Abstract
Dibutyltin dilaurate functions as a stabilizer for polyvinyl chloride. In this study, experimental rats were intragastrically administered 5, 10, or 20 mg/kg dibutyltin dilaurate to model sub-chronic poisoning. After exposure, our results showed the activities of superoxide dismutase and glutathione peroxidase decreased in rat brain tissue, while the malondialdehyde and nitric oxide content, as well as nitric oxide synthase activity in rat brain tissue increased. The cell cycle in the right parietal cortex was disordered and the rate of apoptosis increased. DNA damage was aggravated in the cerebral cortex, and the ultrastructure of the right parietal cortex tissues was altered. The above changes became more apparent with exposure to increasing doses of dibutyltin dilaurate. Our experimental findings confirmed the neurotoxicity of dibutyltin dilaurate in rat brain tissues, and demonstrated that the poisoning was dose-dependent.
Keywords: dibutyltin dilaurate, oxidative damage, cell cycle, apoptosis, ultrastructure, DNA damage, brain injury, sub-chronic poisoning
Research Highlights
-
(1)
After dibutyltin dilaurate sub-chronic poisoning, the activities of superoxide dismutase and glutathione peroxidase in rat brain tissue decreased, while the malondialdehyde, nitric oxide content and nitric oxide synthase activity increased.
-
(2)
After dibutyltin dilaurate sub-chronic poisoning, the percentage of cells at G0/G1 phase in rat brain tissue increased, while that of cells at S phase and G2+M phase decreased. Also, the apoptotic rate of brain cells increased, and DNA damage was aggravated.
-
(3)
20 mg/kg dibutyltin dilaurate exposure resulted in apparent neuropil cavitation in rat brains, as shown by cavitation and other ultrastructural changes, with glial filaments dissolving within the axon.
INTRODUCTION
Organic tin is a metal organic compound that has important industrial application potential, by serving as a polyvinyl chloride stabilizer, and as antifouling coatings in pesticides and fungicides. The primary role of tin is as a polyvinyl chloride stabilizer which accounts for 70% of the output of total organic tin compounds. Since tributyltin can induce sexual aberration in marine life[1], organic tin as a pollutant has received increasing attention. Research and emerging regulations restrict the production and use of organic tin[2]. In industry, organic tin has been widely used in ship and marine construction as an antifouling coating.
Therefore, its dialysis into the ocean may pollute bays and ports, which results in tin being found in seafood. In agriculture, organic tin is used as an insecticide, and is directly sprayed onto crops and accordingly enters the environment. Ritsema et al[3] found a strong adsorption and complexation effect of organic tin on soil. These compounds can be accumulated via biological enrichment and enter the human body through the food chain, thus impacting human health[4]. In addition, organic tin compounds, and especially tributyltin, can be toxic to the mammalian reproductive, neurological and immune systems[5]. Tributyltin exposure, which can lead to the apoptosis of rat mesencephalic neural stem cells cultured in vitro[6], induces oxidative damage through the inhibition of glutathione S-transferase activity in in vitro cultured hippocampal slices[7]. Intraperitoneal injection of tributyltin chloride also leads to toxicity and the apoptosis of olfactory cells[8]. Organic tin compounds are strongly toxic against Scenedesmus obliquus and Daphnia magna[9]. Dibutyltin dilaurate is an organic tin compound, with a lower toxicity than tributyltin[10]. Dibutyltin dilaurate mainly serves as a promoting agent in the rubber industry, as a polyethylene plastic stabilizer, and as a catalyst for aldol condensation and esterification. Dibutyltin dilaurate also plays a crucial role in marine antifouling coatings, wood preservative protection, fluorescent dyes, photochemical reactions, and ceramic and polymer modifications[11]. Organic tin compounds can affect nervous system development and function because of their neurotoxicity[12]. Although people are exposed to dibutyltin dilaurate through daily life and work exposure, its cerebral neurotoxicity is seldom reported. Dibutyltin dilaurate can damage the phosphatidylinositol signaling system in rat brains and decrease the levels of diacylglycerol and inositol phosphates in the cerebral cortex[13]. High-dose (40 mg/kg) dibutyltin dilaurate increased the contents of cerebral polyamine and spermine levels in the pons, hypothalamus and frontal cortex, which led to synaptic dysfunction and aggravated poisoning symptoms[14]. This study aims to observe the effects of dibutyltin dilaurate on oxidative damage, lipid peroxidation, changes of cell cycle and apoptosis, DNA damage and ultrastructure changes in rat brain tissue, in a broader attempt to reveal the mechanism(s) underlying dibutyltin dilaurate's neurotoxicity.
RESULTS
Quantitative analysis of experimental animals
Forty rats were randomly divided into a normal control group and dibutyltin dilaurate groups at high, medium and low doses. Respectively, they were intragastrically administered corn oil (1 mL/200 g), and 5, 10, and 20 mg/kg dibutyltin dilaurate (median lethal dose 175 mg/kg[15]). After exposure, two rats in each of the high dose and medium dose groups had mild euphoria and fought each other to the death. All 40 rats were analyzed in the results.
Dibutyltin dilaurate reduced superoxide dismutase and glutathione peroxidase activities, and increased the malondialdehyde content in rat brain tissue
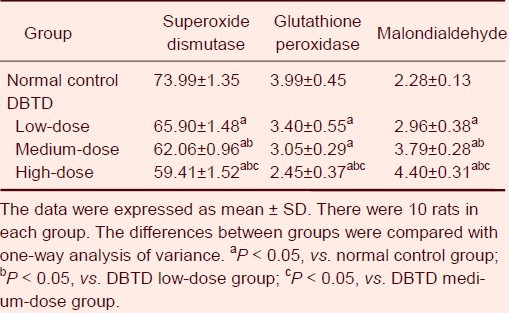
Compared with the normal control group, the activity of superoxide dismutase and glutathione peroxidase in rat brain tissues dose-dependently decreased with the increasing doses of intragastric dibutyltin dilaurate (P < 0.05), while the content of malondialdehyde gradually increased (P < 0.05; Table 1).
Table 1.
Effect of dibutyltin dilaurate (DBTD) on superoxide dismutase activity (U/mg), glutathione peroxidase activity (U/mg) and malondialdehyde content (nmol/mg) in rat brain tissue
Dibutyltin dilaurate increased nitric oxide content and nitric oxide synthase activity in rat brain tissue
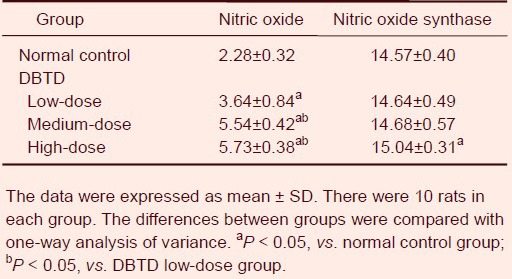
Compared with the normal control group, the nitric oxide content and the activity of nitric oxide synthase in rat brain tissues dose-dependently increased with intragastric dibutyltin dilaurate treatment (P < 0.05; Table 2).
Table 2.
Effect of dibutyltin dilaurate (DBTD) on nitric oxide content (μmol/g) and nitric oxide synthase activity (μmol/g) in rat brain tissue
Effect of dibutyltin dilaurate on cell cycle and apoptosis in the rat right parietal cortex
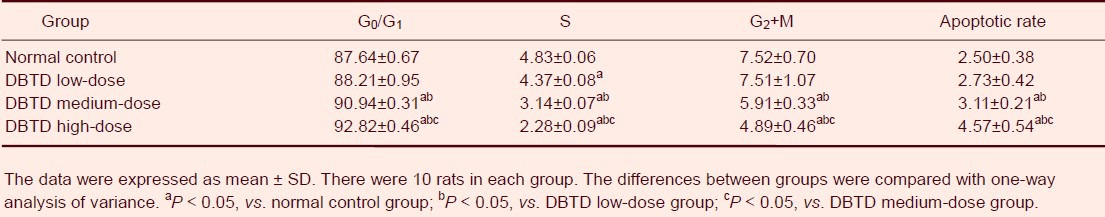
Along with dibutyltin dilaurate exposure, the percentage of cells at the G0/G1 phase in the right parietal cortex gradually increased (P < 0.05), and was significantly higher in the high-dose and medium-dose dibutyltin dilaurate groups compared with the normal control group (P < 0.05). The percentage of cells in the S phase and G2+ M phase gradually decreased (P < 0.05), and was significantly lower in the high-dose and medium-dose dibutyltin dilaurate groups compared with the normal control group (P < 0.05). The apoptotic rate of cells in the rat right parietal cortex gradually increased with increasing doses, and the high-dose and medium-dose dibutyltin dilaurate groups showed significantly higher apoptotic rates than the normal control group (P < 0.05; Table 3).
Table 3.
Effect of dibutyltin dilaurate (DBTD) on cell cycle and apoptosis in the rat right parietal cortex (percentage of cells to total cell number)
Effect of dibutyltin dilaurate on DNA damage in rat cerebral cortex cells
Low-dose dibutyltin dilaurate induced the DNA smearing phenomenon in rat cerebral cortex cells and DNA damage was aggravated with exposure to the low dose (P < 0.05). The total DNA damage rate in the high-dose dibutyltin dilaurate group reached 79.7%, and was significantly increased in the dibutyltin dilaurate groups compared with the normal control group (P < 0.05; Figure 1, Table 4).
Figure 1.
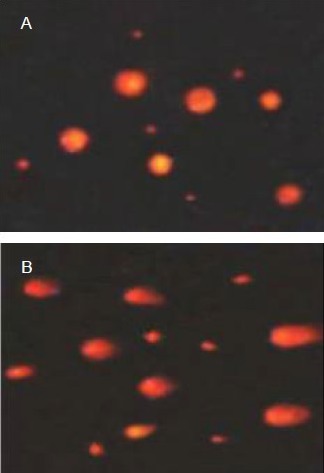
Effect of dibutyltin dilaurate on DNA damage in rat cerebral cortical cells (single cell gel electrophoresis, × 200).
(A) In the normal control group, rat cerebral cortical cells were round, with no DNA smearing.
(B) In the high-dose dibutyltin dilaurate group, rat cerebral cortical cells exhibited nucleus fragmentation, DNA damage and DNA smearing.
Table 4.
Dibutyltin dilaurate on DNA damage in rat cerebral cortical cells
Dibutyltin dilaurate damaged the ultrastructure of the rat right parietal cortex
In the normal control group, the rat right cerebral parietal cortex neuropil showed normal structure with a large number of synapses. The neuropil also displayed visible presynaptic membranes, postsynaptic membranes and synaptic clefts; the neuronal nuclei had clear nuclear membranes, intact cytoplasmic organelles, and evenly distributed nuclear chromatin. Glial cells exhibited normal structure, with a large nucleus, evenly distributed nuclear chromatin, and mitochondria in the cytoplasm. In the low-dose dibutyltin dilaurate group, the rat right cerebral parietal cortex neuropil structure was normal. In the neuropil, capillary peripheral edema was visible, and protrusions surrounding blood vessels occurred in the mitochondria cristae. This cavitation change was demonstrated because the neurofilament fracture was dissolved, and an increasing number of dark neurons was observed. The dark neurons contained cellulose inclusion bodies in the nuclei, while the cytoplasm was deeply colored, and mitochondrial cavitation and crest fractures were observed. Glial cells demonstrated obvious cavitation, even in the cytoplasm and nuclei. In the high-dose dibutyltin dilaurate group, rat right cerebral parietal cortex neuropil cavitation was apparent. Cavitation changes included glial filaments being dissolved within the axon, and deformed neuronal nuclei. Many lysosomes were visible and the mitochondria swelled, while the number of organelles decreased. Intranuclear heterochromatin was also condensed in glial cells, and occasionally, mitochondrial cavitation was observed (Figure 2).
Figure 2.
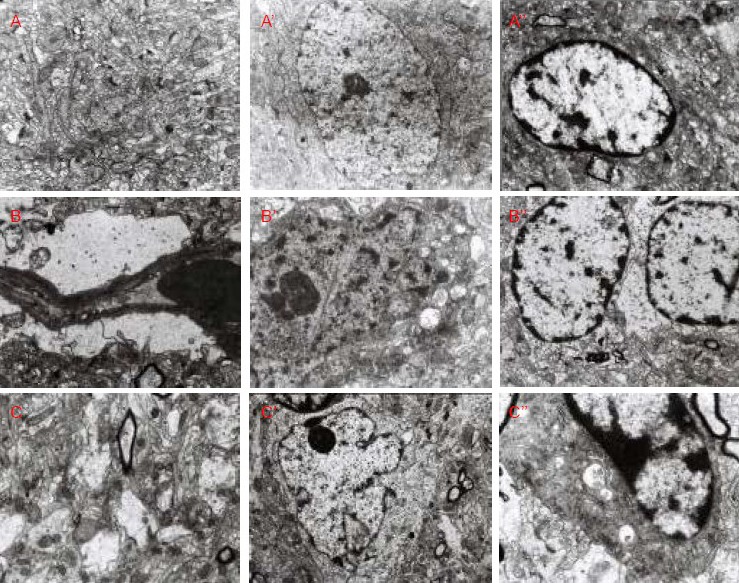
Ultrastructure of rat right parietal cortex (transmission electron microscope).
In the normal control group, rat right cerebral parietal cortical neuropil (A, × 10 000), neurons (A’, × 6 000), and glial cells (A’’, × 7 500) showed normal ultrastructure.
In the low-dose dibutyltin dilaurate group, the capillary ultrastructure was normal (B, × 10 000), but neuronal damage was visible (B’, × 12 000), and glial cells demonstrated cavitation (B’’, × 7 500).
In the high-dose dibutyltin dilaurate group, neuropil cavitation (C, × 10 000), neuronal deformation (C’, × 5 000), and glial cell cavitation (C’’, × 15 000) were visible.
DISCUSSION
The development of modern agriculture and industrial production allows for a wide application of organic tin compounds[16]. Metal ions can induce the generation of reactive oxygen, resulting in oxidative damage to DNA cells, protein and other biological macromolecules[17]. The human body contains oxidative and antioxidative systems[18], which maintain a dynamic balance. When exogenous chemical substances enter into an organism, excessive free radicals may induce lipid peroxidation in the biofilms, damage membrane structure, cause overflow of the cell contents, and ultimately cell death[19].
Malondialdehyde was shown to be significantly increased in the brain tissues of rats with neurodegenerative diseases, such as dementia and Parkinson's syndrome[20,21]. Our experimental results showed that dibutyltin dilaurate exposure significantly decreased the activities of superoxide dismutase and glutathione peroxidase, but significantly increased the content of malondialdehyde compared with the normal control group. These results show that dibutyltin dilaurate might increase the production of lipid peroxidation in brain tissue, decrease the activities of antioxidants such as superoxide dismutase and glutathione peroxidase, disorder the balance between oxidative damage and the antioxidant system, and ultimately cause lipid peroxidation injury in brain cells.
The appropriate amount of nitric oxide meets the needs of physiological function, but redundancies or inadequacies lead to brain tissue damage[22]. Nitric oxide damages the central nervous system by inducing apoptosis[23]. A growing number of animal experiments and clinical studies show that nitric oxide is an important cause of cell necrosis or apoptosis[24]. Nitric oxide freely crosses through cell membranes and regulates cellular functions[25]. Nitric oxide can damage and crack DNA through base deamination[26]. In this study, the nitric oxide content in the brain tissue of dibutyltin dilaurate-exposed rats was significantly higher than that in normal control rats. Also, the activity of nitric oxide synthase increased. Because nitric oxide synthase contributes to the generation of endogenous nitric oxide, the rise of nitric oxide synthase activity raises nitric oxide content. A large concentration of nitric oxide could induce apoptosis, through lipid peroxidation, mitochondrial damage, and/or the antioxidant effect[27], which relies on DNA damage.
Results of this study showed a significant increase in the percentage of dibutyltin dilaurate-treated cells at the G1 phase, while cells at the S phase and G2 phase significantly decreased, dose-dependently. This suggests a G1 arrest hindered the transportation from G1 phase to S phase. The number of cells in the DNA synthetic phase decreased, thereby affecting cell mitosis and inhibiting DNA synthesis. This reduction in S phase cells disturbed the normal development of brain cells and caused cell apoptosis. In vitro experimental results demonstrated that organic tin can increase the generation of thymus cell reactive oxygen species and cause intracellular calcium overload. Also, tin exposure induced apoptosis and oxidative damage in PC12 cells[8,28]. In vivo studies confirmed that tributyltin induced the apoptosis of nerve cells through producing reactive oxygen species[29]. Similar to previously published experimental results, the cell apoptosis rate significantly increased with increasing doses of dibutyltin dilaurate. Our results indicate that dibutyltin dilaurate may alter the cell cycle and accordingly induce the apoptosis of nerve cells. In addition, the apoptosis rate is associated with lipid peroxidation injury and a pro-apoptotic effect on nitric oxide. DNA damage is the main molecular mechanism underlying chemical toxicity[30]. Results of this study showed that brain nerve cells in normal rats demonstrated very little of the DNA smearing phenomenon. After rats were treated with low doses of dibutyltin dilaurate, single or double strand breakages appeared in the DNA, while high doses caused apoptosis in the nerve cells. Higher dibutyltin dilaurate exposures demonstrated more broken DNA chains and alkali denaturation fragments, because of the excessive production of nitric oxide. There is little evidence reporting the mechanism underlying DNA damage-induced apoptosis. Other studies have proposed the mechanism associated with DNA damage could be the sustained activation of poly-ADP-ribose polymerase, which leads to massive depletion of intracellular nicotinamide adenine dinucleotide, reduced contents of coenzyme I and II, and damage to antioxidant capacity. These outcomes eventually increase Ca2+ influx and induce apoptosis[31]. Therefore, dibutyltin dilaurate-induced cell apoptosis may occur because of dibutyltin dilaurate-induced DNA damage.
In this study, the ultrastructure of brain tissue was altered after low-dose of dibutyltin dilaurate exposure. Glial cells that had adhered to the capillary walls began to swell in the protrusions, with apparent cavitations. In addition, large amounts of dark neuron cells were visible. These ultrastructure changes in the cerebral cortex suggested that even low-dose dibutyltin dilaurate can damage glial cells, cross through blood-brain barrier, and lead to slight changes in neurons. High-dose dibutyltin dilaurate induced the apparent cavitation of cerebral cortex neuropil. High-dose dibutyltin dilaurate also caused neuronal nuclei deformation and swelling of mitochondria, reduced the number of cell organelles, and showed apoptotic-like changes, which contributed to the pro-apoptotic effect of nitric oxide and lipid peroxidation damage. Tributyltin can cause neurodegenerative changes, and its molecular mechanism depends on the generation of reactive oxygen species[32]. This is consistent with our experimental findings, and all above changes indicate that increased doses cause more apparent ultrastructural changes in the cerebral cortex, and aggravate neuronal and glial cell damage. We speculate that dibutyltin dilaurate can pass through the blood-brain barrier, induce the apoptosis of glial cells and neurons, damage the rat nervous system, and is neurotoxic.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
Experiments were performed at the Experimental Center, School of Public Health, Jilin University, China from March 2005 to January 2007.
Materials
Animals
Forty Wistar clean rats, aged 10 weeks, male or female, weighing 190 ± 10 g, were provided by the Animal Experimental Center of Jilin University, China with license No. SCXK (Ji) 2007-0003. The animals were housed in separated noise-free cages, at 20–25°C with a relative humidity of 40–60%, a 12-hour light-dark cycle, and a light intensity of 15–20 lx, with free access to water and food. Our experimental proposals were in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[33].
Drugs
Dibutyltin dilaurate is a light yellow, oily liquid, with a molecular formula of (C4H9)2Sn(C11H23COO)2. Dibutyltin dilaurate contains 18.96% Sn, and is insoluble in water but soluble in organic solvent[34]. Dibutyltin dilaurate is a chemically active, moderately toxic organic tin compound, with a specific gravity of 1.05 g/cm3. The half lethal dose in rats is 175 mg/kg[15]. In this study, different doses of dibutyltin dilaurate solution were prepared with corn oil (Sigma, St. Louis, MO, USA).
Methods
Dibutyltin dilaurate poisoning
High-, medium- and low-dose dibutyltin dilaurate groups of rats were intragastrically administered 20, 10 and 5 mg/kg dibutyltin dilaurate, respectively. The normal control group rats were given 1 mL/200 g corn oil. Rats were weighed before each exposure, 5 days per week, for 7 weeks.
Sampling
48 hours after the last exposure, rats were anesthetized with ether, and femoral vein blood samples were harvested. After the rats were killed, brain tissue was excised and preserved. Left brain tissue pieces (0.1–0.2 g) were weighed and placed into a 2-mL tube, mixed with 9 times volume of PBS, and ultrasonically homogenized at 4°C. Then a 10% homogenate was prepared and centrifuged at 3 000 r/min for 10 minutes. The supernatant was collected for further use.
Detection of lipid peroxidation in brain tissue
The activity of superoxide dismutase in brain tissue homogenates was determined using the xanthine oxidase method[35]. Glutathione peroxidase activity was determined using the enzyme dynamics method[35], and the amount of the lipid peroxidation product, malondialdehyde, was determined using the thiobarbituric acid reaction method. Nitric oxide content was determined using the nitrate reductase method[36], while nitric oxide synthase activity was analyzed using hemoglobin spectrophotometry[36]. The protein levels in brain homogenates were measured using the Coomassie brilliant blue method[37]. All reagent kits were provided by the Nanjing Jiancheng Biological Engineering Research Institute (Nanjing, Jiangsu Province, China).
Flow cytometry detection of cell cycle and apoptosis
Brain tissues from rat right parietal cortices[38] were rinsed with 0.01 mM PBS twice, grinded with a slide, triturated with PBS solution repeatedly, and centrifuged at 1 500 r/min for 5 minutes. After the supernatant was removed, the tissue was rinsed with PBS twice and filtered through a 200-mesh nylon. Each sample was prepared into a 1 × 107/mL single cell suspension, which was fixed with 70% cold ethanol. Then single cell suspensions were removed and centrifuged at 1 500 r/min for 5 minutes, followed by the removal of ethanol. Samples were again rinsed with PBS twice and centrifuged as above. After the supernatant was discarded, a 0.5 mL solution was obtained and incubated with 0.01% RNase (200 μL). The solution was stained with propidium iodide (0.5 mL of 0.05 g/L, containing 0.03% Triton X-100) at 4°C in the dark for 30 minutes. The samples were detected with FACScan (Becton Dickinson, Franklin Lakes, NJ, USA). A thousand cells were collected using CellQuest software (Becton Dickinson) and the data were analyzed with ModFit software (Becton Dickinson). The cell percentage and apoptosis rate at each phase were recorded.
Single cell gel electrophoresis assay for the detection of DNA damage in cerebral cortical cells
The single cell gel electrophoresis assay was performed by the modified Singh method[39]. After ethidium bromide staining, 50 cells in each slide (two slides in each group) were observed under UFX-II fluorescence microscopy (Nikon, Tokyo, Japan) using the 549 nm excitation filter and the 590 nm emission filter. We microscopically found DNA smearing shaped like a comet in the damage. DNA damage was divided into five levels as follows: level 0 (< 5%), no damage, no smearing, only a round nucleus-formed fluorescent head; level 1 (5–20%), low damage, a small amount of smearing; level 2 (20–40%), moderate damage, apparent smearing; level 3 (40–95%), great damage, severe smearing; level 4 (> 95%), severe damage, forming a fragment[39].
Transmission electron microscopic observation of the rat cerebral cortex ultrastructure
Right inferior parietal cortical tissues were cut into 1-mm3 pieces, prefixed with 2.5% glutaraldehyde, rinsed with 0.1 M phosphoric acid solution, postfixed with 1% osmium tetroxide, rinsed with 0.1 M phosphoric acid solution, dehydrated with ethanol, embedded in Epon812 epoxy resin, and cut into semi-thin sections (0.8 μm thickness) using a LKB-III microtome. Then 50-nm ultrathin sections were prepared for uranyl acetate and lead citrate double staining. Under transmission electron microscopy (JEM-1200EX; JEOL, Tokyo, Japan), the ultrastructural changes in the rat cerebral cortex were observed.
Statistical analysis
The data were statistically analyzed with SPSS 10.0 software (SPSS, Chicago, IL, USA) and expressed as mean ± SD. The difference between groups was compared with one-way analysis of variance.
Footnotes
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Ethics Committee, the Experimental Animal Center of Jilin University, China.
(Edited by Zou LY, Xu JB/Yang Y/Song LP)
REFERENCES
- [1].Matthiessen P, Gibbs PE. Critical appraisal of the evidence for tributyltin-mediated endocrine disruption in mollusks. Environ Toxicol Chem. 1998;17(1):37–43. [Google Scholar]
- [2].Gibbs PE, Bryan GW. Reproductive failure in populations of the dog-whelk, nucella lapillus, caused by imposex induced by tributyltin from antifouling paints. J Mar Biol Assoc U.k. 1986;66(4):767–777. [Google Scholar]
- [3].Ritsema R, De Smaele T, Moens L, et al. Determination of butyltins in harbour sediment and water by aqueous phase ethylation GC-ICP-MS and hydride generation GC-AAS. Environ Pollut. 1998;99(2):271–277. doi: 10.1016/s0269-7491(97)00128-0. [DOI] [PubMed] [Google Scholar]
- [4].Zeng HC, Chen FR. Environmental estrogens and health. Shiyong Yufang Yixue. 2003;10(5):818. [Google Scholar]
- [5].Shi XH, Wei SQ, Li J. 7. Vol. 26. Ziran Kexue Ban: Chongqing Daxue Xuebao; 2003. Behaviors and effects of organotin compounds in the environment; pp. 104–108. [Google Scholar]
- [6].Suzuki JS, Ishido M. Transcriptome of tributyltin-induced apoptosis of the cultured rat mesencephalic neural stem cells. Toxicology. 2011;287(1-3):61–68. doi: 10.1016/j.tox.2011.06.001. [DOI] [PubMed] [Google Scholar]
- [7].Ishihara Y, Kawami T, Ishida A, et al. Tributyltin induces oxidative stress and neuronal injury by inhibiting glutathione S-transferase in rat organotypic hippocampal slice cultures. Neurochem Int. 2012;60(8):782–790. doi: 10.1016/j.neuint.2012.03.004. [DOI] [PubMed] [Google Scholar]
- [8].Tomiyama K, Nakashima H, Arakawa Y, et al. Mechanism underlying the olfactory disturbance induced by an intraperitoneal injection of tributyltin chloride in rats. Toxicology. 2010;276(2):110–114. doi: 10.1016/j.tox.2010.07.014. [DOI] [PubMed] [Google Scholar]
- [9].Wang SS, Feng L. Toxicity of organic tin compounds and its influencing factors. Anquan yu Huanjing Xuebao. 2005;5(3):12–15. [Google Scholar]
- [10].Wang JL, Ge B, Liu LL. Tributyltin pollution in marine environment. Huanjing Jiance Guanli yu Jishu. 2009;21(6):15–19. [Google Scholar]
- [11].Liu HY, Lin S, Ren JM. Synthesis and application of organotin polymer. Jiangxi Nongye Daxue Xuebao. 2003;25(5):797–800. [Google Scholar]
- [12].Jenkins SM, Ehman K, Barone S., Jr Structure-activity comparison of organotin species: dibutyltin is a developmental neurotoxicant in vitro and in vivo. Brain Res Dev Brain Res. 2004;151(1-2):1–12. doi: 10.1016/j.devbrainres.2004.03.015. [DOI] [PubMed] [Google Scholar]
- [13].Subramoniam A, Husain R, Seth PK. Reduction of phosphoinositides and diacylglycerol levels in repeatedly dibutyltin-dilaurate-treated rat brain. Toxicol Lett. 1991;57(3):245–250. doi: 10.1016/0378-4274(91)90198-f. [DOI] [PubMed] [Google Scholar]
- [14].Khaliq MA, Husain R, Seth PK, et al. Effect of dibutyltin dilaurate on regional brain polyamines in rats. Toxicol Lett. 1991;55(2):179–183. doi: 10.1016/0378-4274(91)90132-p. [DOI] [PubMed] [Google Scholar]
- [15].Klimmer OR. Use of organotin compounds from a experimental-toxicological point of view. Arzneimittelforschung. 1969;19(6):934–939. [PubMed] [Google Scholar]
- [16].Champ MA. A review of organotin regulatory strategies, pending actions, related costs and benefits. Sci Total Environ. 2000;258(1-2):21–71. doi: 10.1016/s0048-9697(00)00506-4. [DOI] [PubMed] [Google Scholar]
- [17].Gennari A, Viviani B, Galli CL, et al. Organotins induce apoptosis by disturbance of [Ca(2+)](i) and mitochondrial activity, causing oxidative stress and activation of caspases in rat thymocytes. Toxicol Appl Pharmacol. 2000;169(2):185–190. doi: 10.1006/taap.2000.9076. [DOI] [PubMed] [Google Scholar]
- [18].Hai CX. Xi’an: Fourth Military Medical University Press; 2006. Radicals Medicine. [Google Scholar]
- [19].Livingstone DR. Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Mar Pollut Bull. 2001;2(8):656–666. doi: 10.1016/s0025-326x(01)00060-1. [DOI] [PubMed] [Google Scholar]
- [20].Schmidley JW. Free radicals in central nervous system ischemia. Stroke. 1990;21(7):1086–1090. doi: 10.1161/01.str.21.7.1086. [DOI] [PubMed] [Google Scholar]
- [21].Lu JM, Zhou HG, Bao YC, et al. Experimental study on cytotoxicity of levodopa in Parkinson's disease rat. Linchuang Shenjing Bing Xue Zazhi. 2004;17(5):355. [Google Scholar]
- [22].Su ZD, Li Y. Nitric oxide and central nervous system injury. Nanjing Junyi Xueyuan Xuebao. 2000;22(3):173–175. [Google Scholar]
- [23].Liu L, Zhao SF. Hyperbaric oxygen inhibits NO synthesization and improves cerebral ischemic cell apoptosis. Zhongguo Weixunhuan. 2005;9(1):21–24. [Google Scholar]
- [24].Zhang L, Dai EL. Influence of Tongluoyizhi Powder on NO and NOS in brain tissue in rat with vascular dementia. Gansu Zhongyi Xueyuan Xuebao. 2005;22(4):25–26. [Google Scholar]
- [25].Hughes KJ, Chambers KT, Meares GP, et al. Nitric oxides mediates a shift from early necrosis to late apoptosis in cytokine-treated β-cells that is associated with irreversible DNA damage. Am J Physiol Endocrinol Metab. 2009;297(5):E1187–1196. doi: 10.1152/ajpendo.00214.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].An LM, Niu YJ, An BH, et al. Effects on nitric oxide synthase expression in rat brain induced by lead. Huanjing yu Jiankang Zazhi. 2004;21(5):296–297. [Google Scholar]
- [27].Bolaños JP, Peuchen S, Heales SJ, et al. Nitric oxide-mediated inhibition of the mitochondrial respiratory chain in cultured astrocytes. J Neurochem. 1994;63(3):910–916. doi: 10.1046/j.1471-4159.1994.63030910.x. [DOI] [PubMed] [Google Scholar]
- [28].Viviani B, Rossi AD, Chow SC, et al. Organotin compounds induce calcium overload and apoptosis in PC12 cells. Neurotoxicology. 1995;16(1):19–25. [PubMed] [Google Scholar]
- [29].Qu M, Zhou Z, Chen C, et al. Lycopene protects against trimethyltin-induced neurotoxicity in primary cultured rat hippocampal neurons by inhibiting the mitochondrial apoptotic pathway. Neurochem Int. 2011;59(8):1095–1103. doi: 10.1016/j.neuint.2011.10.005. [DOI] [PubMed] [Google Scholar]
- [30].Adeeko A, Li D, Forsyth DS, et al. Effects of in utero tributyltin chloride exposure in the rat on pregnancy outcome. Toxicol Sci. 2003;74(2):407–415. doi: 10.1093/toxsci/kfg131. [DOI] [PubMed] [Google Scholar]
- [31].Zeng HC, Chen F, Long DX, et al. Toxicity study on tributyltin chloride on cultured mouse embryos in vitro. Zhongguo Zhiye Yixue. 2005;32(6):11–13. [Google Scholar]
- [32].Geloso MC, Corvino V, Michetti F. Trimethyltin-induced hippocampal degeneration as a tool to investigate neurodegenerative processes. Neurochem Int. doi: 10.1016/j.neuint.2011.03.009. in press. [DOI] [PubMed] [Google Scholar]
- [33].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [34].Wang CH, Liang YX, Ren DF. Dibutyltin dilaurate on occupational exposure to the health of workers. Bengbu Yixueyuan Xuebao. 1997;22(2):118–119. [Google Scholar]
- [35].Pang ZJ. Beijing: People's Medical Publishing House; 2000. Research Methods of Radicals Medicine. [Google Scholar]
- [36].Miao WL, Zhao YQ, Hou YL, et al. Changes of nitric oxide and nitric oxide synthase in rabbits with acute renal failure. Hebei Beifang Xueyuan Xuebao. 2005;22(1):1–3. [Google Scholar]
- [37].Zor T, Selinger Z. Linearization of the Bradford protein assay increases its sensitivity: theoretical and experimental studies. Anal Biochem. 1996;236(2):302–308. doi: 10.1006/abio.1996.0171. [DOI] [PubMed] [Google Scholar]
- [38].Keenan JP, Wheeler M, Platek SM, et al. Self-face processing in a callosotomy patient. Eur J Neurosci. 2003;18(8):2391–2395. doi: 10.1046/j.1460-9568.2003.02958.x. [DOI] [PubMed] [Google Scholar]
- [39].Singh NP, McCoy MT, Tice RR, et al. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp Cell Res. 1988;175(1):184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]


