Abstract
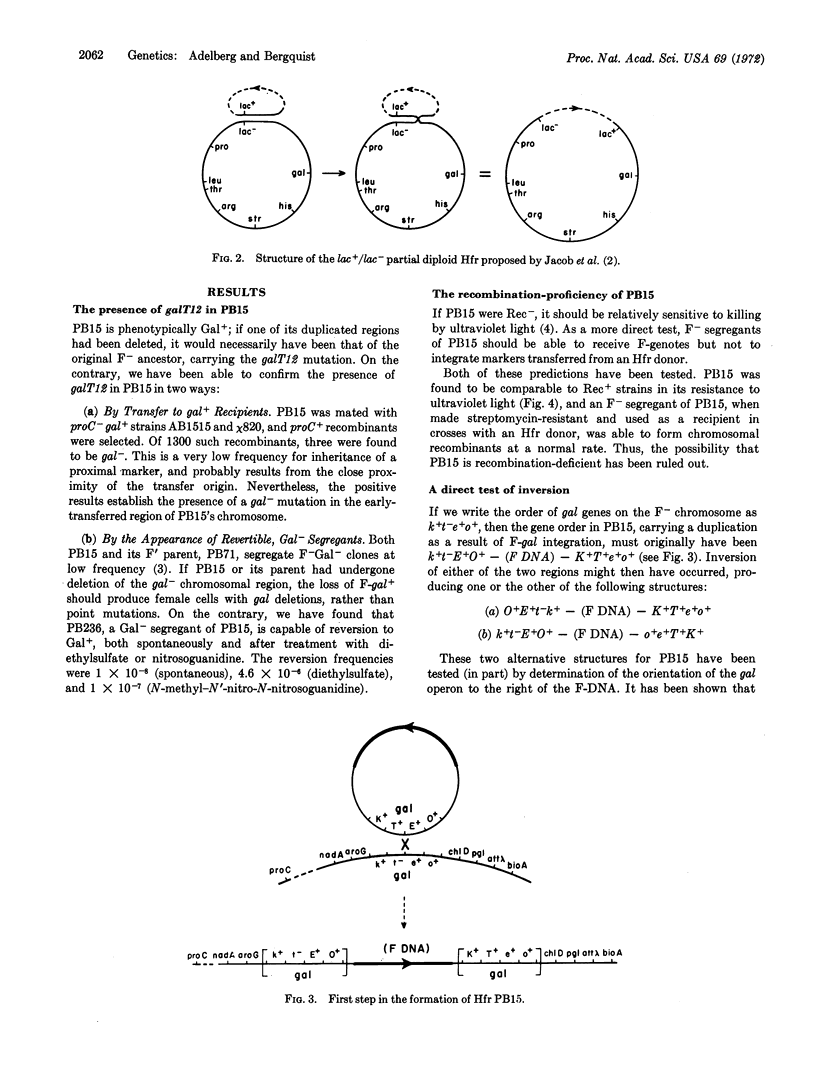
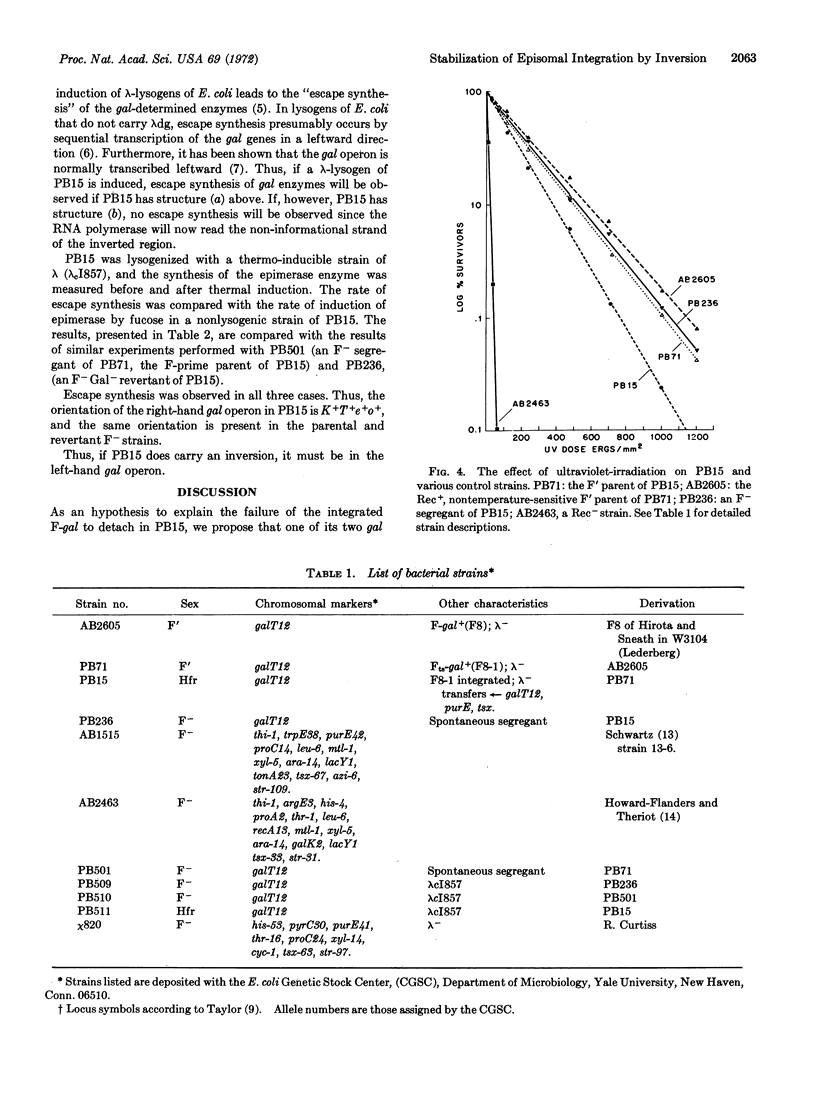
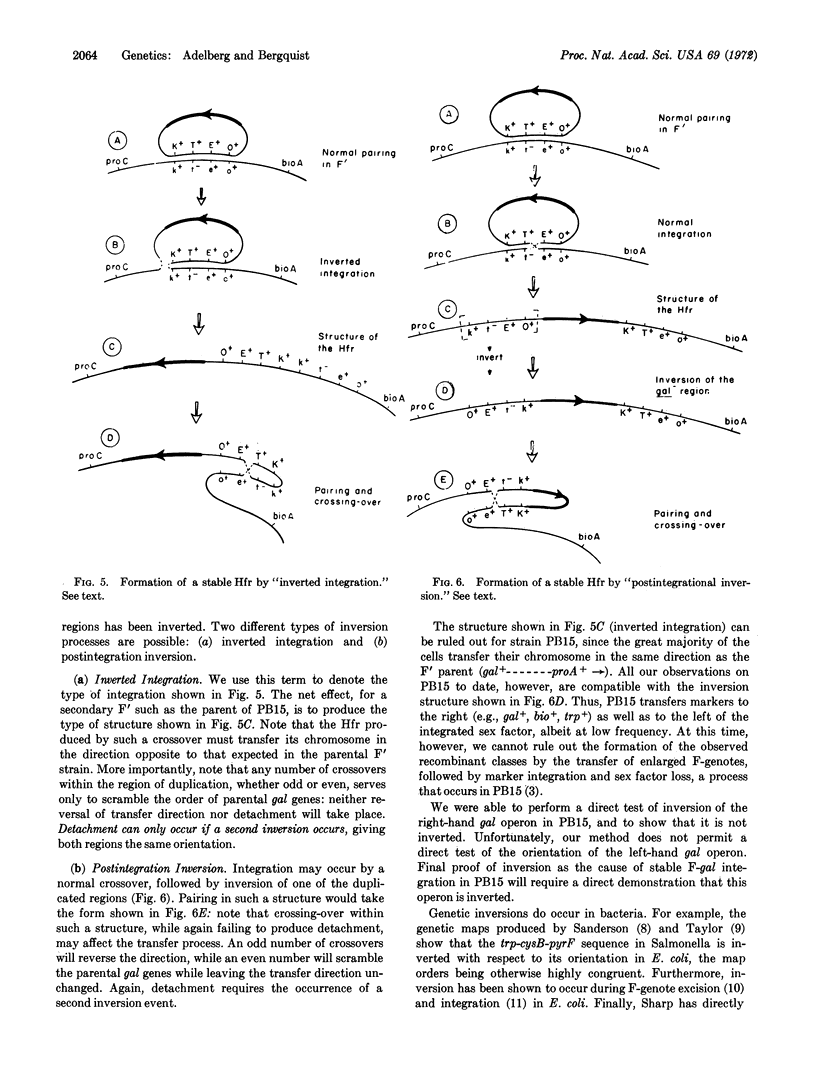
In F′ cells of Escherichia coli K12, recombination in the region of genetic duplication between chromosome and F-genote produces a rapid alteration between the integrated and detached states of the episome. Nevertheless, F′ strains can give rise to Hfr cells in which the F-genote has undergone stable integration. We have found that one such Hfr has retained the genetic duplication as well as the recombination proficiency of the parental F′ strain; to account for the failure of recombination to detach the episome in this Hfr, we have postulated the inversion of one of the duplicated regions. A general model is presented, showing that an inversion must prevent episome detachment by any combination of genetic crossovers.
Keywords: E. coli, recombination, crossover
Full text
PDF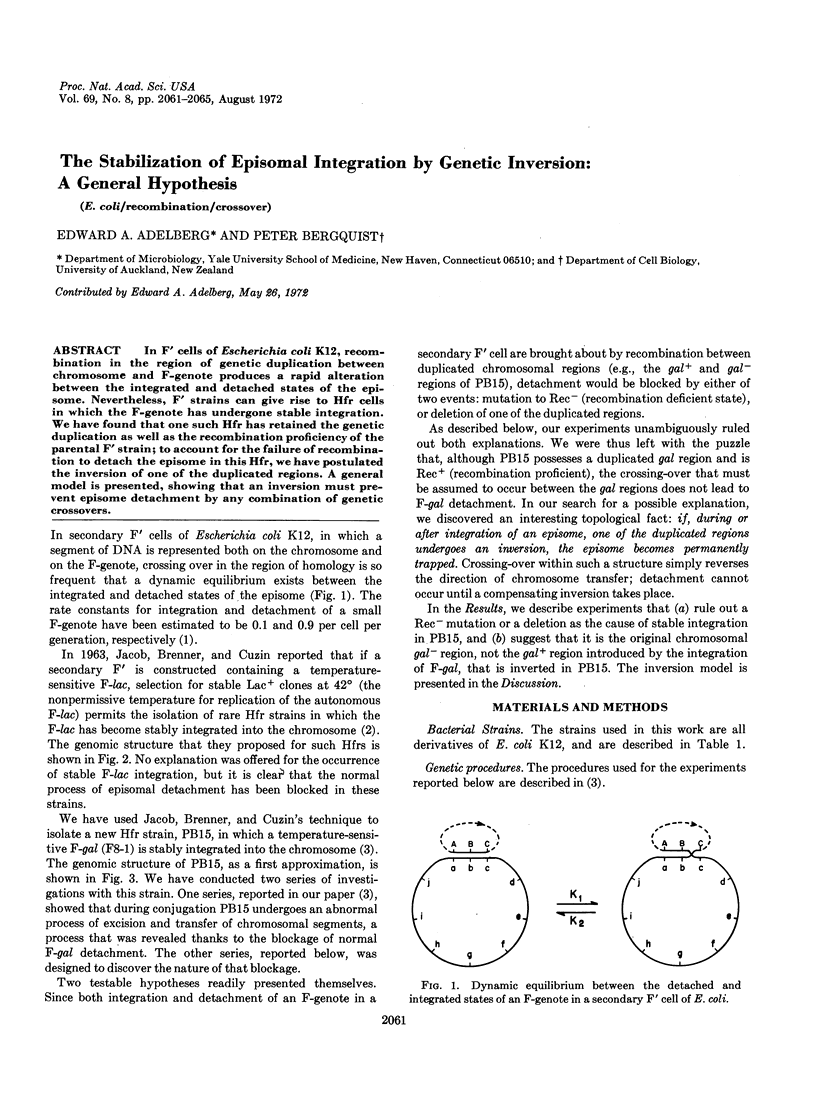
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADELBERG E. A., PITTARD J. CHROMOSOME TRANSFER IN BACTERIAL CONJUGATION. Bacteriol Rev. 1965 Jun;29:161–172. doi: 10.1128/br.29.2.161-172.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BUTTIN G. M'ECANISMES R'EGULATEURS DANS LA BIOSYNTH'ESE DES ENZYMES DU M'ETABOLISME DU GALACTOSE CHEZ ESCHERICHIA COLI K12. III. L'"EFFET DE D'ER'EPRESSION" PROVOQU'E PAR LE D'EVELOPPEMENT DU PHAGE LAMBDA. J Mol Biol. 1963 Dec;7:610–631. doi: 10.1016/s0022-2836(63)80108-4. [DOI] [PubMed] [Google Scholar]
- Beckwith J. R., Signer E. R., Epstein W. Transposition of the Lac region of E. coli. Cold Spring Harb Symp Quant Biol. 1966;31:393–401. doi: 10.1101/sqb.1966.031.01.051. [DOI] [PubMed] [Google Scholar]
- Berg C. M., Curtiss R., 3rd Transposition derivatives of an Hfr strain of Escherichia coli K-12. Genetics. 1967 Jul;56(3):503–525. doi: 10.1093/genetics/56.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guha A., Tabaczyński M., Szybalski W. Orientation of transcription for the galactose operon as determined by hybridization of gal mRNA with the separated DNA strands of coliphages lambda-dg. J Mol Biol. 1968 Jul 14;35(1):207–213. doi: 10.1016/s0022-2836(68)80048-8. [DOI] [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWARTZ N. M. SUPPRESSION OF A LAC O-O MUTATION IN ESCHERICHIA COLI. J Bacteriol. 1964 Oct;88:996–1001. doi: 10.1128/jb.88.4.996-1001.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E. Current linkage map of Salmonella typhimurium. Bacteriol Rev. 1970 Jun;34(2):176–193. doi: 10.1128/br.34.2.176-193.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]


