased. In particular, this relationship of radial pressure decrease found at the stenotic ulcerated bulb was most profound during periods of maximal flow velocity (peak systole).
The development of low pressure at the stenosis during systole pulls fluid from the adjacent ulcer cavity.
This combination of events, slowly swirling fluid in the ulcer cavity allowing platelet aggregates and thrombus to form, and the intermittent Bernoulli effect sucking these aggregates into the adjacent rapidly flowing blood within the stenosis, helps explain how human ulcerated atherosclerotic carotid artery plaques lead to embolic stroke.
Acknowledgement
We gratefully acknowledge Shelley Van Buren for her invaluable assistance in preparing the manuscript.
References
- 1.Bharadvaj BK, Mabon RF, Giddens DP. Steady flow in a model of the human carotid bifurcation: Part I - Flow visualization. J Biomech. 1982;15(5):349–362. doi: 10.1016/0021-9290(82)90057-4. [DOI] [PubMed] [Google Scholar]
- 2.Dewey CF. Fluid mechanics of arterial flow. Adv Exp Med Biol. 1979;115:55–104. [Google Scholar]
- 3.Fox JA, Hugh AE. Static zones in the internal carotid artery: Correlation with boundary layer separation and stasis in model flows. Br J Radiol. 1976;43:370–376. doi: 10.1259/0007-1285-43-510-370. [DOI] [PubMed] [Google Scholar]
- 4.Karino T, Goldsmith HL, et al. Flow patterns in vessels of simple and complex geometries. Ann NY Acad Sci. 1987;516:422–441. doi: 10.1111/j.1749-6632.1987.tb33063.x. [DOI] [PubMed] [Google Scholar]
- 5.Kerber CW, Knox K, et al. Flow Dynamics in the human carotid bulb. Int J Neuroradiol. 1996;2(5):422–429. [Google Scholar]
- 6.Kerber CW, Liepsch D. Flow dynamics for radiologists. II. Practical considerations in the live human. Am J Neuroradiol. 1994;15(6):1076–1086. [PMC free article] [PubMed] [Google Scholar]
- 7.Ku DN, Giddens DP. Pulsatile flow in a model carotid bifurcation. Arteriosclerosis. 1983;3(1):31–39. doi: 10.1161/01.atv.3.1.31. [DOI] [PubMed] [Google Scholar]
- 8.Motomiya M, Karino T. Flow patterns in the human carotid artery bifurcation. Stroke. 1984;15(1):50–56. doi: 10.1161/01.str.15.1.50. [DOI] [PubMed] [Google Scholar]
- 9.Imbesi SG, Kerber CW. Why do ulcerated atherosclerotic carotid artery plaques embolize? A flow dynamics study. Am J Neuroradiol. 1998;19:761–766. [PMC free article] [PubMed] [Google Scholar]
- 10.Kerber CW, Heilman CB, Zanetti PH. Transparent elastic arterial models. I. A brief technical note. Biorheology. 1989;26(6):1041–1049. doi: 10.3233/bir-1989-26607. [DOI] [PubMed] [Google Scholar]
- 11.Liepsch D, Zimmer R. A method for the preparation of true-to-scale inflexible and natural elastic human arteries. Biomed Techn. 1978;23(10):227–230. doi: 10.1515/bmte.1978.23.10.227. [DOI] [PubMed] [Google Scholar]
- 12.Liepsch D, Morabec ST. Pulsatile flow of non-Newtonian fluid in distensible models of human arteries. Biorheology. 1984;21(4):571–586. doi: 10.3233/bir-1984-21416. [DOI] [PubMed] [Google Scholar]
- 13.Mann DE, Tarbell JM. Flow of non-Newtonian blood analog fluids in rigid curved and straight artery models. Biorheology. 1990;27(5):711–733. doi: 10.3233/bir-1990-27508. [DOI] [PubMed] [Google Scholar]
- 14.Berne RM, Levy MN. Haemodynamics, in: Physiology. St. Louis: Mosby; 1983. pp. 473–484. [Google Scholar]
- 15.Serway RA. Physics for Scientists & Engineers. ed 4. Philadelphia: W.B. Saunders; 1996. pp. 433–435. [Google Scholar]
- 16.Keller HM, Merer WE, et al. Noninvasive measurement of velocity profiles and blood flow in the common carotid artery by pulsed Doppler ultrasound. Stroke. 1976;7:370–377. doi: 10.1161/01.str.7.4.370. [DOI] [PubMed] [Google Scholar]