Abstract
Previous studies have shown that transplanted enteric glia enhance axonal regeneration, reduce tissue damage, and promote functional recovery following spinal cord injury. However, the mechanisms by which enteric glia mediate these beneficial effects are unknown. Neurotrophic factors can promote neuronal differentiation, survival and neurite extension. We hypothesized that enteric glia may exert their protective effects against spinal cord injury partially through the secretion of neurotrophic factors. In the present study, we demonstrated that primary enteric glia cells release nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor over time with their concentrations reaching approximately 250, 100 and 50 pg/mL of culture medium respectively after 48 hours. The biological relevance of this secretion was assessed by incubating dissociated dorsal root ganglion neuronal cultures in enteric glia-conditioned medium with and/or without neutralizing antibodies to each of these proteins and evaluating the differences in neurite growth. We discovered that conditioned medium enhances neurite outgrowth in dorsal root ganglion neurons. Even though there was no detectable amount of neurotrophin-3 secretion using ELISA analysis, the neurite outgrowth effect can be attenuated by the antibody-mediated neutralization of each of the aforementioned neurotrophic factors. Therefore, enteric glia secrete nerve growth factor, brain-derived neurotrophic factor, glial cell line-derived neurotrophic factor and neurotrophin-3 into their surrounding environment in concentrations that can cause a biological effect.
Keywords: spinal cord injury, dorsal root ganglia, enteric glia, neurotrophic factor, neurite outgrowth, regeneration, cell culture, immunohistochemistry, central nervous system, neuroregeneration
Research Highlights
-
(1)
Primary enteric glia release nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor into culture medium.
-
(2)
Enteric glia stimulate neurite branching of dorsal root ganglion neurons.
-
(3)
The beneficial effect of enteric glia on neurite of dorsal root ganglion neurons is, in part, regulated by the release of neurotrophic factors including nerve growth factor, brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor, and neurotrophin-3.
-
(4)
This study provides new insight and a foundation for further study on mechanisms by which enteric glia exert their beneficial effects in vitro and in vivo.
Abbreviations
ENS, enteric nervous system; EG, enteric glia; CNS, central nervous system; DRG, dorsal root ganglion; NGF, nerve growth factor; BDNF, brain-derived neurotrophic factor; NT-3, neurotrophin-3; Trk, tropomyosin-related kinase; GDNF, glial cell line-derived neurotrophic factor; MPZ, myelin protein zero
INTRODUCTION
The enteric nervous system (ENS) is a series of interconnected plexuses that exists between muscle layers throughout the gastrointestinal tract. It is considered to be the largest and most complex component of the peripheral nervous system[1]. The ENS is composed of two major populations: enteric neurons and enteric glia (EG) cells. Since EG play a very important role in the maintenance of tissue integrity and the modulation of neuronal activities in the gastrointestinal tract[2,3,4,5,6] and share morphological, structural and functional properties with astrocytes of the central nervous system (CNS)[7,8,9,10,11] as well as sharing some properties with olfactory ensheathing glia[12], they are a particularly interesting source of material for transplantation into the injured CNS. Furthermore, EG are theoretically available in large quantities and can be obtained from the patient's own intestine.
Some investigators have transplanted myenteric plexus into the brain and spinal cord. They found that the myenteric plexus not only integrated into the CNS, but also attracted host axons to sprout and grow into the graft[13,14,15]. Following this study, our group examined the potential of purified EG to promote ingrowth of transected dorsal root ganglion (DRG) axons into the rat spinal cord[16]. EG transplantation to the site of truncation promoted DRG growth deep into the spinal cord. Importantly, this growth was shown to result in the recovery of a previously lost reflex dependent on DRG innervation[17]. We have reported that EG accelerated the blood-brain barrier formation after transplantation into the spinal cord[18]. Moreover, we have also reported that EG transplantation into the injured rat spinal cord resulted in reduced lesion cavity size and improved functional outcome[19]. However, the mechanism by which EG exert these beneficial effects is not known.
Neurotrophic factors are secreted proteins that are implicated in neuronal growth, differentiation, survival and plasticity[20,21]. Many of these homodimeric disulfide- linked secreted proteins, including nerve growth factor (NGF), brain-derived neurotrophic factor (BDNF) and neurotrophin-3 (NT-3), bind and signal through the tropomyosin-related kinase (Trk) tyrosine receptor kinases. NGF was first characterized as a substance able to stimulate robust neurite and neural growth in mouse sympathetic ganglia[22] and has been shown to potently stimulate neurite extension in dissociated and intact DRG neurons[23,24,25]. EG have been shown to increase NGF secretion in response to co-culture with inflammatory mediators[6]. BDNF was initially purified from pig brain[26] and was shown to support embryonic chick neurons in culture. More recently, BDNF has been shown to play significant roles in neuronal recovery after damage in the peripheral nervous system[27], notably in sensory neurons. Another member of the neurotrophin family, NT-3, was initially discovered by virtue of its homology to both NGF and BDNF[28,29]. It has been shown to enhance axonal regrowth into the CNS after dorsal root crush[30]. Glial cell line-derived neurotrophic factor (GDNF) has been shown to be of great importance in the development of the ENS as well as the renal system and portions of the sensory ganglia[31,32,33]. Further, it has been shown to affect neuronal recovery and myelination after spinal cord injury[34] and enhance neurite outgrowth in damaged peripheral nerves[35].
Therefore, we hypothesized that EG mediated their neurorestorative effects at least partially through the secretion of neurotrophic factors. In the present study, we tested whether EG secrete NGF, BDNF, GDNF or NT-3, and the biological relevance of this secretion, by incubating dissociated DRG neuronal cultures in EG-conditioned medium with and/or without the addition of neutralizing antibodies to each of these proteins, and evaluated any difference in neurite growth.
RESULTS
Confirmation of glial cell identity
Extracted cells (Figure 1) showed positive glial fibrillary acidic protein (GFAP) reactivity (Figure 1A) with no discernable myelin protein zero (MPZ) staining detected (Figure 1B) and very little background staining (Figure 1B). In the gut, the presence of GFAP, concomitant with the absence of MPZ, is considered to be indicative of EG[3,9,36,37,38]. It was concluded that EG were successfully extracted and the cultures consisted entirely of GFAP labeled cells, which is consistent with our previous reports[16,18,39]. Cells from this extraction were used to generate EG-conditioned medium.
Figure 1.
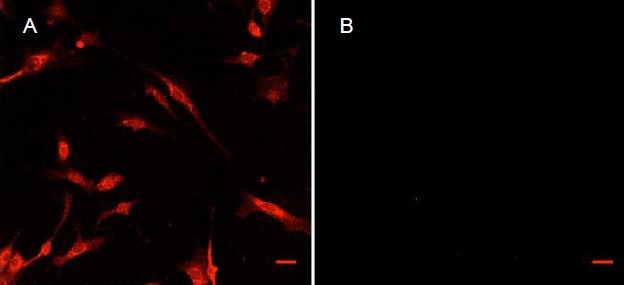
Images under a confocal microscope showing immunohistochemical staining of the intermediate filament and glial fibrillary acidic protein (GFAP) in isolated enteric glia.
(A) and (B) are images of detected fluorescence at 560–610 nm with a laser excitation at 594 nm. The secondary antibody in all cases was to rabbit IgG and was incubated with cells at 1:2 000 for 1 hour, in the dark. The primary antibody was: (A) rabbit anti-GFAP, 1:500; (B) rabbit anti-myelin protein zero, 1:100.
Staining (red, labeled GFAP) in (A) is mostly apparent on cytoskeletal elements surrounding the nucleus and throughout the cytoplasm. While microscope settings are maintained during imaging of (B), no fluorescence is visible excluding a slight signal in the lower left quadrant of (B). Scale bars: 30 μm for both A and B
Effect of EG-conditioned medium on dissociated DRG neurons
In culture, dissociated DRG neurons appeared as cells with roughly spherical cell bodies and widely varying numbers of comparatively long processes. In general, EG-conditioned medium seemed to increase both average neurite length and branching in large DRG neurons (Figures 2D, E). Neurites were generally shorter and underwent less branching in neurons cultured in neurobasal medium. In large neurons, EG-conditioned medium (+CDN) significantly increased neurite count relative to that seen in control (supplemented neurobasal medium [–CDN]) both at day 1 (P < 0.001) and at day 3 (P < 0.001; Figure 2F). In small neurons, EG-conditioned medium increased neurite count in a similar fashion (P < 0.001; Figures 2A–C).
Figure 2.
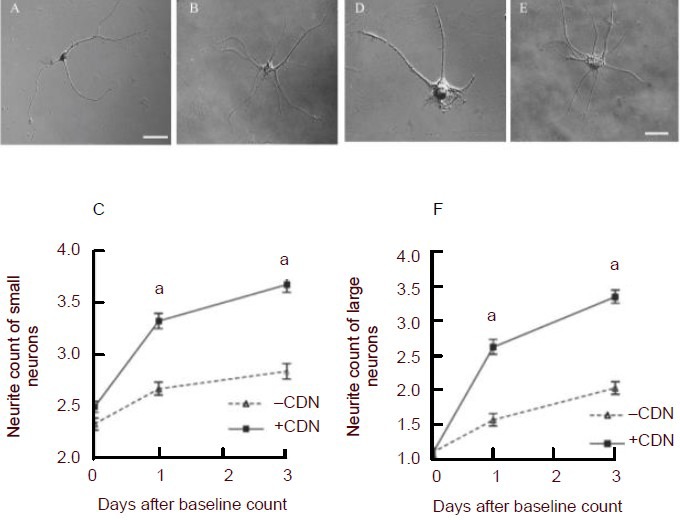
Examples of small (A, B, C) and large dorsal root ganglion (DRG) neurons (D, E, F) demonstrating the effects of incubation with enteric glia-conditioned medium.
(A) and (B) are differential interference contrast (DIC) images of single, dissociated small DRG neurons, and (D) and (E) are images of single dissociated large DRG neurons under a Nikon Eclipse 80i microscope.
(A) and (D) show typical neurons that had been cultured in neural basal medium. (B) and (E) show neurons that were cultured in enteric glia-conditioned medium. (C) and (F) show average neurite counts (± SEM) in DRG neurons (C, small; F, large) plotted against time.
Squares (+CDN) represent counts taken from DRG neurons incubated in enteric glia-conditioned medium, whereas diamonds (–CDN) represent counts taken from DRG neurons incubated in standard, unconditioned neurobasal medium. aP < 0.001, vs. –CDN. Neurite counts were analyzed using the Mann-Whitney U test with the Bonferroni correction applied. Scale bars: 30 μm for all of the images.
EG release NGF, BDNF and GDNF
EG cells cultured in vitro release NGF, BDNF and GDNF under controlled conditions, with their respective concentrations reaching 250, 96.4 and 53.8 pg/mL of culture medium respectively after 48 hours (Figure 3). There was no detectable release of NT-3 from cultured EG cells (data not shown). No neurotrophic factors were detectable in the groups with culture medium only (data not shown).
Figure 3.
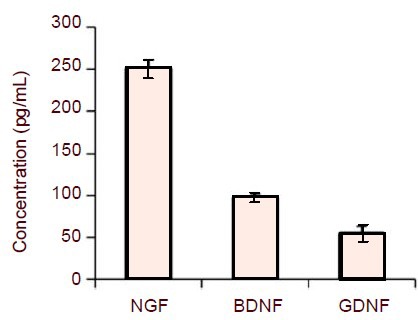
Enzyme-linked immunosorbent assay (ELISA) analysis of neurotrophic factor release of enteric glia (EG) cells.
EG cells were maintained in a standard culture medium containing 10% fetal bovine serum for 24 hours. Medium was changed to one with 2% fetal bovine serum, cells were cultured continually for another 48 hours, and then medium was removed and examined for neurotrophic factor concentration by ELISA.
EG cells released NGF, BDNF and GDNF under controlled conditions, with their respective concentrations reaching 250, 96.4 and 53.8 pg/mL respectively. All bars represent mean ± SEM of five independent experiments.
NGF: Nerve growth factor; BDNF: brain-derived neurotrophic factor; GDNF: glial cell line-derived neurotrophic factor.
Role of neurotrophic factors in effect of EG- conditioned medium
In both small and large neurons, the addition of anti-NGF neutralizing antibody to EG-conditioned medium resulted in a lowered neurite count compared with that seen in the controls without the added anti-NGF antibody (P < 0.01 at days 1 and 3, Figures 4(1)A and 4(1)B). Interestingly, the addition of anti-NGF neutralizing antibody to neurobasal medium also resulted in a significantly lower neurite count compared with that seen in neurons cultured in pure neurobasal medium for small neurons (Figure 4(1)A, P < 0.01 at days 1 and 3), but not for large neurons (Figure 4(1)B).
Figure 4.
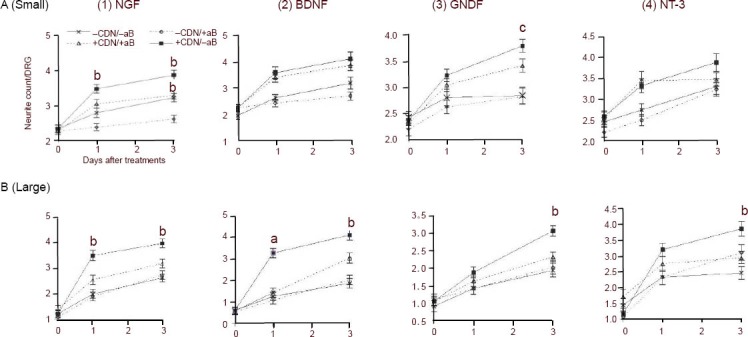
Effect of neutralization of (1) NGF, (2) BDNF, (3) GDNF and (4) NT-3 on neurite counts in either (A) small or (B) large dissociated DRG neurons incubated with or without EG-conditioned medium.
DRG: Dorsal root ganglion; EG: enteric glia; NGF: nerve growth factor; BDNF: brain-derived neurotrophic factor; GDNF: glial cell line-derived neurotrophic factor; NT-3: neurotrophin-3.
(A) Average neurite counts (± SEM) in small DRG neurons plotted over time. (B) Average neurite counts (± SEM) in large DRG neurons plotted over time. Diamonds (–CDN/+aB) represent counts taken from DRG neurons incubated in standard neurobasal medium with neutralizing antibodies. Crosses (–CDN/–aB) represent counts taken from DRG neurons incubated in standard neurobasal medium without neutralizing antibodies. Triangles (+CDN/+aB) represent counts taken from DRG neurons incubated in EG-conditioned medium with neutralizing antibodies. Squares (+CDN/–aB) represent counts taken from DRG neurons incubated in EG-conditioned medium without antibodies.
(1) In both small and large neurons, the addition of anti-NGF neutralizing antibody to EG-conditioned medium resulted in a lowered neurite count compared with that seen in control (bP < 0.01 at days 1 and 3).
(2) Neutralizing antibody to BDNF had no effect on neurite count in small DRG neurons cultured in either conditioned or unconditioned medium, nor did the antibody affect neurite count relative to baseline (A). The antibody had a markedly different effect in large DRG neurons. Neurite count in neurons cultured in conditioned medium was significantly higher than those cultured in conditioned medium with antibody on both day 1 (aP < 0.001) and day 3 (bP < 0.01) (B).
(3) The effect of GDNF antibody on small and large DRG neurons cultured in EG-conditioned medium was not fully clear until three days after baseline count, at which time there was a significant difference (small: cP < 0.05; large: bP < 0.01) in neurite counts of the EG-conditioned medium treatment groups.
(4) In small neurons, anti-NT-3 neutralizing antibody had no significant effect on neurite count in neurons cultured in EG-conditioned medium (A). In large neurons, it resulted in a decrease in the absolute neurite count (bP < 0.01; B) by day 3.
Neurite counts were analyzed with the Kruskal-Wallis test to determine whether at least two groups at the same time point were different. When it was determined that at least two groups were different, pairwise comparisons were conducted using the Mann-Whitney U test with the Bonferroni correction applied.
Neutralizing antibody to BDNF had no effect on neurite count in small DRG neurons cultured in either conditioned or unconditioned medium, nor did the antibody affect neurite count relative to baseline in the unconditioned medium (Figure 4(2)A).
The antibody had a markedly different effect in large DRG neurons. At day 1, the neurite count in neurons cultured in conditioned medium with antibody was significantly (P < 0.001) lower than those cultured in conditioned medium alone (Figure 4(2)B), but was not significantly different from that of neurons cultured in unconditioned medium with or without antibody. By day 3, the neurite count in neurons cultured in conditioned medium without antibody was significantly (P < 0.01) greater than that seen in neurons cultured in conditioned medium with antibody and was also significantly (P < 0.01) greater than that seen in neurons cultured in neurobasal medium with or without antibody to BDNF (Figure 4(2)B).
The effect of anti-GDNF neutralizing antibody on small DRG neurons cultured in EG-conditioned medium was not fully clear until 3 days after the baseline count (Figure 4(3)A), at which time there was a significant (P < 0.05) difference in neurite counts between groups. The effect of anti-GDNF neutralizing antibody on large DRG neurons was also only apparent at day 3 (Figure 4(3)B), where the neurite count in neurons bathed in conditioned medium without antibody was significantly different (P < 0.01) from the group incubated in conditioned medium with the antibody. Anti-GDNF neutralizing antibody had no significant effect on the neurite count in neurons cultured in neurobasal medium (day 3, Figure 4(3)A).
Anti-NT-3 neutralizing antibody had no significant effect on neurite counts at day 1 in both small and large neurons cultured in either EG-conditioned or neurobasal medium (Figures 4(4)A and B). By day 3, there was still no effect of the neutralizing antibody on neurite counts in small neurons cultured in both conditioned and unconditioned medium (Figure 4(4)A). However, anti-NT-3 antibody had a more pronounced effect in large neurons, where its addition resulted in a decrease in the absolute neurite count on neurons cultured in EG-conditioned medium (P < 0.01; Figure 4(4)B) by day 3, but resulted in an increase in absolute neurite count (P < 0.05; Figure 4(4)B) in neurons cultured in unconditioned medium by day 3.
DISCUSSION
In summary, it is a novel finding that EG-conditioned medium was able to enhance neurite growth from dissociated DRG neurons, and this is the first study to demonstrate that EG cells from primary culture release not only NGF and GDNF, but also BDNF, with their respective concentrations reaching 250, 96.4 and 53.8 pg/mL. Although NT-3 release could not be detected using ELISA, there is biologically relevant activity as shown by the NT-3 neutralization assay. By the end of the experiment, we showed that neutralizing any of either NGF, BDNF, GDNF or NT-3 resulted in a lowered neurite count in both large and small neurons cultured in the conditioned medium.
It was hypothesized that EG would secrete each of NGF, BDNF, NT-3 and GDNF into their environment. Given the information regarding neurotrophic factor expression in DRG neurons discussed above, it was anticipated that neutralization of NGF, GDNF, BDNF or NT-3 would primarily affect the neurite counts of small neurons in the cases of NGF or GDNF neutralization, and large neurons when BDNF or NT-3 were blocked. It was interesting to note that neutralization of NGF resulted in a lowered neurite count even in control neurons. The addition of anti-NGF to DRG cultures without conditioned medium caused an even greater reduction in neurite outgrowth than in those treated with antibody and conditioned medium. Since cultures were kept in a defined medium that contained no NGF, we can attribute the expression and secretion of NGF to the cells within the culture. Low levels of NGF secretion by the DRG culture would be consistent with the findings of Lee et al[40], who found strong NGF immunoreactivity in axotomized and intact DRG neurons. NGF might have been secreted by the neurons themselves, although, as mentioned, only relatively low levels of NGF secretion have been found in DRG neurons. NGF might also have been secreted in the culture by Schwann cells[41] that had not been fully eliminated by the pre-plating step: total elimination of all glial contaminants would have necessitated the use of various toxins to eliminate dividing cells, which might have altered neuronal behavior and survival in culture[42]. Moreover, Madduri et al[35] reported that axonal branching of DRG was strongly promoted by adding NGF even at the very low concentration of 0.01 ng/mL (10 pg/mL) and the optimal dose for maximum neurite outgrowth was 1–10 ng/mL when NGF alone was added to the DRG neurons. It is worth noting that high doses of NGF delayed nerve regeneration by retarding the production of growth associated protein 43 in the early phase after axotomy[43].
In the present study, the neutralizing NGF antibody did not have an obvious effect in the first 24 hours. This might be explained by the fact that both Shen et al[44] and Li et al[45] found TrkA expression to be substantially downregulated the first week after injury in vivo. Since extraction from the rat and plating to culture dishes involves both axotomization and cutting of the dendritic processes of DRG neurons, it could be inferred that a similar reaction to injury takes place in cultured neurons. This would partially mitigate the effect of NGF on these neurons shortly after injury (both studies did show a gradual upregulation of TrkA in DRG neurons to nearly normal levels 1–2 weeks after injury). Furthermore, Madduri et al[35] also found that NGF tended to increase neurite branching, rather than neurite length. Since the method used to evaluate neurite growth in this study included only primary and secondary neurites, this prominent effect of NGF on dorsal root neurons (that of giving rise to many intertwining branches) might have been missed. We will include those intertwining branches and the length of the neurite branching in our future study.
Interestingly, a different picture emerges when examining the effect of conditioned medium on large DRG neurons. Neutralizing NGF innate to the culture had no effect on neurite counts in the control group (medium only), but it did inhibit the increased neurite counts in the EG- conditioned medium group. As stated above, studies discussing TrkA expression in injured DRG normally show prominent downregulation of the receptor. However, this diminished expression is stated as a proportion of all neurons in the ganglion. TrkA is predominantly found on small- and medium-sized neurons[46]; however, in the present study, the large neurons responded with equally reduced neurite outgrowth in the presence of anti-NGF as the small neurons suggesting that TrkA is expressed equally in these two populations in culture. Further study to evaluate the TrkA expression will be done in future experimentation.
As stated previously, TrkB, the BDNF receptor, tends to be expressed in larger-sized DRG neurons, although its expression is not as specific as, for example, that of TrkC[47]. The results obtained in this study show that neutralizing BDNF has little effect on neurite counts in small neurons, in either conditioned or unconditioned medium. Eliminating BDNF from the solution did, however, result in a pronounced reduction of neurite growth in large neurons, which is consistent with the original hypothesis of this study.
Neutralizing GDNF significantly reduced neurite count in both large and small DRG neurons. GDNF signals through the ret receptor tyrosine kinase; GDNF activation of ret is mediated by GFRα1[21]. Though GFRα1 expression is normally confined to small DRG neurons[48], its expression is highly upregulated in both large and small DRG neurons after injury[49]. Given that GDNF was shown[35] to especially enhance neurite elongation in culture, this might, particularly in large neurons, have contributed to its effects being noted, as neurites were defined here as being at least one cell body diameter in length.
We did not observe a detectable level of NT-3 using ELISA assays, which means that the level of NT-3 in the EG-conditioned medium was lower than 15 pg/mL according to the manufacturer's instructions. We used a NT-3-containing medium as a positive control to exclude any possible technical problems with the assay we used. Surprisingly, our study did show NT-3 antibodies significantly reduced neurite outgrowth of large DRG neurons. As previously discussed, one possible explanation is that the potential synergic effects of multiple neurotrophic factors on neurite outgrowth may have reduced the amount of each growth factor required for neurite outgrowth although more study is required to determine the precise mechanism. TrkC, the high-affinity NT-3 receptor, is the most consistently expressed neurotrophic factor receptor in DRG, being found nearly exclusively in large neurons[48]. In large DRG neurons cultured in EG-conditioned medium, neutralization of NT-3 diminished neurite count, which is consistent with known effects of NT-3 on neurite outgrowth in cells expressing TrkC[50,51]. The increase in neurite count seen in neurons cultured in unconditioned medium with anti-NT-3 neutralizing antibody might be caused through the same effect noted in two earlier studies[24,52]. It was hypothesized in these articles that NT-3 might inhibit NGF-mediated neurite extension by binding to P75 and preventing it from acting as a co-receptor to NGF, thereby reducing its ability to signal downstream. Assuming this hypothesis holds in the scenario encountered here, there must be an upregulation in TrkA in at least some large DRG neurons.
In the present study, we found that the conditioned medium contained multiple growth factors at physiologically relevant levels. Previous studies have also demonstrated that those neurotrophic factors have synergistic effects on neuronal growth[35,53,54]. Given that each specific neutralization of NGF, BDNF, GDNF and NT-3, in at least some of the populations of neurons studied, had an inhibitory effect on branching of DRG neurons cultured in conditioned medium, it can be concluded that EG secrete each of these factors in quantities that are sufficient to have a biologically relevant effect in vitro. This secretion might, therefore, be a mechanism by which they mediate their beneficial effects in vivo. The present study provides new insight and a foundation for further study on mechanisms through which EG exert their beneficial effects in vitro and in vivo.
The neurotrophic factors in the EG-conditioned medium may act in a coordinated or sequential manner on the neurite outgrowth. Therefore, further investigations are required to test the effects of those growth factors, alone and in combination, on nerve branching and elongation, growth kinetics and expression of their functional receptors in order to elucidate the interactions and the mechanisms supporting the enhanced neurite outgrowth by those growth factors.
MATERIALS AND METHODS
Design
A parallel controlled in vitro experiment.
Time and setting
Experiments were performed at McMaster University, Hamilton, Ontario, Canada from May 2009 to January 2011.
Materials
Thirty-five female Wistar rats, aged 6–16 weeks old, weighing 200–300 g, were provided by the Animal Care Facility of McMaster University, Canada. All experiments were performed in accordance with the requirements of the Animals for Research Act of Ontario, Canada and the Guidelines of the Canadian Council on Animal Care.
Methods
Preparation of supplemented neurobasal medium
Neurobasal medium (21103-049), Hank's balanced salt solution (14025-092), B-27 supplement (17504-044), Dulbecco's modified eagle's medium (DMEM)/F12 1:1 (11320-033), dispase (17105-041), L-glutamine (25030149), penicillin/streptomycin (15140-122) and antibiotic-antimycotic 100 × solution (15240-062), dimethyl sulfoxide (11995), and 0.25% trypsin/ethylenediamine tetraacetic acid (25200072) were obtained from Gibco (New York, NY, USA). Fetal bovine serum (F1051), poly-L-lysine (P4832), laminin (L2020), collagenase (C9263), rat-tail collagen (type 1, C7661), and N-acetylcysteine (A1965) were acquired from Sigma (St. Louis, MO, USA). A cell strainer (352350) and tissue culture inserts (353102, 1 μm pore size) for 6-well culture dishes were obtained from BD Falcon. Supplemented neurobasal medium was freshly prepared every day and consisted of 10 mL neurobasal medium, 200 μL B-27, 100 μL L-glutamine and 50 μL penicillin/streptomycin. No neurotrophic factors were added to this supplemented neurobasal/B-27 serum free medium.
EG extraction and culture
EG were extracted according to our previously described method[39]. Briefly, the day before extraction, 3T3 mouse embryonic fibroblasts (American Type Culture Collection, CCL-92) were seeded to collagen-coated tissue culture inserts at 4 × 104 cells/insert in DMEM with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin. On the day of extraction, rats were euthanized with pentobarbital and segments of the small intestine were removed, rinsed in PBS with antibiotics and scraped. Segments were incubated in 6 U/mL dispase for 3 hours and then scraped to further dislodge the myenteric plexus; segments were then discarded. The remaining suspension was centrifuged, rinsed in N-acetylcysteine to remove mucous, filtered to remove neuronal contamination and finally plated to rat tail collagen-coated wells of a 6-well plate. Inserts were placed into wells containing the extracted suspension. Insert and well were then filled with 3 mL DMEM/F12 supplemented with 20% FBS and 1% penicillin/streptomycin. Medium and inserts were changed every other day. When first plated in culture, EG are roughly disc-shaped with a diameter of less than 20 μm. When EG had grown in size so as to have a mean cell diameter of greater than 20 μm and took on an irregular but usually elongated and somewhat fibroblast-like morphology, serum concentration was lowered to 10% FBS and inserts were no longer added to culture. EG were then used immediately or frozen in DMEM/F12 with 10% FBS, 5% dimethyl sulfoxide and 1% penicillin/streptomycin and stored in liquid nitrogen. For cells kept in culture, medium was DMEM/F12 with 10% FBS and 1% penicillin/streptomycin and was changed every 2–3 days. Cells were subcultured using 0.25% trypsin/ethylenediamine tetraacetic acid. Five different EG lines were generated and cells from passages 6–10 were used for experimental purposes.
Confirmation of EG identity
Cellular identity was confirmed by staining for GFAP and MPZ using immunocytochemical staining technique[39,55]. Cells were fixed in methanol for 5 minutes at –20°C. Primary antibody was rabbit anti-rat GFAP (1:500; Zymed 18-0036) or rabbit anti-myelin protein zero (1:100; Abcam ab31851) in PBS containing 0.25% Triton-X-100 and 5% donkey serum. Secondary antibody was donkey anti-rabbit-594 Alexa fluor (1:1 000; Invitrogen, Carlsbad, CA, USA) in PBS with 5% donkey serum.
DRG extraction and culture
DRG from 3- to 16-week-old Wistar rats were cultured and extracted according to a previous study[56]. Briefly, rats were deeply anesthetized with pentobarbital, and dorsal portions of the vertebrae were removed. The spinal cord was pushed aside and DRG from all levels of the spine were collected. DRG were desheathed and then dissociated by a 2-hour incubation in a Hank's balanced salt solution containing 2.5 U/mL dispase/200 U/mL collagenase followed by multiple triturations with a bovine serum albumin-coated cotton-plugged Pasteur pipette. Extracted cells were then centrifuged, resuspended in supplemented neurobasal medium and plated to a Petri dish for 3.5 hours to remove non-neuronal cells: glial cells adhere to the dish surface while neurons do not. Neurons were collected by centrifugation at 2 000 × g for 4 minutes, counted and seeded at 1 × 104 cells per well to poly-L-lysine and laminin-coated wells of a 24-well plate in supplemented neurobasal medium. For immunocytochemical experiments, neurons were seeded at 5 × 104 cells per poly-L-lysine and laminin-coated 34 mm glass coverslip. Before experimentation, neurons were allowed to acclimatize to culture conditions for 3 days. One half of the medium in which the neurons were bathed was removed and replaced with fresh neurobasal medium or conditioned medium every other day.
Neurite counting
Dissociated DRG neurons were broadly divided into two categories based on the size of their cell body. Neurons whose cell body diameter along its longest axis was greater than 30 μm were classified as “large” (Figure 5B), whereas those with cell bodies less than 30 μm in diameter were classified as “small” (Figure 5A). Separate counts were made for large and small neurons. A neurite was defined as a neuronal process that extended at least one cell body diameter from a cell that also had a small swelling–the growth cone–at its tip[57]. Primary neurites, those originating from the neuronal soma, as well as secondary neurites, were included in the count. Ten to twenty dissociated neurons per well were examined for neurites, with each replicate group consisting of at least three wells, and three different animals were used for each group.
Figure 5.
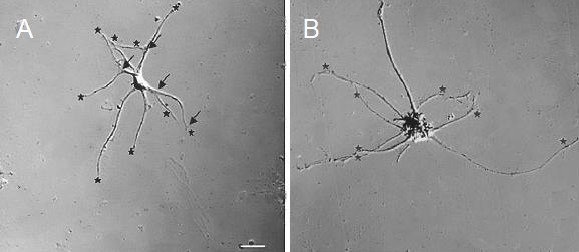
Differential interference contrast micrographs of small (A) and large (B) dissociated dorsal root ganglion neurons.
Small neurons were defined as having a cell body whose longest diameter was less than 30 μm; large neurons had a cell body whose longest diameter was at least 30 μm. The neuron in (A) has extended six primary neurons, four of which have branched, with only three giving rise to secondary neurites that are longer than a cell body diameter. The “count” for this particular neuron would, therefore, be nine.
In the neuron featured in (B), eight primary neurites are visible (as indicated by stars), but none of them has branches that are longer than a cell body diameter. Therefore, the “count” for this particular neuron is eight. Counted neurites are marked with stars, and branch points are marked with arrows. Scale bar: 20 μm for both A and B
Generation of EG-conditioned medium
EG derived from Wistar rats were seeded to wells of a 6-well plate at 4 × 104 cells/well in DMEM/F12 (1:1) supplemented with 10% FBS and 1% penicillin/streptomycin. After 24 hours, cells were rinsed with PBS and bathed in DMEM/F12 (1:1) containing 2% FBS and 1% penicillin/streptomycin. Twenty-four hours after that, cells were again rinsed in PBS and bathed in supplemented neurobasal medium. After 24 more hours, the medium was considered to be conditioned and was centrifuged (4 minutes at 2 000 × g) to remove any particulates, then used immediately for the neurite outgrowth study.
Determination of the release of neurotrophic factors NGF, NT-3, BDNF and GDNF by EG using ELISA
In a separate study, EG that had been isolated and grown to confluence were trypsinized. After trypsinization, EG cells were plated at a concentration of 4 × 104 cells/well in 6-well plates. The cells were grown in 3 mL of DMEM with 10% FBS and 1% penicillin/streptomycin for 24 hours. The cells were then washed in PBS (pH 7.4) and re-suspended in 2 mL of the basal medium for 48 hours. Subsequently, the cells were centrifuged and the growth factors were measured in the supernatant using commercially available ELISA kits for NGF, BDNF, NT-3 and GDNF (Promega, Madison, WI, USA) using the protocols described by the manufacturer. It was only the EG that were initially incubated in FBS, and they were rinsed multiple times with PBS before final incubation in NBA + B27. This comprised the base of the “conditioned” medium. The unconditioned medium (NBA + B27 only) served as a control medium.
Growth factors biological relevance test by neutralizing antibodies
The goal of these experiments was to determine whether EG secreted any NGF, BDNF, GDNF or NT-3 into their culture medium by capitalizing on the antibody-antigen interaction. By definition, a neutralizing antibody will bind to and abolish the biological activity of the protein to which it adheres. We tested whether incubation with a neutralizing antibody to one of the aforementioned neurotrophic factors would affect neurite number in DRG neurons cultured in either supplemented neurobasal medium or EG-conditioned medium. Neurons were permitted to acclimatize to culture conditions for 3 days. On the third day in culture, a baseline neurite count was made and culture medium was replaced with either EG-conditioned medium (CDN) or fresh supplemented neurobasal medium (NBA + B27 only). Neutralizing antibodies to one of either the NGF (R&D Systems AF-556-NA, 1 μg/mL), BDNF (Millipore AB1513, 2.5 μg/mL), GDNF (R&D Systems AF-212-NA, 1 μg/mL) or NT-3 (Chemicon Intl. AB1780SP, 2 μg/mL) concentrations were prepared as directed by the manufacturer's instructions. In accordance with the experimental design, some groups of cells received corresponding antibodies for neutralization according to procedures previously described[58,59,60]. The remaining groups that did not receive antibodies served as controls. For instance, to assess for the presence of GDNF in conditioned medium, we used anti-GDNF antibody. A similar process was carried out for NGF, BDNF and NT-3. The end result was multiple replicates of four wells, one well containing EG-conditioned medium without antibody, another with EG-conditioned medium with antibody, a third with supplemented neurobasal medium without antibody and the fourth containing supplemented neurobasal medium with antibody. In these experiments, neurites were counted 1 day and again 3 days after the baseline count.
Statistical analysis
Neurite counts were collected from four separate groups (with and/or without conditioned medium, with and/or without antibody) and analyzed with the Kruskal-Wallis test to determine whether at least two groups at the same time point were different. When it was determined that at least two groups were different, pairwise comparisons were completed using the Mann-Whitney U test with the Bonferroni correction applied when necessary. Analyses were performed with SPSS Advanced Statistics17.0 (IBM, Markham, ON, Canada). Data were expressed as mean ± SEM, and P < 0.05 was considered statistically significant.
Acknowledgments
We thank Ms. Ann Kolkin for her helpful suggestions and for her critical editorial review. We also thank Ms. Xinjie Huang for her excellent work on cell culture and sample processing.
Footnotes
Funding: This study was supported by Canadian Spinal Research Organization, No. #84831.
Conflicts of interest: None declared.
Ethical approval: The study protocol was approved by the Animal Research Ethics Board of McMaster University, Canada.
(Edited by Boyce VS, Toulouse A/Song LP)
REFERENCES
- [1].Benarroch EE. Enteric nervous system: functional organization and neurologic implications. Neurology. 2007;69(20):1953–1957. doi: 10.1212/01.wnl.0000281999.56102.b5. [DOI] [PubMed] [Google Scholar]
- [2].Gabella G. Fine structure of the myenteric plexus in the guinea-pig ileum. J Anat. 1972;111(Pt 1):69–97. [PMC free article] [PubMed] [Google Scholar]
- [3].Ruhl A. Glial cells in the gut. Neurogastroenterol Motil. 2005;17(6):777–790. doi: 10.1111/j.1365-2982.2005.00687.x. [DOI] [PubMed] [Google Scholar]
- [4].Bassotti G, Villanacci V, Fisogni S, et al. Enteric glial cells and their role in gastrointestinal motor abnormalities: introducing the neuro-gliopathies. World J Gastroenterol. 2007;13(30):4035–4041. doi: 10.3748/wjg.v13.i30.4035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].von Boyen GB, Steinkamp M, Geerling I, et al. Proinflammatory cytokines induce neuro-trophic factor expression in enteric glia: a key to the regulation of epithelial apoptosis in Crohn's disease. Inflamm Bowel Dis. 2006;12(5):346–354. doi: 10.1097/01.MIB.0000219350.72483.44. [DOI] [PubMed] [Google Scholar]
- [6].von Boyen GB, Steinkamp M, Reinshagen M, et al. Nerve growth factor secretion in cultured enteric glia cells is modulated by proinflammatory cytokines. J Neuroendocrinol. 2006;18(11):820–825. doi: 10.1111/j.1365-2826.2006.01478.x. [DOI] [PubMed] [Google Scholar]
- [7].Gershon MD, Bursztajn S. Properties of the enteric nervous system: limitation of access of intravascular macromolecules to the myenteric plexus and muscularis externa. J Comp Neurol. 1978;180(3):467–488. doi: 10.1002/cne.901800305. [DOI] [PubMed] [Google Scholar]
- [8].Bishop AE, Carlei F, Lee V, et al. Combined immunostaining of neurofilaments, neuron specific enolase, GFAP and S-100. A possible means for assessing the morphological and functional status of the enteric nervous system. Histochemistry. 1985;82(1):93–97. doi: 10.1007/BF00502095. [DOI] [PubMed] [Google Scholar]
- [9].Jessen KR, Mirsky R. Glial cells in the enteric nervous system contain glial fibrillary acidic protein. Nature. 1980;286(5774):736–737. doi: 10.1038/286736a0. [DOI] [PubMed] [Google Scholar]
- [10].Ferri GL, Probert L, Cocchia D, et al. Evidence for the presence of S-100 protein in the glial component of the human enteric nervous system. Nature. 1982;297(5865):409–410. doi: 10.1038/297409a0. [DOI] [PubMed] [Google Scholar]
- [11].Lomas JP, Dunning J. Best evidence topic report. S-100b protein levels as a predictor for long-term disability after head injury. Emerg Med J. 2005;22(12):889–891. doi: 10.1136/emj.2005.031732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Doucette R. Glial influences on axonal growth in the primary olfactory system. Glia. 1990;3(6):433–449. doi: 10.1002/glia.440030602. [DOI] [PubMed] [Google Scholar]
- [13].Jaeger CB, Toombs JP, Borgens RB. Grafting in acute spinal cord injury: morphological and immunological aspects of transplanted adult rate enteric ganglia. Neuroscience. 1993;52(2):333–346. doi: 10.1016/0306-4522(93)90161-8. [DOI] [PubMed] [Google Scholar]
- [14].Lawrence JM, Raisman G, Mirsky R, et al. Transplantation of postnatal rat enteric ganglia into denervated adult rat hippocampus. Neuroscience. 1991;44(2):371–379. doi: 10.1016/0306-4522(91)90062-s. [DOI] [PubMed] [Google Scholar]
- [15].Tew EM, Anderson PN, Saffrey MJ, et al. Transplantation of the postnatal rat myenteric plexus into the adult rat corpus striatum: an electron microscopic study. Exp Neurol. 1994;129(1):120–129. doi: 10.1006/exnr.1994.1153. [DOI] [PubMed] [Google Scholar]
- [16].Jiang S, Wang J, Khan MI, et al. EG promote regeneration of transected dorsal root axons into spinal cord of adult rats. Exp Neurol. 2003;181(1):79–83. doi: 10.1016/s0014-4886(02)00030-4. [DOI] [PubMed] [Google Scholar]
- [17].Jiang S, Khan MI, Wang J, et al. EG promote functional recovery of CTM reflex after dorsal root transection. Neuroreport. 2003;14(10):1301–1304. doi: 10.1097/01.wnr.0000077547.91466.e1. [DOI] [PubMed] [Google Scholar]
- [18].Jiang S, Khan MI, Lu Y, et al. Acceleration of blood-brain barrier formation after transplantation of enteric glia into spinal cords of rats. Exp Brain Res. 2005;162(1):56–62. doi: 10.1007/s00221-004-2119-3. [DOI] [PubMed] [Google Scholar]
- [19].Jiang S, Khan MI, Bain JR, et al. Locally transplanted enteric glia improve functional and structural recovery in a rat model of spinal cord injury. Neural Regen Res. 2009;4(9):710–716. [Google Scholar]
- [20].Arevalo JC, Wu SH. Neurotrophin signaling: many exciting surprises. Cell Mol Life Sci. 2006;63(13):1523–1537. doi: 10.1007/s00018-006-6010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cui Q. Actions of neurotrophic factors and their signaling pathways in neuronal survival and axonal regeneration. Mol Neurobiol. 2006;33(2):155–179. doi: 10.1385/MN:33:2:155. [DOI] [PubMed] [Google Scholar]
- [22].Cohen S, Levi-Montalcini R, Hamburger V. A nerve growth-stimulating factor isolated from sarcom as 37 and 180. Proc Natl Acad Sci U S A. 1954;40(10):1014–1018. doi: 10.1073/pnas.40.10.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jones DM, Tucker BA, Rahimtula M, et al. The synergistic effects of NGF and IGF-1 on neurite growth in adult sensory neurons: convergence on the PI 3-kinase signaling pathway. J Neurochem. 2003;86(5):1116–1128. doi: 10.1046/j.1471-4159.2003.01925.x. [DOI] [PubMed] [Google Scholar]
- [24].Kimpinski K, Mearow K. Neurite growth promotion by nerve growth factor and insulin-like growth factor-1 in cultured adult sensory neurons: role of phosphoinositide 3 kinase and mitogen activated protein kinase. J Neurosci Res. 2001;63(6):486–499. doi: 10.1002/jnr.1043. [DOI] [PubMed] [Google Scholar]
- [25].Levi-Montalcini R, Cohen S. Effects of the extract of the mouse submaxillary salivary glands on the sympathetic system of mammals. Ann N Y Acad Sci. 1960;85:324–341. doi: 10.1111/j.1749-6632.1960.tb49963.x. [DOI] [PubMed] [Google Scholar]
- [26].Barde YA, Edgar D, Thoenen H. Purification of a new neurotrophic factor from mammalian brain. EMBO J. 1982;1(5):549–553. doi: 10.1002/j.1460-2075.1982.tb01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Geremia NM, Pettersson LM, Hasmatali JC, et al. Endogenous BDNF regulates induction of intrinsic neuronal growth programs in injured sensory neurons. Exp Neurol. 2010;223(1):128–142. doi: 10.1016/j.expneurol.2009.07.022. [DOI] [PubMed] [Google Scholar]
- [28].Hohn A, Leibrock J, Bailey K, et al. Identification and characterization of a novel member of the nerve growth factor/brain-derived neurotrophic factor family. Nature. 1990;344(6264):339–341. doi: 10.1038/344339a0. [DOI] [PubMed] [Google Scholar]
- [29].Maisonpierre PC, Belluscio L, Squinto S, et al. Neurotrophin-3: a neurotrophic factor related to NGF and BDNF. Science. 1990;247(4949 Pt 1):1446–1451. doi: 10.1126/science.247.4949.1446. [DOI] [PubMed] [Google Scholar]
- [30].Hanna-Mitchell AT, O’Leary D, Mobarak MS, et al. The impact of neurotrophin-3 on the dorsal root transitional zone following injury. Spinal Cord. 2008;46(12):804–810. doi: 10.1038/sc.2008.57. [DOI] [PubMed] [Google Scholar]
- [31].Moore MW, Klein RD, Farinas I, et al. Renal and neuronal abnormalities in mice lacking GDNF. Nature. 1996;382(6586):76–79. doi: 10.1038/382076a0. [DOI] [PubMed] [Google Scholar]
- [32].Pichel JG, Shen L, Sheng HZ, et al. Defects in enteric innervation and kidney development in mice lacking GDNF. Nature. 1996;382(6586):73–76. doi: 10.1038/382073a0. [DOI] [PubMed] [Google Scholar]
- [33].Sanchez MP, Silos-Santiago I, Frisen J, et al. Renal agenesis and the absence of enteric neurons in mice lacking GDNF. Nature. 1996;382(6586):70–73. doi: 10.1038/382070a0. [DOI] [PubMed] [Google Scholar]
- [34].Zhang L, Ma Z, Smith GM, et al. GDNF-enhanced axonal regeneration and myelination following spinal cord injury is mediated by primary effects on neurons. Glia. 2009;57(11):1178–1191. doi: 10.1002/glia.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Madduri S, Papaloizos M, Gander B. Synergistic effect of GDNF and NGF on axonal branching and elongation in vitro. Neurosci Res. 2009;65(1):88–97. doi: 10.1016/j.neures.2009.06.003. [DOI] [PubMed] [Google Scholar]
- [36].Jessen KR, Mirsky R. Astrocyte-like glia in the peripheral nervous system: an immunohistochemical study of enteric glia. J Neurosci. 1983;3(11):2206–2218. doi: 10.1523/JNEUROSCI.03-11-02206.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Georgiou J, Charlton MP. Non-myelin-forming perisynaptic schwann cells express protein zero and myelin-associated glycoprotein. Glia. 1999;27(2):101–109. doi: 10.1002/(sici)1098-1136(199908)27:2<101::aid-glia1>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- [38].Bhattacharyya A, Frank E, Ratner N, et al. P0 is an early marker of the Schwann cell lineage in chickens. Neuron. 1991;7(5):831–844. doi: 10.1016/0896-6273(91)90285-8. [DOI] [PubMed] [Google Scholar]
- [39].Middlemiss PJ, Jiang S, Wang J, et al. A method for purifying enteric glia from rat myenteric plexus. In Vitro Cell Dev Biol Anim. 2002;38(4):188–190. doi: 10.1290/1071-2690(2002)038<0188:AMFPEG>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- [40].Lee SE, Shen H, Taglialatela G, et al. Expression of nerve growth factor in the dorsal root ganglion after peripheral nerve injury. Brain Res. 1998;796(1-2):99–106. doi: 10.1016/s0006-8993(98)00335-7. [DOI] [PubMed] [Google Scholar]
- [41].Zhou XF, Deng YS, Chie E, et al. Satellite-cell-derived nerve growth factor and neurotrophin-3 are involved in noradrenergic sprouting in the dorsal root ganglia following peripheral nerve injury in the rat. Eur J Neurosci. 1999;11(5):1711–1722. doi: 10.1046/j.1460-9568.1999.00589.x. [DOI] [PubMed] [Google Scholar]
- [42].Neuwelt EA, Glasberg M, Frenkel E, et al. Neurotoxicity of chemo-therapeutic agents after blood-brain barrier modification: neuropathological studies. Ann Neurol. 1983;14(3):316–324. doi: 10.1002/ana.410140310. [DOI] [PubMed] [Google Scholar]
- [43].Hirata A, Masaki T, Motoyoshi K, et al. Intrathecal administration of nerve growth factor delays GAP 43 expression and early phase regeneration of adult rat peripheral nerve. Brain Res. 2002;944(1-2):146–156. doi: 10.1016/s0006-8993(02)02739-7. [DOI] [PubMed] [Google Scholar]
- [44].Shen H, Chung JM, Chung K. Expression of neurotrophin mRNAs in the dorsal root ganglion after spinal nerve injury. Brain Res Mol Brain Res. 1999;64(2):186–192. doi: 10.1016/s0169-328x(98)00314-3. [DOI] [PubMed] [Google Scholar]
- [45].Li L, Deng YS, Zhou XF. Downregulation of TrkA expression in primary sensory neurons after unilateral lumbar spinal nerve transection and some rescuing effects of nerve growth factor infusion. Neurosci Res. 2000;38(2):183–191. doi: 10.1016/s0168-0102(00)00153-x. [DOI] [PubMed] [Google Scholar]
- [46].Snider WD, Silos-Santiago I. Dorsal root ganglion neurons require functional neurotrophin receptors for survival during development. Philos Trans R Soc Lond B Biol Sci. 1996;351(1338):395–403. doi: 10.1098/rstb.1996.0034. [DOI] [PubMed] [Google Scholar]
- [47].Mu X, Silos-Santiago I, Carroll SL, et al. Neurotrophin receptor genes are expressed in distinct patterns in developing dorsal root ganglia. J Neurosci. 1993;13(9):4029–4041. doi: 10.1523/JNEUROSCI.13-09-04029.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Kashiba H, Uchida Y, Senba E. Distribution and colocalization of NGF and GDNF family ligand receptor mRNAs in dorsal root and nodose ganglion neurons of adult rats. Brain Res Mol Brain Res. 2003;110(1):52–62. doi: 10.1016/s0169-328x(02)00584-3. [DOI] [PubMed] [Google Scholar]
- [49].Bennett DL, Boucher TJ, Armanini MP, et al. The glial cell line-derived neurotrophic factor family receptor components are differentially regulated within sensory neurons after nerve injury. J Neurosci. 2000;20(1):427–437. doi: 10.1523/JNEUROSCI.20-01-00427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Lamballe F, Klein R, Barbacid M. trkC, a new member of the trk family of tyrosine protein kinases, is a receptor for neurotrophin-3. Cell. 1991;66(5):967–979. doi: 10.1016/0092-8674(91)90442-2. [DOI] [PubMed] [Google Scholar]
- [51].Tsoulfas P, Soppet D, Escandon E, et al. The rat trkC locus encodes multiple neurogenic receptors that exhibit differential response to neurotrophin-3 in PC12 cells. Neuron. 1993;10(5):975–990. doi: 10.1016/0896-6273(93)90212-a. [DOI] [PubMed] [Google Scholar]
- [52].Gavazzi I, Kumar RD, McMahon SB, et al. Growth responses of different subpopulations of adult sensory neurons to neurotrophic factors in vitro. Eur J Neurosci. 1999;11(10):3405–3414. doi: 10.1046/j.1460-9568.1999.00756.x. [DOI] [PubMed] [Google Scholar]
- [53].Erickson JT, Brosenitsch TA, Katz DM. Brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor are required simultaneously for survival of dopaminergic primery sensory neurons in vivo. J Neurosci. 2001;21(2):581–589. doi: 10.1523/JNEUROSCI.21-02-00581.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Airaksinen MS, Saarma M. The GDNF family: signaling, biological functions and therapeutic value. Nat Rev Neurosci. 2002;3(5):383–394. doi: 10.1038/nrn812. [DOI] [PubMed] [Google Scholar]
- [55].Jiang S, Bendjelloul F, Ballerini P, et al. Guanosine reduces apoptosis and inflammation associated with restoration of function in rats with acute spinal cord injury. Purinergic Signal. 2007;3(4):411–421. doi: 10.1007/s11302-007-9079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hall AK. Rodent sensory neuron culture and analysis. Curr Protoc Neurosci. 2006;(Chapter 3: Unit 3.19) doi: 10.1002/0471142301.ns0319s36. [DOI] [PubMed] [Google Scholar]
- [57].Gunning PW, Shooter EM, Austin L, et al. Differential and coordinate regulation of the eukaryotic small molecular weight RNAs. J Biol Chem. 1981;256(13):6663–6669. [PubMed] [Google Scholar]
- [58].Alonso M, Bekinschtein P, Cammarota M, et al. Endogenous BDNF is required for long-term memory formation in the rat parietal cortex. Learn Mem. 2005;12(5):504–510. doi: 10.1101/lm.27305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Freeman AY, Pierce RC. Neutralization of neutrophin-3 in the ventral tegmental area or nucleus accumbens differentially modulates cocaine-induced behavioral plasticity in rats. Synapse. 2002;46(2):57–65. doi: 10.1002/syn.10123. [DOI] [PubMed] [Google Scholar]
- [60].Hasan W, Jama A, Donohue T, et al. Sympathetic hyperinnervation and inflammatory cell NGF synthesis following myocardial infarction in rats. Brain Res. 2006;1124(1):142–154. doi: 10.1016/j.brainres.2006.09.054. [DOI] [PMC free article] [PubMed] [Google Scholar]


