Summary
We describe three children infected by the human immunodeficiency virus (HIV 1), and one child suffering from familial mucocutaneous candidiasis; who all had multiple, fusiform subarachnoid intracranial aneurysms.
Because infectious causative agents were never detected at the level of the lesions, a classical “mycotic” origin of these aneurysms seemed unlikely. Despite the fact that these aneurysms have the same angiographic appearance, they have different etiologies (immune and infectious). These data open the discussion on the reciprocal role of an infectious or immune initial trigger acting on a vascular (endothelial) target. The specificities of the target in terms of location and response enhance specific topographic characteristics (phenotypes) of the cerebral vasculature.
Key words: intracranial aneurysms, children, chronic mucocutaneous candidiasis, acquired immunodeficiency syndrome, multiple aneurysms, fusiform aneurysms
Introduction
In children, the neurovascular manifestations of AIDS differ markedly from those in adults. Whereas vascular complications in adults usually consist of vascular occlusions associated with distal emboli, and complications of thrombocytopenia, in children, cerebrovascular lesions are mostly due to arteritis composed of arterial sclerosis, vascular occlusions, and the formation of intracranial aneurysms2,9,10. These aneurysms are typically multiple, subarachnoid, and fusiform, involving the proximal arteries of the base of the brain and preferentially the supraclinoidal part of the internal cerebral artery (ICA). Although these lesions were initially considered relatively specific8, we observed similar features in chronic mucocutaneous candidiasis (CMCC). CMCC is a rare familial disorder of unknown etiology, characterized by recurrent infections of the mucous membranes, nails, and skin with Candida albicans3,7.
We report here this case of CMCC in a immunodepressed child, and compare it to the cases of three HIV-infected children with multiple fusiform aneurysms. The very similar neuroradiological aspect of these cases was remarkable and raised several questions regarding the etiology and mechanism of these lesions.
Reports
Case n° 1
A 7 year-old boy was admitted in emergency for moderate headaches and vomiting of acute onset without fever. He had a long history of recurrent buccal candidiosis, with episodes of onyxis lesions due to Candida albicans. CMCC had been diagnosed at the age of five. Biological and immunological investigations were unremarkable except for a slight deficit in Ig G2 sub-class. His mother, and one of his brothers presented the same mucocutaneous symptomatology.
On admission his clinical examination was normal. Cutaneous examination failed to disclose candidiosis at that time. Biological tests were normal without elevation of the erythrocyte sedimentation rate or CRP; different serologies including Candida albicans were negative.
CT showed diffuse subarachnoid haemorrhage and suspect presence of an aneurysm in the posterior fossa. Cerebral angiography diagnosed multiple fusiform dilatations, involving the left supraclinoidal ICA (figure 1A), the Al-A2 junction of the left anterior cerebral artery (ACA), as well as the basilar artery (figure 1B), the left posterior cerebral artery (PCA) and both vertebral arteries. It was suspected that these lesions could correspond to a vasculopathy due to CMCC and the child was given antifungal therapy for several weeks. A control angiography performed six months later showed an aneurysm on the left posterior cerebral artery that had appeared despite the well-conducted medical treatment. None of these aneurysms was deemed treatable.
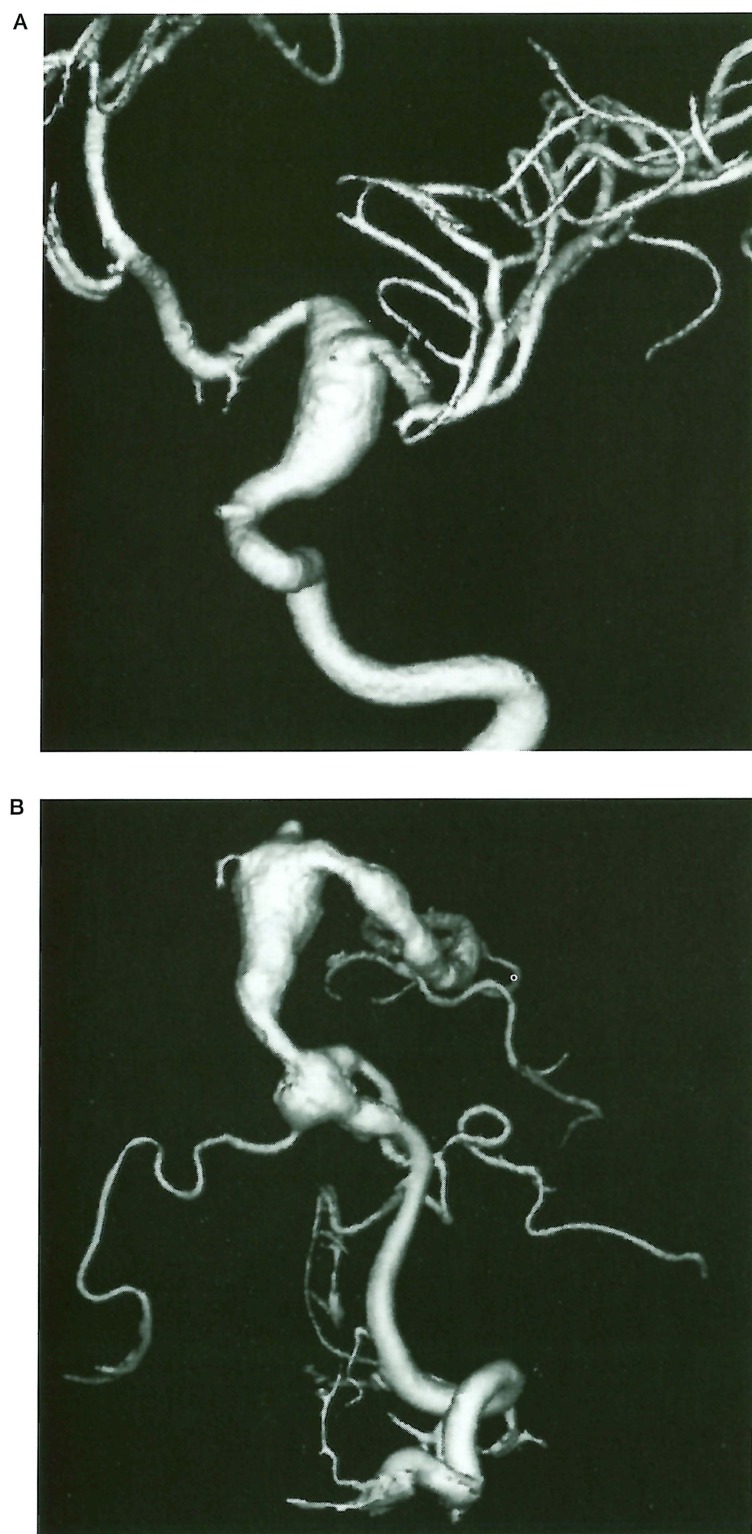
Figure 1.
Case 1, 7-year-old boy with CMCC. A) 3D Angiography of the left internal carotid artery showing a fusiform arterial dilatation of the supraclinoidal internal carotid artery. B) 3D Angiography of the left vertebral artery: large fusiform aneurysm of the basilar artery involving the proximal part of the left posterior cerebral artery, associated with another aneurysm of the same type in the distal part of the left vertebral artery at the vertebrobasilar junction.
One year later, the boy presented a new subarachnoid haemorrhage. MRI and angiography showed worsening of the previously described lesions.
The child died a few weeks later from massive intracerebral haemorrhage. At pathology, rupture of an aneurysm of the left ICA was suspected.
Case n° 2
A 4-year-old African boy was HIV infected by blood transfusions because of paludism. The infection was diagnosed after several bacterial pulmonary infections at that time. When he was 14, he suddenly developed right faciobrachial hemiparesis.
CT and MRI revealed a recent infarct in the left internal capsule and basal ganglia; older stroke stigmata were found in the left occipital and temporal lobes. Diffuse dilatations of the left ICA, and of the left middle cerebral artery (MCA) were also demonstrated. Cerebral angiography diagnosed fusiform aneurysms of the left supracavernous ICA, extending towards the proximal part of both MCA and ACA (figure 2A), like the same lesions on both PCAs (figure 2B). Thrombosis in the left distal MCA territory was also confirmed. Analysis of the CSF, blood, and urine failed to reveal any bacterial, mycobacterial, viral or fungal infection.
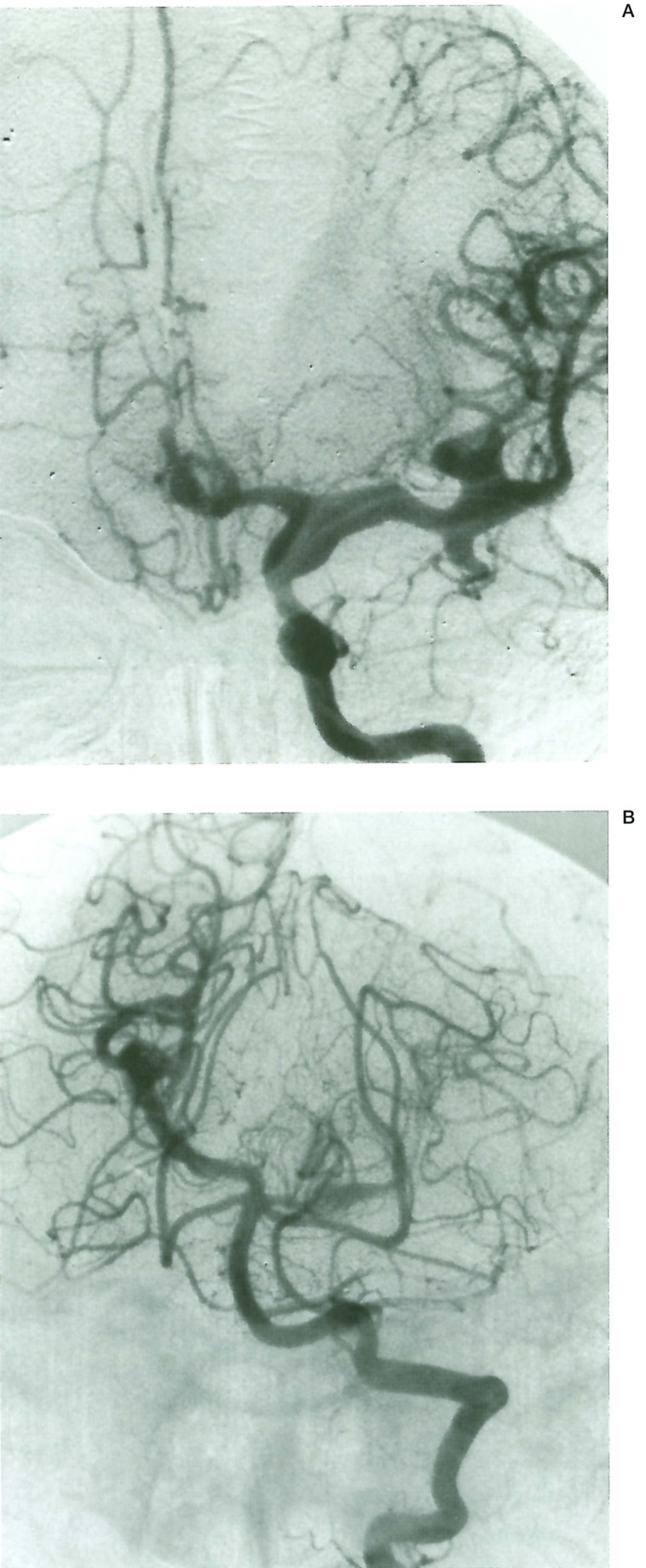
Figure 2.
Case 2,14 year old boy with AIDS. A) Left internal carotid angiogram. A-P view. Multiple fusiform arterial dilatations affecting the supraclinoid internal carotid, middle, and anterior cerebral arteries. B) Left vertebral artery. A-P view. Similar multifocal fusiform aneurysms are visible on the right P2 and left PI segments of both posterior cerebral arteries.
Case n° 3
This 8 year-old boy was infected antenatally with HIV. He presented a severe immunologic deficit with a decreased level of T4 lymphocytes, and was treated for two years by AZT. He never presented any infection.
Neurological evaluations were normal until the patient suffered three generalized tonoclonic seizures without any post ictal deficit, but followed two days later by an acute stroke with right hemiparesis. CT revealed several diffuse dilatations of the right supracavernous ICA. Cerebral angiography confirmed fusiform dilatations of the right distal ICA extending to the posterior communicating artery, and to the MCA (figure 3A). Identical features were observed on the left A1 segment. Occlusion of an insular branch of the left MCA was also demonstrated (figure 3B).
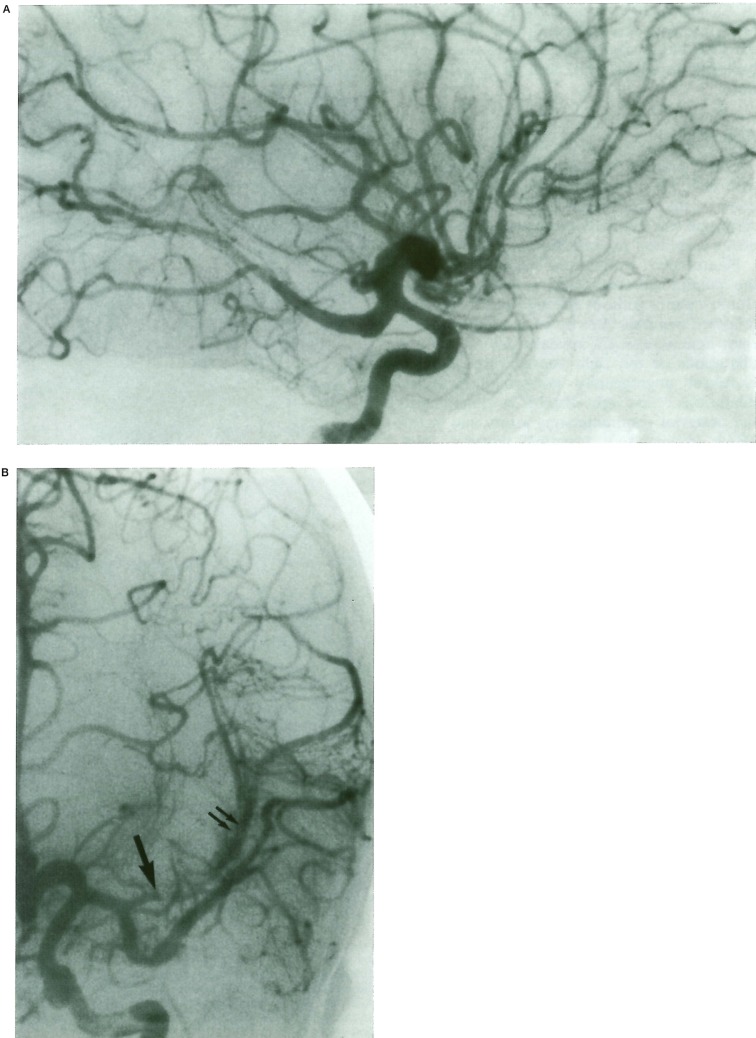
Figure 3.
Case 3, 8-year-old boy with AIDS. A) Right internal carotid injection. Lateral view. Fusiform aneurysm of the supracavernous internal carotid artery, involving the posterior communicating and cerebral artery. B) Left internal carotid injection. A-P view. Fusiform aneurysm of the Al segment of the anterior cerebral artery, associated with thrombosis of the proximal portion of the left insular branch of the MCA (arrow). A collateral circulation on the cortex of the insula is already installed (double arrows). Fusiform dilatations on the temporal branch of the MCA are detected.
Case nº 4
A 9-year-old girl antenatally infected with HIV had a long history of multiple lung infections and viral meningitis.
During one of her hospitalizations for meningo-encephalitis, she presented a left hemiparesis of acute onset. CT revealed focal infarction in the right internal capsule, and suspected abnormal intracranial vascular dilatations. Cerebral angiography demonstrated fusiform aneurysms involving the main cerebral arteries (figure 4), and showed that these were associated with irregular stenotic aspects of distal cortical branches of both MCAs. No vascular thrombosis was observed.
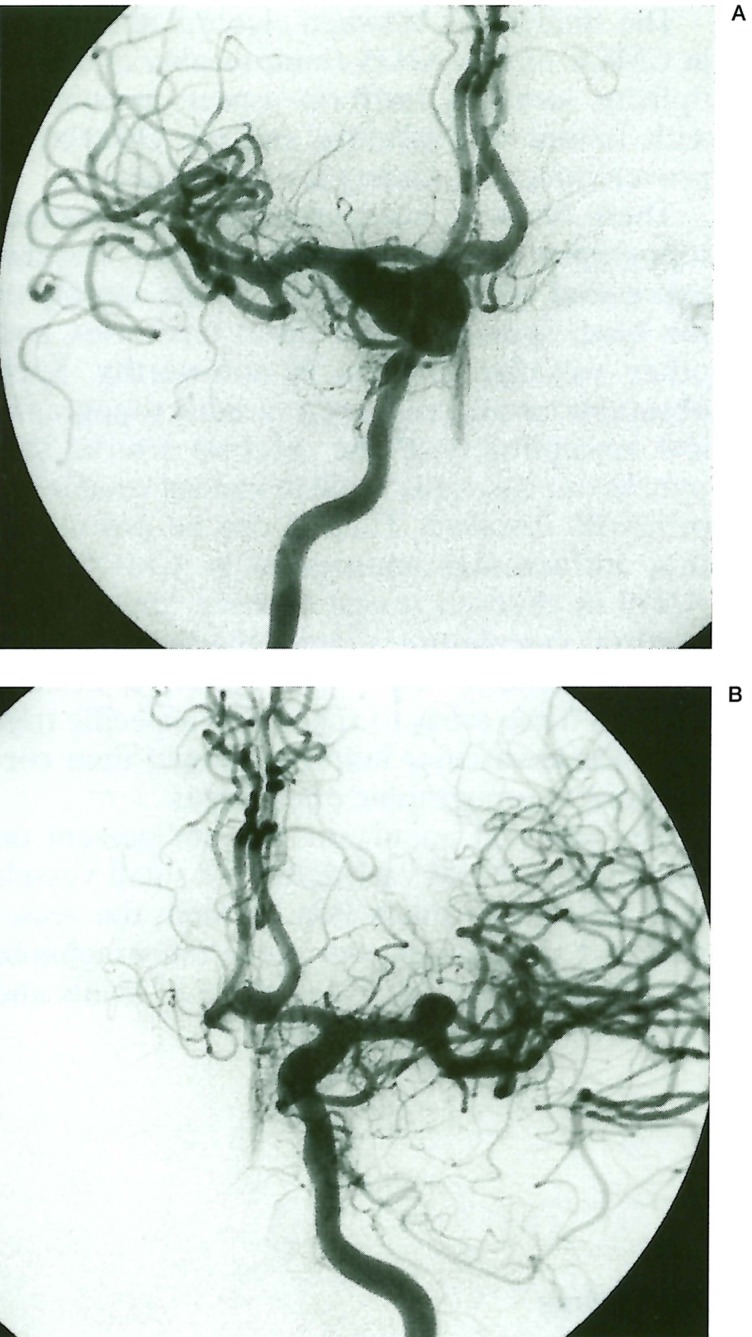
Figure 4.
Case 4, 9-year-old girl with AIDS. Right (A) and left (B) internal carotid injection. A-P views. Fusiform aneurysms of both internal cerebral arteries (predominant on the right side), with extension on the Ml segment of both middle cerebral arteries and bifurcation branches. A similar fusiform lesion is also detected at the left Al-A2 junction.
Chemical and bacteriological analysis of CSF failed to reveal any infection. The level of T4 lymphocytes was 280.
Discussion
Only 20 cases of cases of HIV infected children with multiple and fusiform intracranial aneurysms have been described in the literature (including the three reported here). The lesions observed were almost the same type: they consisted in multifocal fusiform arterial dilatations involving the major vessels of the circle of Willis, with a predominance for the ICA in its supraclinoïd portion, and the basilar artery.
These lesions did not affect the distal parts of the cerebral arteries (A3, M3, and P3 segments i.e.), these arterial segments showing mostly typical lesions of arteritis including stenosis and thrombosis.
None of the patients had evidence of coagulopathy, collagen vascular disease or familial hereditary disease, but most of them suffered severe immunosuppressive disorders or syndromes with a long history of multiple opportunistic infections preceding the diagnosis of cerebral arteriopathy.
CMCC is a familial disease with a primary immunodeficiency disorder that must be distinguished from the opportunistic candidiosis seen in globally immunocompromised patients (steroid therapies, chemotherapies...). The association of CMCC with cerebral vasculitis or aneurysm is very rare, and only three cases have been described to date: one 19-year-old woman with fusiform aneurysms of the major vessels of the circle of Willis 7 a 21-year-old man with thromboses and stenoses of the distal territory of the MCA and ACA without any aneurysm 3, a 38-year-old-man with a fusiform aneurysm associated with vasculitis 3. None of these patients presented bacterial, fungal or viral infections at the time of the diagnosis, nor any coagulopathy, metabolic abnormalities or CNS infection.
The pathogenesis of fusiform aneurysms in HIV infected children has been discussed by several authors2,8,9, and remains controversial. Some claimed the vasculopathy was secondary to an infectious cause2,8.
In a review of the the literature, Dubrovsky2 found two patients with mycobacterium avium intracellular infection, one with cytomegalovirus (CMV) infection, four patients with herpes zoster virus (HZV) infection and two others with elevated spot-checked HZV antibody titers. On the other hand, Kure 4 and Park 8 found a major HIV transmembrane glycoprotein (gp4l) in the walls of aneurysmal arteries suggesting a direct cause of the HIV in the formation of the arterial dilatations. Lang5 did immunohistochemical studies but failed to detect glycoprotein gp4l in the wall of such lesions.
The mycotic origin (primary affection of the arterial wall by an infectious agent) of these aneurysms is unlikely: histological and bacteriological analyses of these lesions have rarely been performed but, when available, failed to detect the presence of any infectious agent. Furthermore, in all these patients, none had a laboratory confirmed CNS infection at the time of the diagnosis, and no temporal relationship was noted between the time of the diagnosis of the aneurysmal lesions and the infectious episodes. No patient improved despite well-conducted medical treatment.
Shah10 suggested that the aneurysmal dilatations, like the arterial thromboses and stenoses seen in this population with severe immune deficiencies, were in fact due to lesions of panarteritis resulting in the destruction of the lamina elastica with subintimal fibrosis. This author postulated that these lesions resulted from ischaemia of the arterial wall because of an inflammatory reaction involving adventitia and vasa-vasorum.
The similarities between cerebral aneurysms in CMCC and in AIDS (multifocality and multiplicity, location, fusiform aspect, association with lesions of vasculitis, and the absence of proven infectious cause) are remarkable.
These fusiform aneurysms present the same topographic distribution and a similar vascular expression with involvement of the vessels of the base. Sparing of the distal territories, and other vascular systems is noteworthy. Such observations may suggest a variable topographical susceptibility of the cerebral arterial system. In our cases, the midline ventral vessels are primarily involved. Thus, it can be postulated that intracranial aneurysms in CMCC and AIDS in children reveal exposed areas of the cerebral vasculature, sharing the same “identity” or vulnerability 1.The immune disease exposes these areas to specific or aspecific triggers. These vascular markings would then correspond to topographic phenotypes.
The fact that aneurysms are not present on the distal cerebral vasculature or small vessels may reflect the interaction between the vessel wall and the perivascular space (subarachnoid for the large arteries of the circle of Willis and aneurysms, in opposition to subpial for smaller arteries, and thrombosis/stenosis). Apart from their topography, the vulnerability of these arterial segments seems time-related: their expression evolves from fusiform ectasia (in the pediatric population) to occlusive arteritis (in adult patients). All these observations suggest that, more than any “direct” effect on the vessel, vascular biology is the major key to the formation of these particular diseases, like many others6.
The fact that the lesions do not regress despite medical therapy (as mycotic aneurysms often do) may indicate that these fusiform dilatations are not directly related to a pathogenic agent, but result from an active biological response to an unknown trigger beyond the classically advocated infectious or haemodynamic agents.
Acknowledgements
We wish to thank our colleagues from Pediatric Neurology in Bicêtre (Prof. Tardieu) and Pediatric Neurosurgery in Necker (Prof. Zerah) for their trust and confidence in the management of these patients.
References
- 1.Campos C, Churojana A, et al. Multiple intracranial arterial aneurysms: A congenital metameric disease? Interventional Neuroradiology. 1998;4:293–299. doi: 10.1177/159101999800400405. [DOI] [PubMed] [Google Scholar]
- 2.Dubrovsky T, Curless R, et al. Cerebral aneurysmal arteriopathy in childhood AIDS. Neurology. 1998;51:560–565. doi: 10.1212/wnl.51.2.560. [DOI] [PubMed] [Google Scholar]
- 3.Groubi M, Dalas I, et al. Cerebral vasculitis associated with chronic muco-cutaneous Candidiasis. J Pediatr. 1998;133:571–574. doi: 10.1016/s0022-3476(98)70072-1. [DOI] [PubMed] [Google Scholar]
- 4.Kure K, Park Y, et al. Immunohistochemical localization of an HIV epitope in cerebral aneurysmal arteriopathy in pediatric acquired immunodeficiency syndrome (AIDS) Pediatr Pathol. 1989;9:655–667. doi: 10.3109/15513818909022373. [DOI] [PubMed] [Google Scholar]
- 5.Lang C, Jacobi G, et al. Rapid development of giant aneurysm at the base of brain in an 8-year-old boy with perinatal HIV infection. Acta Histochem Suppl. 1992;42:83–90. [PubMed] [Google Scholar]
- 6.Leroy D, Dompmartin A, et al. Aneurysm associated with chronic muco-cutaneous candidiasis during long-term therapy with ketoconazole. Dermatologica. 1989;178:43–46. doi: 10.1159/000248386. [DOI] [PubMed] [Google Scholar]
- 7.Lasjaunias P. A revised concept of the congenital nature of cerebral arteriovenous malformations. Interventional Neuroradiology. 1997;3:275–281. doi: 10.1177/159101999700300401. [DOI] [PubMed] [Google Scholar]
- 8.Park YD, Belman AL, Kim TS. Stroke in pediatric acquired immunodeficiency syndrome. Ann Neurol. 1990;28:303–311. doi: 10.1002/ana.410280302. [DOI] [PubMed] [Google Scholar]
- 9.Philippet P, Blanche S, et al. Stroke and cerebral infarcts in children infected with human immunodeficiency virus. Arch Pediatr Adolesc Med. 1994;148:965–970. doi: 10.1001/archpedi.1994.02170090079015. [DOI] [PubMed] [Google Scholar]
- 10.Shah SS, Zimmerman RA, et al. Cerebrovascular complications of HIV in children. Am J Neuroradiol. 1996;17:1913–1917. [PMC free article] [PubMed] [Google Scholar]