Abstract
Many studies have been dedicated to the development of scaffolds for improving post-traumatic nerve regeneration. The goal of this study was to assess the effect on nerve regeneration, associating a hybrid chitosan membrane with non-differentiated human mesenchymal stem cells isolated from Wharton's jelly of umbilical cord, in peripheral nerve reconstruction after crush injury. Chromosome analysis on human mesenchymal stem cell line from Wharton's jelly was carried out and no structural alterations were found in metaphase. Chitosan membranes were previously tested in vitro, to assess their ability in supporting human mesenchymal stem cell survival, expansion, and differentiation. For the in vivo testing, Sasco Sprague adult rats were divided in 4 groups of 6 or 7 animals each: Group 1, sciatic axonotmesis injury without any other intervention (Group 1-Crush); Group 2, the axonotmesis lesion of 3 mm was infiltrated with a suspension of 1 250–1 500 human mesenchymal stem cells (total volume of 50 μL) (Group 2-CrushCell); Group 3, axonotmesis lesion of 3 mm was enwrapped with a chitosan type III membrane covered with a monolayer of non-differentiated human mesenchymal stem cells (Group 3-CrushChitIIICell) and Group 4, axonotmesis lesion of 3 mm was enwrapped with a chitosan type III membrane (Group 4-CrushChitIII). Motor and sensory functional recovery was evaluated throughout a healing period of 12 weeks using sciatic functional index, static sciatic index, extensor postural thrust, and withdrawal reflex latency. Stereological analysis was carried out on regenerated nerve fibers. Results showed that infiltration of human mesenchymal stem cells, or the combination of chitosan membrane enwrapment and human mesenchymal stem cell enrichment after nerve crush injury provide a slight advantage to post-traumatic nerve regeneration. Results obtained with chitosan type III membrane alone confirmed that they significantly improve post-traumatic axonal regrowth and may represent a very promising clinical tool in peripheral nerve reconstructive surgery. Yet, umbilical cord human mesenchymal stem cells, that can be expanded in culture and induced to form several different types of cells, may prove, in future experiments, to be a new source of cells for cell therapy, including targets such as peripheral nerve and muscle.
Keywords: stem cells, mesenchymal stem cells, Wharton jelly, umbilical cord, biomaterials, chitosan, axonotmesis, functional analysis, rat, karyotype analysis, stereological analysis
Research Highlights
-
(1)
The sought for effective new therapeutic strategies for improving peripheral nerve regeneration represents one of the hot topics in biomedicine because of the high number of lesions affecting peripheral nerves.
-
(2)
In this study, it was tested in vivo the application of human mesenchymal stem cells from Wharton's jelly of the umbilical cord associated to hybrid chitosan, focusing on its effect in promoting nerve regeneration in axonotmesis.
-
(3)
The results open interesting perspectives in regenerative medicine/tissue engineering emerging areas for the clinical employment of human mesenchymal stem cells and chitosan in peripheral nerve reconstruction.
-
(4)
These results can be interesting for an interdisciplinary readership.
Abbreviations
GPTMS, γ-glycidoxypropyltrimethoxysilane; ESCs, embryonic stem cells; MSCs, mesenchymal stem cells; SFI, sciatic functional index; SSI, static sciatic index; WRL, withdrawal reflex latency
INTRODUCTION
A full understanding of nerve regeneration, especially complete functional achievement and organ reinnervation after nerve injury, still remains the principle goal of regenerative biology. The reliability of animal models is crucial for peripheral nerve research. Because of its peripheral nerve size, the rat sciatic nerve has been the most commonly experimental model used in this kind of studies[1]. The induction of a crush injury in rat sciatic nerve provides a very realistic and useful model of damage for the study of the role of numerous factors in regenerative processes[2]. Focal crush causes axonal interruption but preserves the connective sheaths (axonotmesis). After axonotmesis injury, regeneration is usually successful, after a short (1–2 days) latency, axons regenerate at a steady rate towards the distal nerve stump, supported by the reactive Schwann cells and the preserved endoneural tubules enhance axonal elongation and facilitate adequate reinnervation[3].
Chitosan has attracted particular attention in medical areas due to its biocompatibility, biodegradability, and low toxicity, low cost, enhancement of wound-healing and antibacterial effects[2,4]. In addition, the potential usefulness of chitosan in nerve regeneration has been demonstrated both in vitro and in vivo[5,6]. Chitosan is a partially deacetylated polymer of acetyl glucosamine obtained after the alkaline deacetylation of chitin[5,6]. While chitosan matrices have low mechanical strength under physiological conditions and are unable to maintain a predefined shape after transplantation, their mechanical properties can be improved by modification with a silane agent, namely γ-glycidoxypropyltrimethoxysilane (GPTMS), one of the silane-coupling agents which has epoxy and methoxysilane groups. The epoxy group reacts with the amino groups of chitosan molecules, while the methoxysilane groups are hydrolyzed and form silanol groups. Finally, the silanol groups are subjected to the construction of a siloxane network due to the condensation. Thus, the mechanical strength of chitosan can be improved by the cross-linking between chitosan, GPTMS and siloxane network. By adding GPTMS and employing a freeze-drying technique, we have previously obtained chitosan type III membranes (hybrid chitosan membranes) with pores of about 110 µm diameter and about 90% of porosity, and which were successful in improving sciatic nerve regeneration after axonotmesis[2,4]. Significant differences in water uptake between commonly used chitosan and our hybrid chitosan type III were previously reported and it has been shown that they retain about two times as much biological fluid[2,4,7].
Tissue engineering associates biomaterials, like chitosan, to cellular systems, able to differentiate into neuron-like cells, which might improve peripheral nerve regeneration, in terms of motor, sensory and histomorphometric parameters. Schwann cells, mesenchymal stem cells (MSCs), embryonic stem cells (ESCs), bone marrow stromal cells are the most studied cells candidates. With only a few exceptions, adult stem cell are difficult to expand in culture, and multipotency is a property that remains mostly observed in vivo, whereas ESCs show a remarkable capacity to differentiate into a wide range of cell types in vitro[8,9]. Most human ESC lines have a normal karyotype. Chromosomal abnormalities are common in embryonic carcinomal cells and mouse ESCs, and karyotypic changes often enhance their proliferative capacity while shortening the population doubling time. Such epigenetic changes are associated with prolonged culture of ESCs[10,11] and are observed not only in naturally occurring ESCs, but also in ESCs cloned using somatic cell nuclear transfer[12]. It has also been reported that the acquisition of chromosomal abnormalities may be related to the laboratory manipulations of cells. Established cell lines must be maintained under stringent culture conditions and be checked often for the acquisition of chromosomal abnormalities, although the incidence of such instability is not fully understood. So we decided to check the karyotype of the Wharton's jelly MSCs used for nerve regeneration associated with the hybrid chitosan membranes type III. The cellular systems implanted into the injured nerve may produce growth factors or extracellular matrix molecules, or may modulate the inflammatory process, to improve nerve regeneration[4,13,14,15,16]. We previously focused our research in N1E-115 cell line differentiated in vitro[4,13,15,16]. To implant cultured cells (N1E-115 cells, MSCs, Schwann cells, and other cellular systems) into defective nerves (with axonotmesis and neurotmesis injuries), there are two main techniques. The cellular system may be directly injected into the neural scaffold which has been interposed between the proximal and distal nerve stumps or around the crush injury (in neurotmesis and axonotmesis injuries, respectively). It can also be performed by pre-adding the cells to the neural scaffold via injection or co-culture (in most of the cellular systems, it is allowed to form a monolayer) and then the biomaterial with the cellular system is implanted in the injured nerve.
In addition to the ESCs, there are sources rich in non-ESCs, or adult stem cells. These are undifferentiated cells found in differentiated tissues that have limited self-renewal and differentiation capacity, usually restricted to cell types of the tissue that they originally came from. The most common source from which they are isolated is the bone marrow, a mesoderm derived tissue. For decades, it has been known that the bone marrow contains two types of stem cells: hematopoietic ones, which are committed to differentiate into mature blood cells, and the less-differentiated MSCs. MSCs have the ability to differentiate in vivo and in vitro into a variety of adult mesenchymal tissues, such as bone, cartilage, adipose, and muscle and therefore may be considered to be an important counterpart for potential clinical applications. In addition to the cells derived from the bone marrow, the term “adult stem cell” also describes cells obtained from less-mature sources such as the umbilical cord blood, the placenta, and fetal tissues such as the umbilical cord, the liver and pancreas[17]. Extra-embryonic tissues as stem cell reservoirs offer many advantages over both embryonic and adult stem cell sources. Extra-embryonic tissues, collectively known as the afterbirth, are routinely discarded at parturition, so little ethical controversy attends the harvest of the resident stem cell populations. Most significantly, the comparatively large volume of extra-embryonic tissues and easy manipulation hypothetically increases the number of stem cells that can be isolated[18]. The umbilical cord contains two arteries and one vein protected by a proteoglycan rich connective tissue called Wharton's jelly. Within the abundant extracellular matrix of Wharton's jelly resides a recently described stem cell population called umbilical cord matrix stem cells or Wharton's jelly MSCs. In average, 400 000 cells can be isolated per umbilical cord, which is significantly greater than the number of MSCs that can be routinely isolated from adult bone marrow. The phenotypic stromal cells in the Wharton's jelly are fibroblast-like cells[17]. However, cells with the ultra-structural characteristics of myofibroblasts have been found[17]. The MSCs from the umbilical cord express adhesion molecules (CD44, CD105), integrin markers (CD29, CD51), and MSC markers (SH2, SH3) but not markers of hematopoietic differentiation (CD34, CD45)[17], when expanded in culture. In vitro, Wharton's jelly MSCs are capable of differentiation to multiple mesoderm cell types including skeletal muscle and neurons[17,18,19]. Generation of clinically important dopaminergic neurons has also been reported[19]. Human MSCs isolated from Wharton's jelly from the umbilical cord can be easily and ethically obtained and processed compared with embryonic or bone marrow stem cells. These cells may be a valuable source in the repair of the peripheral nerve system. Human MSCs from Wharton's jelly of the umbilical cord possess stem cell properties[20] and it was previously demonstrated that human MSCs could be induced to differentiate into neuron-like cells[19]. The transplanted cells were able to promote local blood vessel formation (local vascularization) and produce neurotrophic factors, like brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor[17,19]. Thus, umbilical cord human MSCs can be expanded in culture and induced to form several different types of cells. They may therefore prove to be a new source of cells for cell therapy, including targets such as peripheral nerve and muscle. This will help to avoid several ethical and technical issues. Previous published results from in vivo experiments showed that enrichment of chitosan membranes with N1E-115 neural cells in axonotmesis and neurotmesis lesions did not have any positive effect on nerve regeneration in comparison to crush controls and, in case of type III chitosan membrane, the presence of transplanted cells even prevented the positive effects of the membrane wrapping alone on nerve regeneration. Probably, these negative results obtained were due to the neoplastic source of the cellular system used for neurotrophic factors delivery albeit the in vitro differentiation into neural cells in the presence of DMSO[2,13,16,21]. Neuronal differentiation of these cells is accompanied by synthesis and delivery of a number of neurotrophic factors that might be useful in promoting axonal elongation[2,13,16,21]. However, the presence of transplanted N1E-115 cells in nerve scaffolds competing for the local blood supply of nutrients and oxygen and by space-occupying effect could have hindered the positive effect of local neurotrophic factor release leading a negative outcome on nerve regeneration[2,13,16,21]. Anyway, it can be hypothesized that membrane enrichment with other cell types like the MSCs, may lead to better results and thus the goal of this study was to assess the effect of the hybrid chitosan membrane type III enriched with non-differentiated human MSCs isolated from umbilical cord Wharton's jelly, in peripheral nerve reconstruction after crush injury. The synergistic effect of a more favorable porous microstructure and physicochemical properties (more wettable and higher water uptake level) of chitosan type III compared to common chitosan, as well as the presence of silica ions, may be responsible for the good results in promoting post-traumatic nerve regeneration[2] suggesting that this material may not just work as a simple mechanical scaffold but instead may work as an inducer of nerve regeneration[2]. The neuroregenerative property of chitosan type III might be explained by a direct stimulation of Schwann cell proliferation, axon elongation and myelination[22,23].
RESULTS
Human MSCs culture and karyotype analysis
Undifferentiated human MSCs from human umbilical cord Wharton's jelly, exhibited a normal star-like shape with a flat morphology in culture (Figure 1). A total of 20 Giemsa-stained metaphases of these cells, were analyzed for numerical aberrations. Sporadic, non-clonal aneuploidy was found in 3 cells (41–45 chromosomes). The other 17 metaphases had 46 chromosomes (Figure 2). The karyotype was determined in a completely analyzed G-banding metaphase. No structural alterations were found. The karyotype analysis to the human MSCs cell line derived from human Wharton jelly demonstrated that this cell line has no neoplastic characteristics and is stable during the cell culture procedures in terms of number and structure of the somatic and sexual chromosomes.
Figure 1.
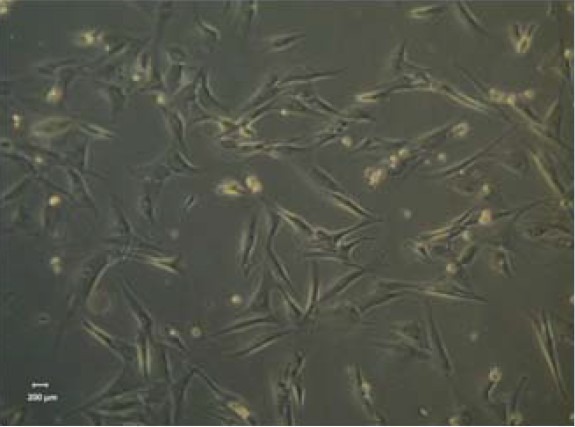
Undifferentiated mesenchymal stem cells, from human umbilical cord Wharton's jelly, exhibiting a star-like shape with a flat morphology (× 100).
Figure 2.

Selected methaphases from human mesenchymal stem cell line from Wharton's jelly, showing the normal number of chromosomes (46, XY) (× 1 000).
Functional analysis of motor deficit and nociceptive function
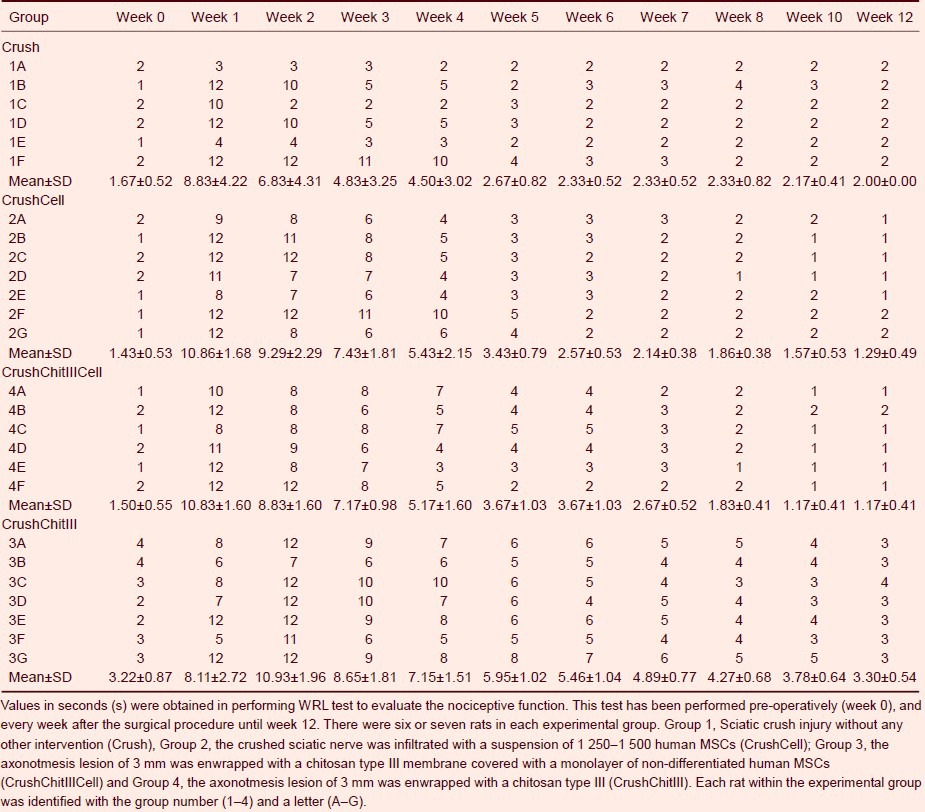
The withdrawal reflex latency (WRL) test was used to assess nociception. In the first 2 weeks post sciatic crush injury, the WRL response was absent (i.e. lack of withdrawal within 12 seconds; the cutoff time to prevent thermal damage to the paw) in a large majority of animals (Table 1). With time, the WRL improved in all animals and at week 12, the WRL values were 2.00 ± 0.00, 1.29 ± 0.18, 1.17 ± 0.17, and 3.3 ± 0.54 seconds, in groups Crush, CrushCell, CrushCellChitIII, and CrushChitIII, respectively. A significant difference was observed between the groups in WRL data (F(3,22) = 6.449, P = 0.001) with delayed recovery in WRL performance in the CrushChitIII group compared to the other three groups (P < 0.05).
Table 1.
Nociceptive function of rats throughout a healing period of 12 weeks
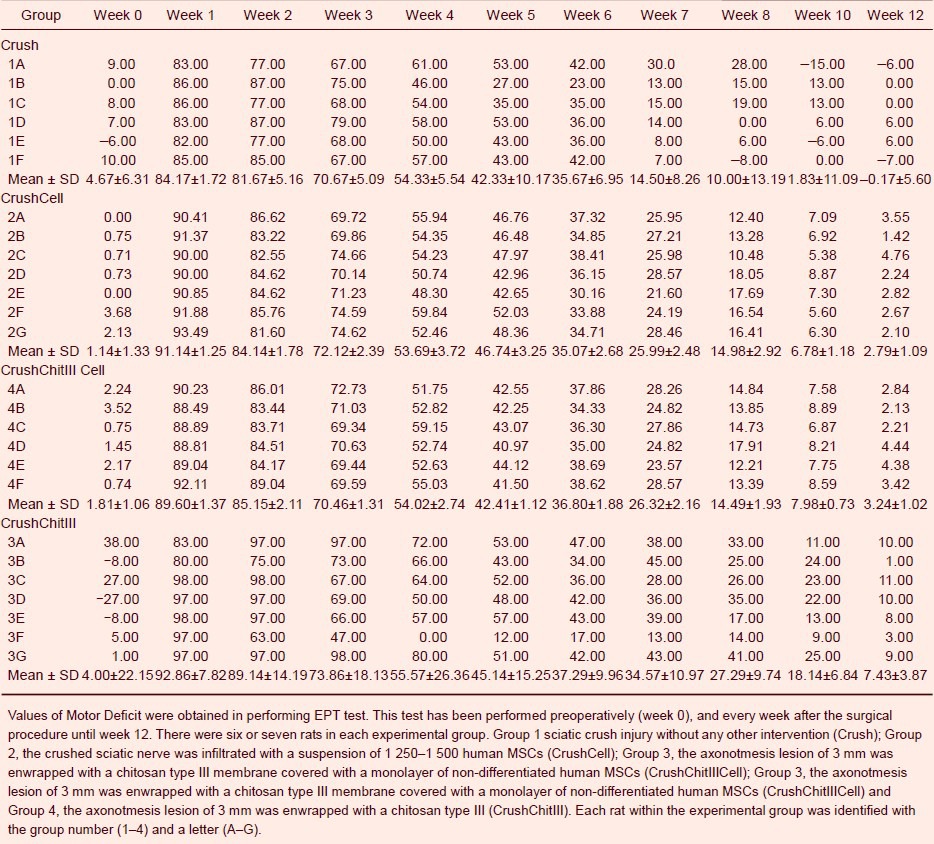
The sciatic nerve crush caused severe muscle force deficit in the affected limb immediately post-surgery. At week 1, the percentage of motor function deficit for the right hindlimb reached over 90% in all groups (Table 2). A gradual recovery of the right hindlimb extensor postural thrust occurred during the 12-week survival time in all groups, so that at week 12, the EPT deficit in the affected side, although not fully reestablished, was reduced to only 4.16 ± 5.60, 2.79 ± 0.41, 3.24 ± 0.42, and 7.43 ± 3.58 in groups Crush, CrushCell, CrushChitIIICell and, CrushChitIII, respectively. EPT performance was similar in all groups [F(3,22) = 1.367, P = 0.279] (Table 2) although at the end of the 12 weeks of recovery, the EPT values were lower in CrushCell and CrushChitIIICell groups.
Table 2.
Motor function of rats throughout a healing period of 12 weeks
Sciatic nerve morphology and stereology
Figure 3 represents the histology of the sciatic regenerating nerves 12 weeks after crush lesion-Group Crush, crush lesion with human MSCs injection-Group CrushCell, crush lesion enwrapped with a chitosan type III membrane covered with human MSCs-Group CrushChitIIICell, crush lesion enwrapped with a chitosan type III membrane-Group CrushChitIII compared to a normal sciatic nerve. Fiber regeneration was good in all experimental groups, though the regenerated nerves presented smaller myelin fibers than the normal nerves without injury, as confirmed by stereological data presented in Table 3.
Figure 3.
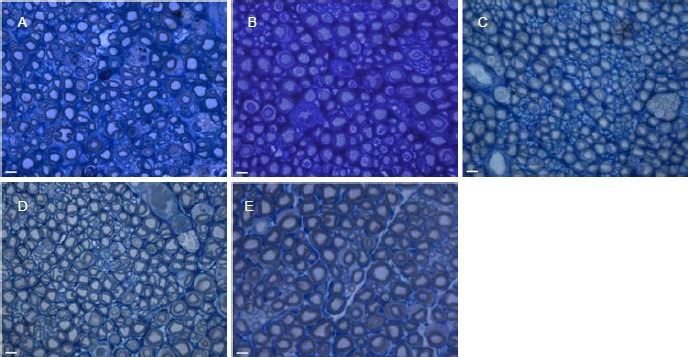
Semithin transverse sections, stained with Toluidine Blue, of sciatic nerve of the different experimental groups.
(A: Group 1–Crush; B: Group 2–CrushCell; C: Group 3–CrushChitIIICell; D: Group 4–CrushChitIII) compared with a normal nerve (E). Scale bars: 5 μm.
Table 3.
Histomorphometrical assessment of normal (control) and regenerated sciatic nerves submitted to a standardized sciatic nerve crush injury with non-serrated clamp (week-12 posttraumatic)
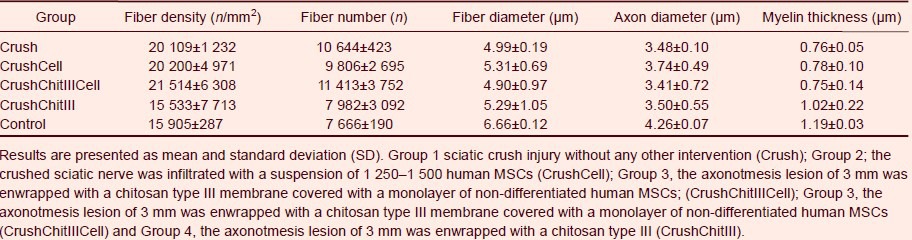
Myelinated fiber density and total number were significantly (P < 0.05) higher than in controls in all nerve regeneration groups except for the CrushChitIII group. Axon and fiber diameter and myelin thickness were significantly (P < 0.05) lower in the 3 experimental groups compared to control group (normal sciatic nerve without injury). Analysis of the inter-group variability among regenerated groups, showed that CrushChitIII group had significantly (P < 0.05) lower fiber density and fiber total number and a higher myelin thickness while no statistically significant differences (P > 0.05) were detectable for the remaining histomorphometrical predictors of nerve regeneration (fiber diameter and axon diameter).
DISCUSSION
Results from in vivo testing previously performed by our research group[2] showed that type III chitosan improved nerve fiber regeneration in comparison to control crushed sciatic nerves. Chitosan type III was developed as a hybrid of chitosan by the addition of GPTMS. Wettability of material surfaces is one of the key factors for protein adsorption, cell attachment and migration[24]. The addition of GPTMS improved the wettability of chitosan surfaces[2,4,22], and therefore chitosan type III is expected to be more hydrophilic than the original chitosan[2,4,22]. Chitosan type III was developed to be more porous, with a larger surface to volume ratio but preserving mechanical strength and the ability to adapt to different shapes. Significant differences in water uptake between commonly used chitosan and our hybrid chitosan type III were previously reported as a consequence of the difference in the ability of the matrix to hold water. In fact, hybrid chitosan-based membranes may retain about twice as much biological fluid as chitosan[7]. A synergistic effect of a more favorable porous microstructure and physicochemical properties (more wettable and higher water uptake level) of chitosan type III and the presence of silica ions may be responsible for the good results in promoting post-traumatic nerve regeneration. The significant improvement of axonal regeneration obtained in crushed sciatic nerves surrounded by chitosan type III membranes suggests that this material may not just work as a simple mechanical scaffold but instead may work as an inducer of nerve regeneration. The neuroregenerative property of chitosan type III might be explained by the action on Schwann cell proliferation, axon elongation and myelinization[2,4,22]. Yet, the expression of established myelin genes such as PMP22, PO and MBP[25,26] may be influenced by the presence of silica ions which exert an effect on several glycoprotein expression[25,26].
Results from in vivo experiments previously performed[2] showed that enrichment of chitosan membranes with N1E-115 neural cells did not have any positive effect on nerve regeneration in comparison to crush controls and, in case of type III chitosan membrane, the presence of transplanted cells even prevented the positive effects of the membrane wrapping alone on nerve regeneration. These results are in agreement with previous experiments that showed that N1E-115 cell population does not have significant effects in promoting axon regeneration and, when N1E-115 cells were cultured inside a poly(lactic-co-glycolic acid) scaffold used to bridge a nerve defect, they can even exert negative effects on nerve fiber regeneration[16,21]. The presence of transplanted N1E-115 cells in nerve scaffolds competing for the local blood supply of nutrients and oxygen and by space-occupying effect could have hindered the positive effect of local neurotrophic factor release leading a negative outcome on nerve regeneration[2,13,16,21]. Thus, N1E-115 cells did not prove to be a suitable candidate cellular system for treatment of nerve injury after axonotmesis and neurotmesis[2,16,21] and their application is limited only to research purposes as a basic scientific step for the development of other cell delivery systems, due to its neoplastic origin.
In this study, we used chitosan type III membrane to deliver human MSCs from the umbilical cord Wharton jelly and we compared this delivery approach with direct injection/infiltration of these human MSCs in suspension, in the rat sciatic nerve axonotmesis model. The cellular systems implanted into the injured nerve may produce growth factors or extracellular matrix molecules, or may modulate the inflammatory process, to improve nerve regeneration or even replace the injured neural and Schwann cells[4,13,14,15,16,27]. The human MSCs karyotype was studied in order to be sure that these cells did not present any number or structure chromosome abnormalities due to isolation and cell culture procedures before in vivo application. This concern was due to the negative effects that N1E-115 cells presented in axonotmesis and neurotmesis injuries, since this cell line has neoplastic characteristics[2,13,16,21]. The karyotype analysis to the human MSCs cell line derived from umbilical cord Wharton jelly demonstrated that this cell line has no neoplastic characteristics and is stable during the cell culture procedures in terms of number and structure of the somatic and sexual chromosomes. Also, the morphologic characteristics of these cells in culture, observed in an inverted microscope, were perfectly normal. These cells presented a star-like shape with a flat morphology, characteristic of MSCs[28].
The functional analysis revealed a gradual recovery of the injured hindlimb EPT during the 12-week healing period in all three experimental groups. In week 12, the percentage motor deficit in the affected hindlimb, although not totally recovered to normal values, reduced especially in CrushCell, and CrushCellChitIII groups. With time, the WRL improved in all animals during the 12-week healing period, and no differences between groups were found in the rate of recovery of this response. By the end of the 12 weeks, all animals from the three experimental groups presented normal WRL values. Anyway, there was no significant delayed recovery in WRL performance in the CrushChitIII group compared to the other three groups.
Similarly, stereological analysis showed no statistically significant differences among the experimental groups for any of the histomorphological of nerve regeneration investigated with the only exception of the group where nerve crush site was enwrapped with chitosan type III membranes alone, in line with previous findings[2]. The neuroregenerative property of chitosan type III might be explained by the action on Schwann cell proliferation, axon elongation and myelinization[2,4,22], which might explain the higher myelin thickness in the regenerated nerves enwrapped with the chitosan type III membrane alone (Group CrushChitIII). Comparing the results obtained in crush injuries where the local lesion was enwrapped with chitosan type III membranes associated to N1E-115 in vitro differentiated cells[2], with the results obtained in this experimental work with the same chitosan membranes associated to human MSCs, we conclude that the negative effects observed with the N1E-115 cell line are not observed with human MSCs. As a matter of fact, the application of human MSCs associated or not to the chitosan membranes showed positive effects concerning the functional recovery (evaluated by EPT and WRL tests), probably due to the modulation of the inflammatory process during the Wallerian degeneration and by the production of growth factors. On the other hand, statistically significant positive effects where observed concerning the higher myelin thickness in the regenerated nerves enwrapped with the chitosan type III membrane alone. As expected, regenerated nerve fibers were organized in microfascicles and smaller when compared to normal control nerves. CrushChitIII group like the control group presented a significantly lower fiber density and fiber total number and a higher myelin thickness in comparison with other experimental groups as it was observed in a previous published work[2].
In conclusion, results of this study suggest that either enrichment of human MSCs alone or the combination of chitosan type III membrane enwrapment and human MSCs infiltration after nerve crush injury provides a slight advantage in comparison to untreated controls. On the other hand, our results confirmed that chitosan type III membranes alone may represent a very promising clinical tool in peripheral nerve reconstructive surgery. Thus, human umbilical cord human MSCs can be expanded in culture and induced to form several different types of cells. They may therefore in future experiments, be tested as a new source of cells for cell therapy, including targets such as peripheral nerve in more serious lesions (neurotmesis with and without loss of nerve tissue) and muscle. Also, a more accurate functional analysis in other to evaluate different therapeutic strategies should be used, considering for instance the use of ankle kinematic analysis during the rat locomotion.
MATERIALS AND METHODS
Design
A randomized controlled animal experiment.
Time and setting
This experimental work was performed from January to July of 2011 in Institute of Biomedical Sciences Abel Salazar, Veterinary Clinics Department, Porto University, Portugal.
Materials
Hybrid chitosan membranes
Chitosan (high molecular weight, Aldrich®, USA) was dissolved in 0.25 M acetic acid aqueous solution to a concentration of 2% (w/v). To obtain type III membranes, GPTMS (Aldrich®, USA) was also added to the chitosan solution and stirred at room temperature for 1 hour. The drying process for type III chitosan membrane was as follows: the solutions were frozen for 24 hours at –20°C and then transferred to the freeze-dryer, where they were left 12 hours to complete dryness. The chitosan type III membranes were soaked in 0.25 N sodium hydroxide aqueous solution to neutralize remaining acetic acid, washed well with distilled water, and freeze dried[2]. All membranes were sterilized with the preferred ethylene oxide gas method[29]. Prior to their use in vivo, membranes were kept during 1 week at room temperature in order to clear any ethylene oxide gas remnants[2].
Methods
Human MSCs culture
Human MSCs from Wharton's jelly umbilical cord matrix were purchased from PromoCell GmbH (C-12971, lot No. 8082606.7). The human MSCs were cultured and maintained in a humidified atmosphere with 5% CO2 at 37 °C. MSC medium, PromoCell (C-28010) was replaced every 48 hours. At 90% confluence, cells were harvested with 0.25% trypsin with EDTA (Gibco, Alfagene, Carcavelos, Portugal) and passed into a new flask for further expansion. MSCs at a concentration of 104cells/cm2 were cultured exhibiting a 90% confluence after 4 days. The application of human MSCs in rats is possible without inducing any immunossupression in the experimental animals.
Karyotype determination of human MSCs from Wharton's jelly
Chromosome analysis on human MSC line from Wharton's jelly was carried out between passages 4 and 5. When confluence was reached, culture medium was changed and supplemented with 4 μg/mL colcemid solution (stock solution, Cat. n⁰. 15212-012, Gibco). After 4 hours, cells were collected and suspended in 8 mL of 0.075 M KCl solution supplemented with bovine fetal serum. Then the suspension was incubated in 37°C for 35 minutes. After centrifugation (1 500 r/min), 8 mL of the fixative methanol: glacial acetic acid at 6:1 was added and mixed together, and the cells were again centrifuged. After two rounds of fixation, two new rounds were performed with the fixative methanol: glacial acetic acid at 3:1. After the last centrifugation, the cell suspension was spread onto very well cleaned slides. Chromosome analysis was performed by one scorer on 20 Giemsa-stained metaphases. Each cell was scored for chromosome number. Routine chromosome G-banding analysis was also carried out for determination of the karyotype.
Surgical procedure
All procedures were performed with the approval of the Veterinary Authorities of Portugal in accordance with the European Communities Council Directive of November 1986 (86/609/EEC). A total of 25 adult male Sasco Sprague rats (Charles River Laboratories, Barcelona, Spain) weighing approximately 250 g at the start of the experiment were used. Animals were divided by 4 experimental groups of 6 or 7 animals each. Experimental groups were set according to treatment after nerve sciatic axonotmesis injury. In Group 1, animals recovered from axonotmesis sciatic injury without any other intervention (Group 1–Crush). In Group 2, axonotmesis sciatic nerve was infiltrated with a suspension of 1 250–1 500 MSCs (total volume of 50 μL) (Group 2–CrushCell). In Group 3, axonotmesis lesion of 3 mm was enwrapped with a chitosan type III membrane covered with a monolayer of non-differentiated human MSCs (Group 3–CrushChitIIICell) and in Group 4, axonotmesis lesion of 3 mm was enwrapped with a chitosan type III membrane (Group 4–CrushChitIII). Standardized crush injury was carried out with the animals placed prone under sterile conditions and skin from the clipped lateral right thigh scrubbed in a routine fashion with antiseptic solution. Surgery procedure was previously described[3,15]. A standard crush injury was performed by a non-serrated clamp (Institute of Industrial Electronic and Material Sciences, University of Technology, Vienna, Austria), exerting a constant force of 54 N for a period of 30 seconds, 10 mm above the bifurcation into tibial and common peroneal nerves, inducing a 3 mm axonotmesis lesion[30]. To prevent autotomy, a deterrent substance was daily applied to rat right foot[31]. Animals were intensively examined for signs of autotomy and contracture and none presented severe wounds (absence of a part of the foot or severe infection) or contractures during the study. No local or systemic signs of rejection or foreign body were observed in the experimental animals transplanted with chitosan type III membranes and human MSCs. There was no need of administrating immunosuppressive treatment to the experimental animals during the entire healing period of 12 weeks after the surgical procedure.
Functional analysis of motor deficit and nociceptive function
All animals were tested preoperatively (week 0), every week until week 8, and then every other week, until week 12. Animals were gently handled, and tested in a quiet environment to minimize stress levels. Motor deficit and nociceptive function were evaluated by measuring EPT and WRL, respectively[3,15,16,21]. For EPT test, the entire body of the rat, excepting the hindlimbs, was wrapped in a surgical towel. Supporting the animal by the thorax and lowering the affected hind limb towards the platform of a digital balance, the EPT was elicited. As the animal was lowered to the platform, it extended the hindlimb, anticipating the contact made by the distal metatarsus and digits. The force in grams (g) applied to the digital platform balance (model TM 560; Gibertini, Milan, Italy) was recorded. The same procedure was applied to the contra-lateral, unaffected limb. For this test, the affected and normal limbs were tested three times, with an interval of 2 minutes between consecutive tests, and the three values were averaged to obtain a final result. Normal (unaffected limb) EPT (NEPT) and experimental EPT (EEPT) values were incorporated into an equation (Equation (1)) to derive the percentage of functional deficit, as described in the literature[32]:

Nociceptive WRL was adapted from hotplate test developed by Masters et al[33] and described elsewhere[3,15,16,21]. Briefly, the rat was wrapped in a surgical towel above its waist and then positioned to stand with the affected hind paw on a hot plate at 56°C (model 35-D, IITC Life Science Instruments, Woodland Hill, CA, USA). WRL is defined as the time elapsed from the onset of hotplate contact to withdrawal of the hind paw and measured with a stopwatch. Normal rats withdraw their paws from the hotplate within 4 seconds or less[34]. The affected limbs were tested 3 times, with an interval of 2 minutes between consecutive tests to prevent sensitization, and the 3 latencies were averaged to obtain a final result. The cutoff time for heat stimulation was set at 12 seconds to avoid skin damage to the foot[35].
Sciatic nerve morphology and stereology
Nerve samples (10-mm-long sciatic nerve segments distal to the crush site and from un-operated controls) were processed for quantitative morphometry of myelinated nerve fibers[36]. Fixation was carried out using 2.5% purified glutaraldehyde and 0.5% saccarose in 0.1 M Sorensen phosphate buffer for 6–8 hours and resin embedding was obtained following Glauerts’ procedure[37]. Series of 2 µm thick semi-thin transverse sections were cut using a Leica Ultracut UCT ultramicrotome (Leica Microsystems, Wetzlar, Germany) and stained by Toluidine blue. Stereology was carried out on a DM4000B microscope equipped with a DFC320 digital camera and an IM50 image manager system (Leica Microsystems, Wetzlar, Germany). Systematic random sampling and D-disector were adopted using a protocol previously described[38,39]. Fiber density and total number were estimated together with fiber and axon diameter and myelin thickness.
Statistical analysis
The results of the functional tests are reported for each time point, including pre-operatively, and each experimental group as means and standard deviation (SD). Differences between time points and between groups were tested by two-way analysis of variance using a mixed model of within- (time of recovery) and between-subjects (experimental groups) factors. Pairwise comparisons between each two groups were undertaken using the post hoc Tukey's HSD test. Statistical significance was accepted at the level of P < 0.05. For stereology, statistical comparisons of quantitative data were subjected to one-way analysis of variance test. Statistical significance was established as P < 0.05. All statistical procedures were performed by using the statistical package SPSS (version 14.0, SPSS, Chicago, IL, USA) except stereological data that were analyzed using the software “Statistica per discipline bio-mediche” (McGraw-Hill, Milan, Italy).
Acknowledgments
We would like to gratefully acknowledge the valuable support by Dr. José Manuel Correia Costa, from National Institute for Health Dr. Ricardo Jorge (INSRJ), Parasitology Laboratory, Porto, Portugal.
Footnotes
Funding: This work was supported by Technology and Science Foundation (FCT), Education and Science Ministry, Portugal, through the financed research project PTDC/DES/104036/2008, and by QREN N° 1 372 - Nucleus I&DT for the Development of Products for Regenerative Medicine and Cell Therapies - Núcleo Biomat & Cell. Andrea Gärtner has a Doctoral Grant from Technology and Science Foundation (FCT), Education and Science Ministry, Portugal, SFRH/BD/70211/2010.
Conflicts of interest: None declared.
Ethical approval: In this study, all procedures were performed with the approval of the Veterinary Authorities of Portugal in accordance with the European Communities Council Directive of November 1986 (86/609/EEC), and the NIH guidelines for the care and use of laboratory animals have been observed.
(Edited by Oliveira J, Haastert K/Zhao LJ/Song LP)
REFERENCES
- [1].Ronchi G, Nicolino S, Raimondo S, et al. Functional and morphological assessment of a standardized crush injury of the rat median nerve. J Neurosci Methods. 2009;179(1):51–57. doi: 10.1016/j.jneumeth.2009.01.011. [DOI] [PubMed] [Google Scholar]
- [2].Amado S, Simoes MJ, Armada da Silva PA, et al. Use of hybrid chitosan membranes and N1E-115 cells for promoting nerve regeneration in an axonotmesis rat model. Biomaterials. 2008;29(33):4409–4419. doi: 10.1016/j.biomaterials.2008.07.043. [DOI] [PubMed] [Google Scholar]
- [3].Luis AL, Amado S, Geuna S, et al. Long-term functional and morphological assessment of a standardized rat sciatic nerve crush injury with a non-serrated clamp. J Neurosci Methods. 2007;163(1):92–104. doi: 10.1016/j.jneumeth.2007.02.017. [DOI] [PubMed] [Google Scholar]
- [4].Simoes MJ, Amado S, Gartner A, et al. Use of chitosan scaffolds for repairing rat sciatic nerve defects. Ital J Anat Embryol. 2010;115(3):190–210. [PubMed] [Google Scholar]
- [5].Chandy T, Sharma CP. Chitosan--as a biomaterial. Biomater Artif Cells Artif Organs. 1990;18(1):1–24. doi: 10.3109/10731199009117286. [DOI] [PubMed] [Google Scholar]
- [6].Senel S, McClure SJ. Potential applications of chitosan in veterinary medicine. Adv Drug Deliv Rev. 2004;56(10):1467–1480. doi: 10.1016/j.addr.2004.02.007. [DOI] [PubMed] [Google Scholar]
- [7].Chen G, Ushida T, Tateishi T. Scaffold design for tissue engineering. Macromol Biosci. 2002;2(2):67–77. [Google Scholar]
- [8].Keller G. Embryonic stem cell differentiation: emergence of a new era in biology and medicine. Genes Dev. 2005;19(10):1129–1155. doi: 10.1101/gad.1303605. [DOI] [PubMed] [Google Scholar]
- [9].Smith AG. Embryo-derived stem cells: of mice and men. Annu Rev Cell Dev Biol. 2001;17:435–462. doi: 10.1146/annurev.cellbio.17.1.435. [DOI] [PubMed] [Google Scholar]
- [10].Draper JS, Smith K, Gokhale P, et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotechnol. 2004;22(1):53–54. doi: 10.1038/nbt922. [DOI] [PubMed] [Google Scholar]
- [11].Eggan K, Rode A, Jentsch I, et al. Male and female mice derived from the same embryonic stem cell clone by tetraploid embryo complementation. Nat Biotechnol. 2002;20(5):455–459. doi: 10.1038/nbt0502-455. [DOI] [PubMed] [Google Scholar]
- [12].Rideout WM, 3rd, Eggan K, Jaenisch R. Nuclear cloning and epigenetic reprogramming of the genome. Science. 2001;293(5532):1093–1098. doi: 10.1126/science.1063206. [DOI] [PubMed] [Google Scholar]
- [13].Amado S, Rodrigues JM, Luis AL, et al. Effects of collagen membranes enriched with in vitro-differentiated N1E-115 cells on rat sciatic nerve regeneration after end-to-end repair. J Neuroeng Rehabil. 2010;7:7. doi: 10.1186/1743-0003-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gu X, Ding F, Yang Y, et al. Construction of tissue engineered nerve grafts and their application in peripheral nerve regeneration. Prog Neurobiol. 2011;93(2):204–230. doi: 10.1016/j.pneurobio.2010.11.002. [DOI] [PubMed] [Google Scholar]
- [15].Luis AL, Rodrigues JM, Amado S, et al. PLGA 90/10 and caprolactone biodegradable nerve guides for the reconstruction of the rat sciatic nerve. Microsurgery. 2007;27(2):125–137. doi: 10.1002/micr.20317. [DOI] [PubMed] [Google Scholar]
- [16].Luis AL, Rodrigues JM, Geuna S, et al. Use of PLGA 90:10 scaffolds enriched with in vitro-differentiated neural cells for repairing rat sciatic nerve defects. Tissue Eng Part A. 2008;14(6):979–993. doi: 10.1089/ten.tea.2007.0273. [DOI] [PubMed] [Google Scholar]
- [17].Wang HS, Hung SC, Peng ST, et al. Mesenchymal stem cells in the Wharton's jelly of the human umbilical cord. Stem Cells. 2004;22(7):1330–1337. doi: 10.1634/stemcells.2004-0013. [DOI] [PubMed] [Google Scholar]
- [18].Marcus AJ, Woodbury D. Fetal stem cells from extra-embryonic tissues: do not discard. J Cell Mol Med. 2008;12(3):730–742. doi: 10.1111/j.1582-4934.2008.00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fu YS, Cheng YC, Lin MY, et al. Conversion of human umbilical cord mesenchymal stem cells in Wharton's jelly to dopaminergic neurons in vitro: potential therapeutic application for Parkinsonism. Stem Cells. 2006;24(1):115–124. doi: 10.1634/stemcells.2005-0053. [DOI] [PubMed] [Google Scholar]
- [20].Yang CC, Shih YH, Ko MH, et al. Transplantation of human umbilical mesenchymal stem cells from Wharton's jelly after complete transection of the rat spinal cord. PLoS One. 2008;3(10):e3336. doi: 10.1371/journal.pone.0003336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Luis AL, Rodrigues JM, Geuna S, et al. Neural cell transplantation effects on sciatic nerve regeneration after a standardized crush injury in the rat. Microsurgery. 2008;28(6):458–470. doi: 10.1002/micr.20524. [DOI] [PubMed] [Google Scholar]
- [22].Shirosaki Y, Tsuru K, Hayakawa S, et al. In vitro cytocompatibility of MG63 cells on chitosan- organosiloxane hybrid membranes. Biomaterials. 2005;26(5):485–493. doi: 10.1016/j.biomaterials.2004.02.056. [DOI] [PubMed] [Google Scholar]
- [23].Yuan Y, Zhang P, Yang Y, et al. The interaction of Schwann cells with chitosan membranes and fibers in vitro. Biomaterials. 2004;25(18):4273–4278. doi: 10.1016/j.biomaterials.2003.11.029. [DOI] [PubMed] [Google Scholar]
- [24].Hench LL, Ethridge EC. New York: Academic Press; 1982. Biomaterials An Interfacial Approach; p. 10. [Google Scholar]
- [25].Kuhn G, Lie A, Wilms S, et al. Coexpression of PMP22 gene with MBP and P0 during de novo myelination and nerve repair. Glia. 1993;8(4):256–264. doi: 10.1002/glia.440080406. [DOI] [PubMed] [Google Scholar]
- [26].Pietak AM, Reid JW, Stott MJ, et al. Silicon substitution in the calcium phosphate bioceramics. Biomaterials. 2007;28(28):4023–4032. doi: 10.1016/j.biomaterials.2007.05.003. [DOI] [PubMed] [Google Scholar]
- [27].Geuna S, Borrione P, Fornaro M, et al. Adult stem cells and neurogenesis: historical roots and state of the art. Anat Rec. 2001;265(3):132–141. doi: 10.1002/ar.1135. [DOI] [PubMed] [Google Scholar]
- [28].Raimondo S, Penna C, Pagliaro P, et al. Morphological characterization of GFP stably transfected adult mesenchymal bone marrow stem cells. J Anat. 2006;208(1):3–12. doi: 10.1111/j.1469-7580.2006.00511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Marreco PR, da Luz Moreira P, Genari SC, et al. Effects of different sterilization methods on the morphology, mechanical properties, and cytotoxicity of chitosan membranes used as wound dressings. J Biomed Mater Res B Appl Biomater. 2004;71(2):268–277. doi: 10.1002/jbm.b.30081. [DOI] [PubMed] [Google Scholar]
- [30].Yoshii S, Oka M, Shima M, et al. 30 mm regeneration of rat sciatic nerve along collagen filaments. Brain Res. 2002;949(1-2):202–208. doi: 10.1016/s0006-8993(02)03149-9. [DOI] [PubMed] [Google Scholar]
- [31].Sporel-Ozakat RE, Edwards PM, Hepgul KT, et al. A simple method for reducing autotomy in rats after peripheral nerve lesions. J Neurosci Methods. 1991;36(2-3):263–265. doi: 10.1016/0165-0270(91)90052-2. [DOI] [PubMed] [Google Scholar]
- [32].Koka R, Hadlock TA. Quantification of functional recovery following rat sciatic nerve transection. Exp Neurol. 2001;168(1):192–195. doi: 10.1006/exnr.2000.7600. [DOI] [PubMed] [Google Scholar]
- [33].Masters DB, Berde CB, Dutta SK, et al. Prolonged regional nerve blockade by controlled release of local anesthetic from a biodegradable polymer matrix. Anesthesiology. 1993;79(2):340–346. doi: 10.1097/00000542-199308000-00020. [DOI] [PubMed] [Google Scholar]
- [34].Hu D, Hu R, Berde CB. Neurologic evaluation of infant and adult rats before and after sciatic nerve blockade. Anesthesiology. 1997;86(4):957–965. doi: 10.1097/00000542-199704000-00026. [DOI] [PubMed] [Google Scholar]
- [35].Varejao AS, Cabrita AM, Geuna S, et al. Functional assessment of sciatic nerve recovery: biodegradable poly (DLLA-epsilon-CL) nerve guide filled with fresh skeletal muscle. Microsurgery. 2003;23(4):346–353. doi: 10.1002/micr.10148. [DOI] [PubMed] [Google Scholar]
- [36].Raimondo S, Fornaro M, Di Scipio F, et al. Chapter 5: Methods and protocols in peripheral nerve regeneration experimental research: part II-morphological techniques. Int Rev Neurobiol. 2009;87:81–103. doi: 10.1016/S0074-7742(09)87005-0. [DOI] [PubMed] [Google Scholar]
- [37].Di Scipio F, Raimondo S, Tos P, et al. A simple protocol for paraffin-embedded myelin sheath staining with osmium tetroxide for light microscope observation. Microsc Res Tech. 2008;71(7):497–502. doi: 10.1002/jemt.20577. [DOI] [PubMed] [Google Scholar]
- [38].Geuna S, Gigo-Benato D, Rodrigues Ade C. On sampling and sampling errors in histomorphometry of peripheral nerve fibers. Microsurgery. 2004;24(1):72–76. doi: 10.1002/micr.10199. [DOI] [PubMed] [Google Scholar]
- [39].Geuna S, Tos P, Battiston B, et al. Verification of the two-dimensional disector, a method for the unbiased estimation of density and number of myelinated nerve fibers in peripheral nerves. Ann Anat. 2000;182(1):23–34. doi: 10.1016/S0940-9602(00)80117-X. [DOI] [PubMed] [Google Scholar]


