Summary
We describe 19 (16.0%) multiple vascular malformations (AVMs) in 119 spinal cord arteriovenous malformations (SCAVMs).
The associated lesions were eight vertebral vascular malformations, two cutaneous, four limbs, four radicular AVMs, three bifocal SCAVMs; one patient had a bifocal cord lesion associated with vertebral and limb localisations. Various syndromic associations were seen: nine Cobb, two Klippel-Trenaunay-Weber, one Parkes Weber. An additional subgroup of unclassified associations is constituted by seven cases with bifocal intradural uni or multimetameric lesions.
In our SCAVMs series, the incidence of multiple vascular lesions is high, in particular multifocal intradural malformations. Metameric distribution is the most frequent type of multiplicity. Identification of the myelomeric level involved in SCAVM allows segmental link between various lesions of mesodermal or neural crest origin to be discussed.
Key words: spinal cord arteriovenous malformation, radicular arteriovenous malformations, multiple arteriovenous malformations, Cobb syndrome, Klippel-Trenaunay-Weber syndrome
Introduction
Spinal cord arteriovenous malformations (SCAVMs) are uncommon 1, their incidence being only about one-tenth of brain arteriovenous malformations (BAVMs)2. Multifocal central nervous system AVMs and AVMs with soft tissue vascular malformation (VM) are a classic association3-14. In this study we focus on this coexistence and discuss the segmental relation between these lesions.
Material and Methods
We reviewed the clinical and radiological charts of 119 SCAVMs referred to us between 1982 and 1996 from which we found 19 patients (16.0%) who had either multiple SCAVM or associated vascular malformations (VMs).
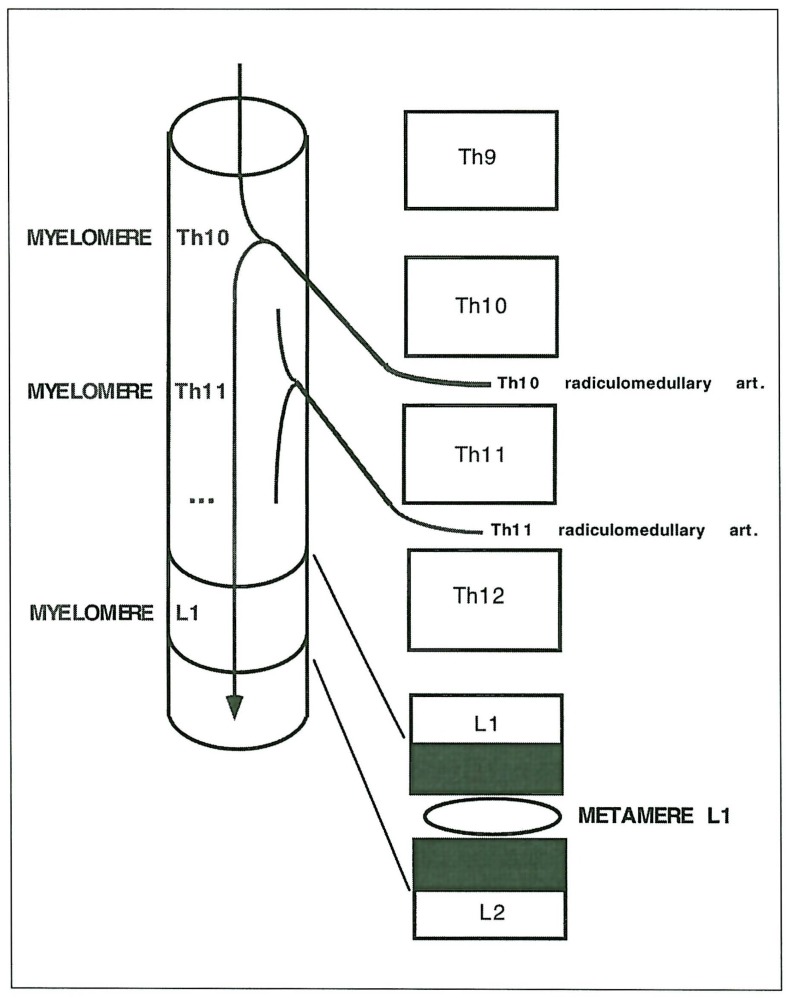
We named vertebral associated lesions those that are usually described as spinal haemangiomas; they were located either at the same metameric level or at an immediatly adjacent one. Axial and para-axial cutaneous lesions were all of the venular type (port wine stain); paraspinal lesions involved the para-axial muscles and sometimes represented extensive high flow lesions; radicular lesions were localised on the spinal nerve intradurally and were supplied by radiculopial or radiculomedullary arteries and drained into spinal cord veins. In one case it was difficult to establish whether the radicular AVM nidus was intradural or dural (spinal dural arteriovenous shunt (DAVS).The denomination of Cobb syndrome was given to SCAVMs associated with vertebral, cutaneous and/or paraspinal lesions in the same or adjacent segment. Klippel Trenaunay syndrome corresponded to (associated) limb venolymphatic lesions without AV shunts, Parkes Weber to (associated) limb localisations with AV shunts whether or not there was an associated venous component. The myelomeric localisation was established from the relation between the segmental artery origin of the radiculopial or medullary artery and the level at which it reached the cord surface. The level of pial origin of the venous outlet at the cord indicated the level at which the SCAVM was located.
Results
The cases are summarized in table 1.The age at referral ranged from 11 months to 44 years, with a mean age of 21 years. There were 13 males and six females. Seven lesions were located in the cervical region, six thoracic, six lumbar or at the conus medullaris.
Table 1.
Summary of the cases
| Case No |
Sex | Age in years |
Revealing symptom |
Location of SCAVM |
Tt | Aassociated lesions |
Syndromes |
|---|---|---|---|---|---|---|---|
| 1 | M | 19 | PND | C | E | vertebral | Cobb (figure 1) |
| 2 | M | 4 | PND | CM | no | cutaneous | Cobb |
| 3 | F | 19 | SAH | C | no | vertebral | Cobb |
| 4 | M | 3 | incidental | T | no | cutaneous | Cobb |
| 5 | M | 40 | PND | T | E | vertebral | Cobb |
| 6 | M | 23 | PND | C | no | vertebral, paraspinal | Cobb |
| 7 | M | 23 | SAH | C | E | vertebral | Cobb |
| 8 | F | 0,9 | PND | T | E | vertebral, paraspinal | Cobb |
| 9 | M | 19 | PND | T | E | vertebral | Cobb |
| 10 | M | 10 | PND | CM | E | limb | KTW |
| 11 | F | 17 | SAH, HM | CM | no | limb | PW |
| 12 | M | 34 | PND | CM | no | limb | KTW |
| 13 | M | 18 | PND | Lx2 | E | bifocal, vertebral, limb | ?, Cobb, PW (figure 2) |
| 14 | M | 13 | PND | Cx2 | E | bifocal | ?, (figure 3) |
| 15 | F | 25 | PND | Tx2 | E | bifocal | ? |
| 16 | M | 34 | PND | C | E | radicular | ?, (figure 6) |
| 17 | F | 24 | SAH | Cx2 | E | radicular | ? |
| 18 | F | 32 | PND | T | E | radicular | ?, (figure 4) |
| 19 | M | 44 | PND | L | op | radicular | ? |
|
PND = Progressive neurological deficit; SAH = Subarachnoid haemorrhage; HM = Haematomyelia; C = Cervical; T = Thoracic; L = Lumbar; CM = Conus medullaris; E = Embolisation; op = surgically operated; KTW = Klippel-Trenaunay; PW = Parkes Weber; SCAVM = spinal cord arteriovenous malformation. | |||||||
The associated lesions were eight vertebrae, two cutaneous (skin discoloration or port wine stain like), four in legs (two Klippel Trenaunay and two Parkes Weber), four intradural radicular AVMs, and three cases of multifocal SCAVMs.
Among these cases, there were nine cases of Cobb syndrome which had vertebral or soft tissue VMs, one case of Parkes-Weber (PW) syndrome (in addition, one case with combined Cobb and PW syndromes was included in the multifocal SCAVMs, figure 2), two Klippel-Trenaunay syndrome. Seven cases had multiple intradural lesions involving the cord or the nerve roots. Among these one could also be described as multimetameric Cobb syndrome as both myelomeric AVMs were associated with other AVMs in their respective segments. One of the multifocal SCAVM cases (nº 15) has already been reported16.
Ilustrative cases
Case 1 (figure 1)
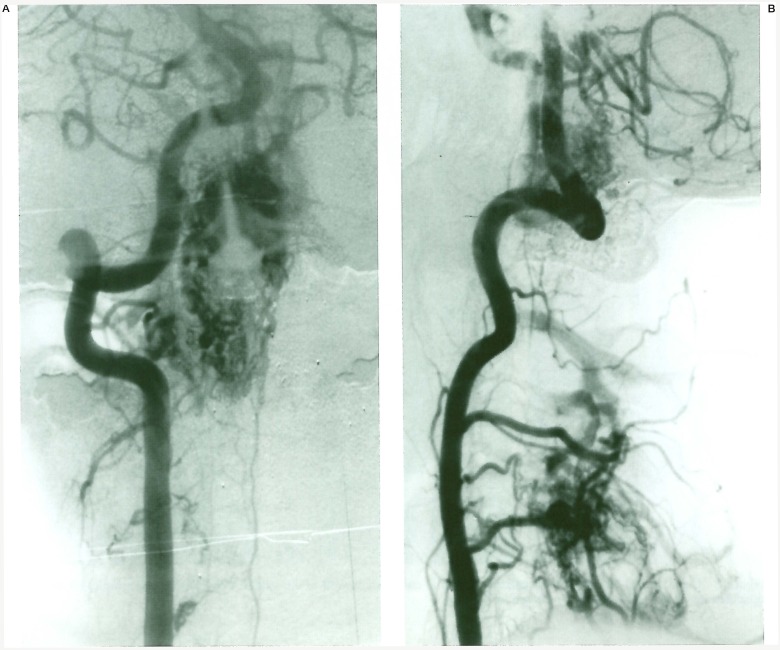
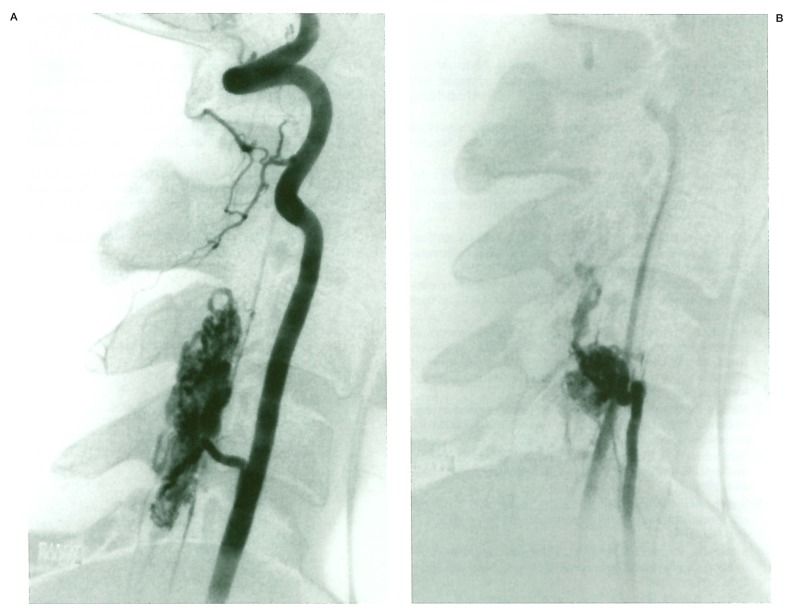
Figure 1.
Case 1. Left vertebral angiogram (A) shows the extensive SCAVM nidus, the right one (B) demonstrates the associated bone AVM.
A 19-year-old man presented with non haemorrhagic progressive right-sided Brown-Sequard syndrome. Magnetic resonance imaging revealed a large SCAVM extending over several cervical cord segments. Left vertebral and right ascending cervical artery injections demonstrated an associated AVM in the spinous process of C4 vertebrae. The patient was embolized in several sessions, and his neurological condition is stable. This case was considered a Cobb syndrome with multimyelomeric involvement of the cervical cord and a single C4 bone localisation.
Case 13 (figure 2)
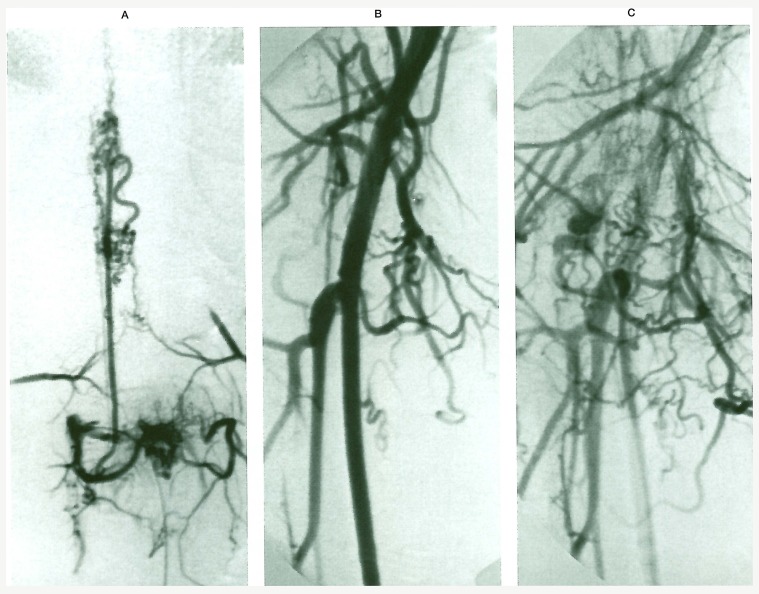
Figure 2.
Case 13. (A) LI injection visualizes the spinal cord nidi as well as the spinal AVM. Iliac arteriography (B and C) opacifies the thigh AVM partially resected in the past.
A 17-year-old man presented with progressive paraparesis of his lower limbs and difficulty in urinating. When he was 12-years-old a difference in the length of his legs (the right leg being longer) and some venous congestion on the proximal part of his right lower limb due toan AVF located in his thigh were noted. The AVF had been operated upon with partial excision. The present spinal angiography revealed a bifocal SCAVM located at LI and L3 myelomeric levels and a vascular lesion in the LI vertebral body.
These two SCAVMs were mainly supplied by the anterior spinal artery originating from LI on the right side. Each of them had a prominent venous outlet, the upper one drained cranially, the lower one caudally, but both seemed to communicate. The vascular lesion at LI vertebral body was supplied by the corresponding bone and dural branches from the first lumbar artery. The injection into the right internal iliac artery showed evidence of a small AVM in the thigh, corresponding to the remainder of the previously operated one. That leg lesion on the right side was considered a PW syndrome. However its localisation in the limb suggested L3 territory. Finally the present case can also be described as a multimetameric “Cobb syndrome”: LI with a spinal cord and bone involvement, and L3 with a spinal cord and soft tissue involvement.
Case 14 (figure 3)
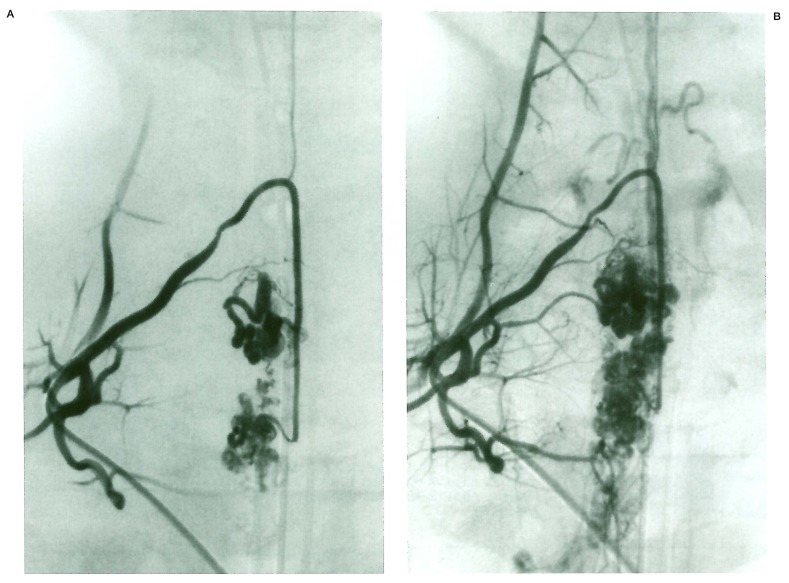
Figure 3.
Case 14. Costocervical injection (A and B) shows bifocal SCAVM with distinct venous drainage.
A 14-year-old boy presented with sudden neck pain and vomiting, but subarachnoid haemorrhage could not be established with certainty. Spinal angiography revealed a bifocal SCAVM located at the cervical spinal cord. Right deep cervical artery injection and right superior intercostal artery injection demonstrated the radiculomedullary and radiculopial arteries supplying both lesions with normal spinal cord tissue identified between both nidi. The venous drainages although separate communicated before leaving the subarachnoid space.
Case 18 (figure 4)
Figure 4.
Case 18. L2 injection (A) demonstrates a large SCAVM. L3 (B) visualises a radicular AVM draining into the same venous outlets (C).
A 33-year-old female presented with a two year history of progressive Brown Sequard involving the low thoracic cord on the left side. MRI failed to demonstrate any haemorrhage but showed extensive intramedullary vascular malformations within the thoracolumbar cord. Angiography demonstrated a SCAVM fed by a radiculomedullary artery at L2 on the right side. The nidus extended over several myelomeres. During spinal angiography, L3 injection showed a radicular AVM draining into a radicular vein joining the upper portion of the SCAVM venous drainage. Both lesions were felt to be metamerically linked.
Discussion and Conclusions
Berenstein et Al reported that 28% patients with SCAVMs had associated VMs or dysplasia and 10% had metameric associated lesions 1. Although dysplasias are excluded from our study, the incidence of SCAVMs associated with other VMs in a metameric distribution is 16%. Noñe of our patients had Rendu-Osler-Weber disease (ROW) and none of our ROW cases had multifocal lesions involving the cord. There were three cases of multiple lesions in the spinal cord in this study, and all together seven multifocal intradural malformations (6%), which were not ROW.
Multifocal SCAVMs are exceptional compared to multifocal cerebral AVMs. The latter have been reported by several authors 3,5,7,8,10,11,13,14. According to Wülinsky et Al, 18 (9%) patients among 203 cerebral vascular lesions could be included in the multiple BAVM category15. In the paediatric population, Iizuka et Al reported that 20% of lesions are multiple5. In our series 5/19 patients were under 15. It is remarkable to note that although we encountered spinal nerve AVMs we saw no cranial nerve malformations.
The involvement of skin, vertebrae and spinal cord by VMs constitutes various syndromes 17-19. There are a few reports of KTW syndrome associated with SCAVMs 1,20,21. Benhaiem-Sigaux et A1 explained the association of the KTW and the SCAVM on a developmental basis21. In fact, the segmented mesodermic origin of the endothelial cells establishes the topographic link between these metameric targets 22. The association of SCAVM and radicular AVM is rare and potentially metameric if the myelomere involved with the SCAVM corresponds to that of the nerve involved. In such a situation, the radiculomedullary or radiculopial arteries directly supplying the SCAVMs (as soon as they reach the cord surface) nourish the radicular AVM (figure 5).
Figure 5.
Schematic representation of the arterial determination of myelomeric levels.
In other situations the SCAVM nidus extends over several myelomeric levels, allowing for different sources of supply for each lesion. In cases with cervical spinal cord and cervical nerve involvement, the role played by the vertebral and cervical arteries has to be carefully analysed (figure 6) to identify the associated lesions. False multifocal lesions can be diagnosed when intradural radicular veins are seen in the vicinity of their transdural course. In our four cases of associated SCAVM and radicular AVM, we considered that the vascular anatomy formally established a metameric link between them.
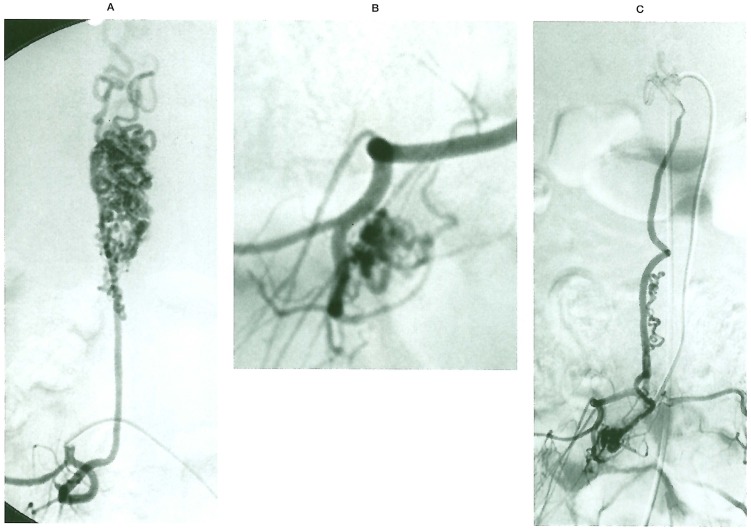
Figure 6.
Case 16. Vertebral injection opacifies a SCAVM at C5 (A) and the thyrocervical trunk injects a C5 nerve AVM (B) draining into the SCAVM outlets.
A peripheral (limb) vascular abnormality could also be included in the metameric group of disorders. Screening of the spinal cord with MRI is thus recommended23 in such a situation. However, MRI failed to assess the myelomeric level, the associated radicular lesions or the SCAVM multifocality in all our cases.
The treatment of patients who have multiple lesions is always difficult. The lesion responsible for the symptoms has to be identified and targeted first. Patients with metameric AVMs that present with spinal cord symptoms are dealt with in the same way as those with isolated SCAVMs. The strategy should be decided on an individual basis depending on the clinical history and the respective angioarchitecture of the various nidi demonstrated.
The discussion on the congenital nature of these lesions and the timing of hypothetical causative triggers is beyond the scope of the present paper. The concept of metameric lesion should be extended to the association between SCAVM and radicular AVM. The absence of dural involvement is remarkable and must be related to the absence of dural sinuses at spinal level. Careful identification of the vascular anatomy can point to the segmental link between the spinal cord, the spinal nerve and the soft tissues.
References
- 1.Berenstein A, Lasjaunias P. Endovascular treatment of spine and spinal cord lesions. vol 5. Berlin Heidelberg New York: Springer-Verlag; 1992. Surgical Neuroangiography. [Google Scholar]
- 2.Cogen P, Stein B. Spinal arteriovenous malformations with significant intramedullary components. J Neurosurg. 1983;59:471–478. doi: 10.3171/jns.1983.59.3.0471. [DOI] [PubMed] [Google Scholar]
- 3.Fukushima T, Hamano K, et al. A case of multiple arteriovenous malformations and diffuse venous abnormalities with facial port-wine stain. Child's Nerv Syst. 1989;5:114–117. doi: 10.1007/BF00571122. [DOI] [PubMed] [Google Scholar]
- 4.Hanieh A, Blumbergs PC, et al. Arteriovenous malformations associated with soft-tissue vascular malformations: case report. J Neurosurg. 1981;54:670–672. doi: 10.3171/jns.1981.54.5.0670. [DOI] [PubMed] [Google Scholar]
- 5.Iizuka Y, Rodesch G, et al. Multiple cerebral arteriovenous shunts in children: report of 13 cases. Child's Nerv Syst. 1992;8:437–444. doi: 10.1007/BF00274404. [DOI] [PubMed] [Google Scholar]
- 6.Mizutani T, Tanaka H, et al. Multiple arteriovenous malformations located in the cerebellum, posterior fossa, spinal cord, dura, and scalp with association of port-wine stain and supratentorial venous anomaly. Neurosurgery. 1992;31:137–141. doi: 10.1227/00006123-199207000-00022. [DOI] [PubMed] [Google Scholar]
- 7.Reddy K, West M, et al. Multiple intracerebral arteriovenous malformations: a case report and a literature review. Surg Neurol. 1987;27:495–499. doi: 10.1016/0090-3019(87)90261-8. [DOI] [PubMed] [Google Scholar]
- 8.Schlachter LB, Fleischer AS, et al. Multifocal intracranial arteriovenous malformations. Neurosurgery. 1980;7:440–444. doi: 10.1227/00006123-198011000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Smith ME, Barth PG, et al. Congenital multiple angiomatosis with brain involvement. Childs Brain. 1981;8:461–467. doi: 10.1159/000120015. [DOI] [PubMed] [Google Scholar]
- 10.Stone JL, Crowell RM, et al. Bilateral parietal arteriovenous malformations: report of a case. Neurosurgery. 1983;5:587–592. doi: 10.1227/00006123-198311000-00019. [DOI] [PubMed] [Google Scholar]
- 11.Tada T, Sugita K, et al. Supra and infratentorial arteriovenous malformations with an aneurysmal dilatation: a case report. Neurosurgery. 1986;5:831–834. doi: 10.1227/00006123-198611000-00021. [DOI] [PubMed] [Google Scholar]
- 12.Tamaki N, Fujita K, et al. Multiple arteriovenous malformations involving the scalp, dura, retina, cerebrum and posterior fossa: case report. J Neurosurg. 1971;34:95–98. doi: 10.3171/jns.1971.34.1.0095. [DOI] [PubMed] [Google Scholar]
- 13.Zellem RT, Buchheit WA. Multiple intracranial arteriovenous malformations: case report. Neurosurgery. 1985;17:88–93. doi: 10.1227/00006123-198507000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Berenstein A. Technique of catheterization and embolisation of the lenticulostriate arteries. J Neurosurg. 1981;54:783–789. doi: 10.3171/jns.1981.54.6.0783. [DOI] [PubMed] [Google Scholar]
- 15.Willinsky RA, Lasjaunias P, et al. Multiple cerebral arteriovenous malformations: Review of our experience from 203 patients with cerebral vascular lesions. Neuroradiology. 1990;32:207–210. doi: 10.1007/BF00589113. [DOI] [PubMed] [Google Scholar]
- 16.Meisel HJ, Lasjaunias P, et al. Multiple arteriovenous malformations of the spinal cord in an adolescent: case report. Neuroradiology. 1996;38:490–493. doi: 10.1007/BF00607286. [DOI] [PubMed] [Google Scholar]
- 17.Kissel P, Dureux JB. Cutaneo-meningo-spinal angiomatosis or syndrome de Cobb. In: Vinken PJ, Bruyn GW, editors. Handbook of Neurology. New York, London: Amsterdam; 1972. pp. 129–445. [Google Scholar]
- 18.Parkes-Weber F. Haemangiectatic hypertrophy of limbs congenital phlebarteriectasis and so-called congenital varicose. Br J Child Dis. 1918;15:13–23. [Google Scholar]
- 19.Klippel M, Trenaunay C. Du Naevus variqueux osteo-hypertrophique. Arch Gen Med. 1900;77:641–672. [Google Scholar]
- 20.Djindjian M, Djindjian R, et al. Spinal cord arteriovenous malformations and the Klippel-Trenaunay-Weber syndrome. Surg Neurol. 1977;8:229–237. [PubMed] [Google Scholar]
- 21.Benhaiem-Sigaux N, Zerah M, et al. A retromedullary arteriovenous fistula associated with the Klippel-Trenaunay-Weber syndrome. Acta Neuropathol (Berl) 1985;66:318–324. doi: 10.1007/BF00690965. [DOI] [PubMed] [Google Scholar]
- 22.Couly G, Coltey P, et al. The angiogenetic potentials of the cephalic mesoderm and the origin of brain and head blood vessels. Mechanisms of development. 1995;53:97–112. doi: 10.1016/0925-4773(95)00428-9. [DOI] [PubMed] [Google Scholar]
- 23.Lasjaunias P. Vascular diseases in neonates, infants and children. Berlin: Springer; 1997. [Google Scholar]