Abstract
Bone marrow mesenchymal stem cells were isolated from New Zealand white rabbits, culture-expanded and differentiated into Schwann cell-like cells. Autologous platelet-rich plasma and Schwann cell-like cells were mixed in suspension at a density of 1 × 106 cells/mL, prior to introduction into a poly (lactic-co-glycolic acid) conduit. Fabricated tissue-engineered nerves were implanted into rabbits to bridge 10 mm sciatic nerve defects (platelet-rich plasma group). Controls were established using fibrin as the seeding matrix for Schwann cell-like cells at identical density to construct tissue-engineered nerves (fibrin group). Twelve weeks after implantation, toluidine blue staining and scanning electron microscopy were used to demonstrate an increase in the number of regenerating nerve fibers and thickness of the myelin sheath in the platelet-rich plasma group compared with the fibrin group. Fluoro-gold retrograde labeling revealed that the number of Fluoro-gold-positive neurons in the dorsal root ganglion and the spinal cord anterior horn was greater in the platelet-rich plasma group than in the fibrin group. Electrophysiological examination confirmed that compound muscle action potential and nerve conduction velocity were superior in the platelet-rich plasma group compared with the fibrin group. These results indicate that autologous platelet-rich plasma gel can effectively serve as a seeding matrix for Schwann cell-like cells to construct tissue-engineered nerves to promote peripheral nerve regeneration.
Keywords: platelet-rich plasma, extracellular matrix, Schwann cells, fibrin, sciatic nerve, peripheral nerve injury, nerve tissue engineering, neural regeneration
Research Highlights
-
(1)
Tissue-engineered nerve was constructed using autologous platelet-rich plasma gel for suspension of Schwann cell-like cells in a poly(lactic-co-glycolic acid) nerve conduit.
-
(2)
Tissue-engineered nerve can elevate the number of nerve fibers, thickness of the myelin sheath, neuron number, compound muscle action potential and nerve conduction velocity.
-
(3)
Platelet-rich plasma gel can be effectively combined with seeded cells to construct tissue-engineered nerve and has a promising application for nerve tissue engineering.
INTRODUCTION
Repair of injured peripheral nerve, particularly a loss of neural tissue leading to gaps, remains a difficult challenge for neuroscience[1]. Different strategies have been developed to improve peripheral nerve regeneration, such as nerve autograft, allograft and tubular biodegradable implants. However, the repair outcomes of these strategies are sub-optimal[2,3].
Tissue engineering strategies, combining regenerative cells with an appropriate delivery vehicle, have proved to be one of the most promising approaches to enhance neural regeneration[4,5,6,7].
To maximize the functionality of tissue-engineered tissue-engineered nerve grafts, a key step is to use an optimized seeding matrix to fill the conduit with cells. This can allow homogenous distribution of cells throughout the conduit and enable cells to continue to proliferate and remain viable[8]. Several proteins, including fibrin gel, collagen and laminin, have been used as a seeding matrix to construct tissue-engineered nerves[8,9,10].
Platelet-rich plasma is defined as an autologous concentration of platelets in a small volume of plasma[11]. Once platelet-rich plasma is activated, it develops a three-dimensional fibrin gel network containing a myriad of growth factors and active proteins within the local environment. Several studies have demonstrated the positive effect of platelet-rich plasma on the reparative process of nerve injuries[12,13].
Based on the efficacy of platelet-rich plasma gel to promote neural regeneration and its main component consisting of fibrin gel and growth factors, we speculated that platelet-rich plasma gel can serve as a superior seeding matrix to construct tissue-engineered nerves in comparison with fibrin gel.
In the present study, we prepared a novel tissue-engineered nerve using autologous platelet-rich plasma gel to suspended Schwann cell-like cells in a poly(lactic-co-glycolic acid) nerve conduit, and investigated its repair efficacy in 10 mm sciatic nerve rabbit defects.
RESULTS
Quantitative analysis of experimental animals
A total of 16 rabbits were included, which were equally and randomly divided into two groups. A 10 mm sciatic nerve injury was established by cutting the sciatic nerve 1 cm from the inferior border of the piriformis muscle. In the platelet-rich plasma and fibrin groups, platelet-rich plasma gel or fibrin were combined with Schwann cell-like cells in a poly(lactic-co-glycolic acid) nerve conduit respectively, for implantation into the sciatic nerve injury model. A total of 16 rabbits were included in the final analysis.
Gross observation of tissue-engineered nerves
Twelve weeks after implantation, poly(lactic-co-glycolic acid) conduits were completely resorbed. No inflammatory reactions were identified and no neuromas were apparent (Figure 1).
Figure 1.
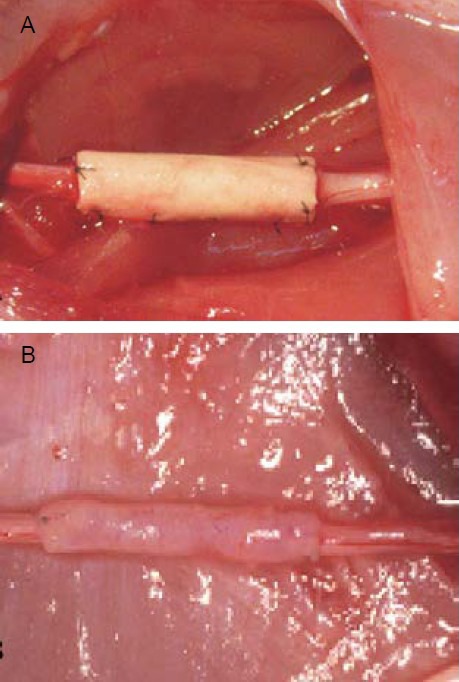
Tissue-engineered nerve using autologous platelet-rich plasma gel for suspension of Schwann cell-like cells in a poly(lactic-co-glycolic acid) nerve conduit was applied to repair the injury.
(A) Immediately after grafting.
(B) 12 weeks after grafting, the conduits were resorbed, no inflammatory reactions were identified and no neuromas were apparent.
Tissue-engineered nerves promoted nerve fiber regeneration and myelination
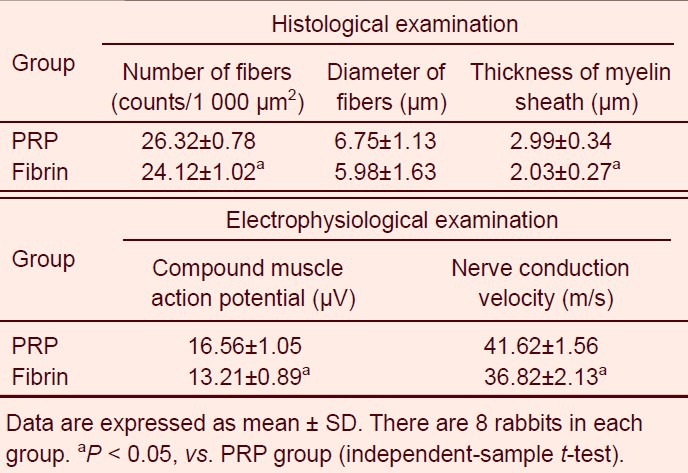
Regenerating nerves from the platelet-rich plasma group were dense and mature, demonstrating a thick and well-arrayed myelin sheath (Figures 2, 3). The platelet-rich plasma group also demonstrated a significant increase in the number of regenerating nerve fibers and thickness of the myelin sheath compared with the fibrin group (P < 0.05). There was no significant difference in the diameter of fibers between the two groups (Table 1).
Figure 2.
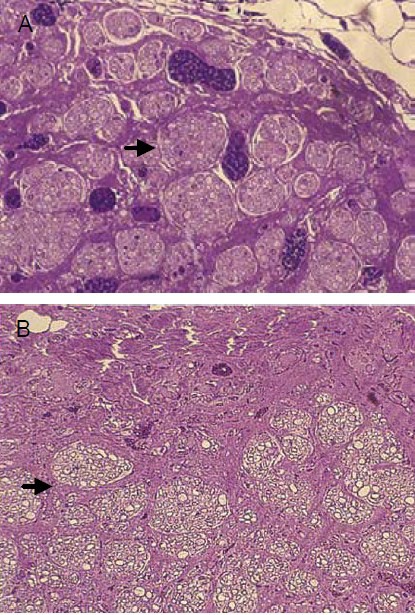
Effect of tissue-engineered nerve prepared using autologous platelet-rich plasma gel for suspension of Schwann cell-like cells in a poly(lactic-co-glycolic acid) nerve conduit for nerve fiber regeneration in rabbits with sciatic nerve injury (toluidine blue staining, × 200).
The platelet-rich plasma group (A) demonstrated denser and more mature regenerating sciatic nerve fibers compared with the fibrin group (B). Arrows highlight regenerated nerves.
Figure 3.
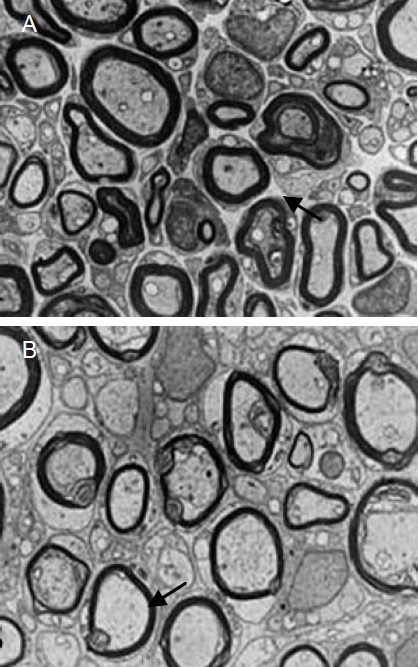
Effects of tissue-engineered nerve prepared using autologous platelet-rich plasma gel for suspension of Schwann cell-like cells in a poly(lactic-co-glycolic acid) nerve conduit for regenerating nerve fiber myelin sheath in rabbits with sciatic nerve injury (transmission electron microscopy, × 4 000).
Regenerating sciatic nerve fibers from the platelet-rich plasma group (A) demonstrated a thicker and well-arrayed myelin sheath compared with the fibrin group (B). Arrows highlight the myelin sheath.
Table 1.
Quantitative analysis of histological and electrophysiological examinations between platelet-rich plasma (PRP) and fibrin groups
Tissue-engineered nerves elevated the number of spinal cord neurons
Sections from the dorsal root ganglion and spinal cord anterior horn, at the surgical side, were found to be positive for Fluoro-gold fluorescence (Figure 4). The average number of Fluoro-gold-positive neurons (n/40 × magnification for 20 fields) in the dorsal root ganglion and spinal cord anterior horn were 42 ± 6 and 163 ± 9 respectively, in the fibrin group, and 58 ± 5 and 185 ± 7 respectively, in the platelet-rich plasma group. The platelet-rich plasma group demonstrated a significant increase in the number of Fluoro-gold-positive neurons compared with the fibrin group (P < 0.05).
Figure 4.
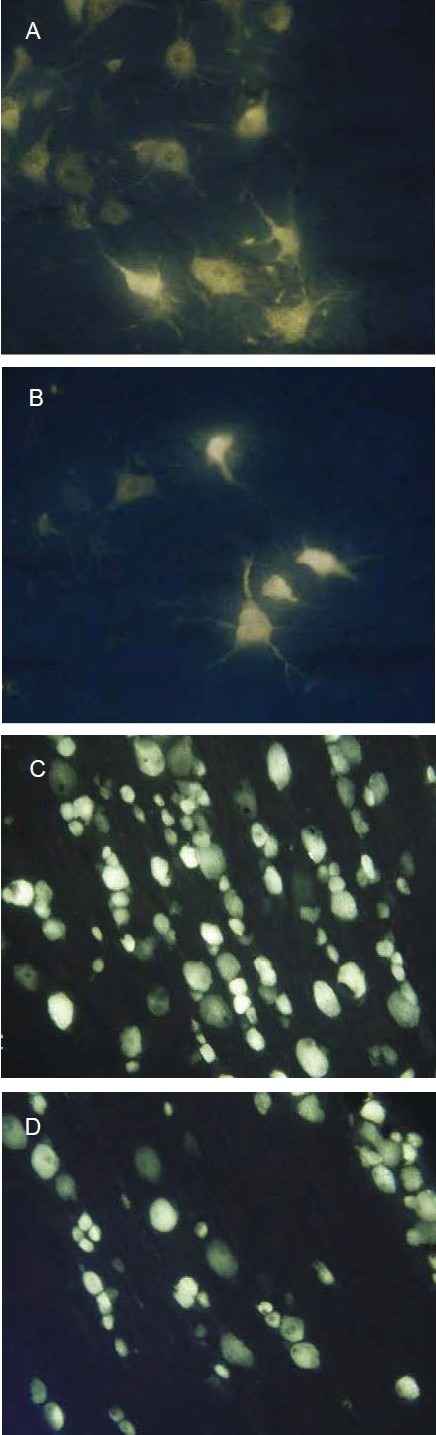
Effect of tissue-engineered nerve prepared using autologous platelet-rich plasma gel for suspension of Schwann cell-like cells in a poly(lactic-co-glycolic acid) nerve conduit on neurons of the spinal cord anterior horn (A, B) and dorsal root ganglion (C, D) in rabbits with sciatic nerve injury (retrograde dye Fluoro-gold labeling, × 100).
The number of Fluoro-gold-positive neurons in the platelet-rich plasma group (A, C) was greater than the fibrin group (B, D).
Tissue-engineered nerves elevated compound muscle action potential and nerve conduction velocity
Compound muscle action potential and nerve conduction velocity were significantly increased in the platelet-rich plasma group compared to the fibrin group (Table 1).
DISCUSSION
In the present study, a novel tissue-engineered nerve was constructed using regenerative cells suspended in an autologous platelet-rich plasma gel within a poly(lactic-co-glycolic acid) nerve conduit. The unique characteristic of this strategy was the use of the platelet-rich plasma gel as a seeding matrix. The rationale for using local application of platelet-rich plasma is that platelet-rich plasma is a rich source of growth factors, including platelet-derived growth factor, transforming growth factor-β and insulin-like growth factor. Furthermore, it contains blood proteins such as fibrinogen[11]. After activation, platelet-rich plasma can develop into a three-dimensional fibrin gel. Fibrin has wide applications in reconstructive surgery and bioengineering[14,15,16]. Fibrin can stimulate angiogenesis, which has been shown to be one of the most important factors for successful regeneration following nerve injury[17,18].
Fibrin can also serve as a natural growth extracellular matrix for regenerating axons[19] and has shown to successfully repair peripheral nerve injuries in experimental animals and humans[20,21]. Furthermore, Kalbermatten et al[8] reported that both Schwann cells and mesenchymal stem cells adhered significantly better, continued to maintain their differentiated state, and were more uniformly distributed throughout the conduits when seeded in fibrin gel in vitro than by delivery in growth medium alone. Transplantation of the nerve conduits in vivo showed that cells in combination with fibrin matrix significantly increased neural regeneration when compared with empty conduits. After activation, platelet-rich plasma develops into a three-dimensional fibrin gel. That is one reason we explored the potential of using platelet-rich plasma gel as a seeding matrix to suspend cells throughout the nerve conduit. In addition, once platelet-rich plasma is activated, a myriad of growth factors are secreted by platelets. All these growth factors have been well demonstrated to be involved in reparative processes. Because they influence many processes common to tissue repair, including angiogenesis, chemotaxis and cell proliferation[11,22,23], it is generally accepted that these growth factors have an essential role in the healing process and tissue formation. However, little is known about suitable combinations, concentrations and application time points of various growth factors in reparative processes. The use of platelet-rich plasma may be an easy and physiological way for application of growth factors. A study reported that culture conditions containing platelet-rich plasma can promote differentiation of rabbit mesenchymal stem cells into Schwann cells in vitro. Several reports have demonstrated that local application of autologous platelet-rich plasma gel has a positive effect on the regeneration of peripheral nerve fibers in animal models[12,13]. We therefore speculated that cells in combination with platelet-rich plasma gel may better enhance neural regeneration with the local addition of growth factors when compared with cells in fibrin matrix alone.
The present study showed that the platelet-rich plasma group demonstrated superior nerve regeneration 12 weeks postoperatively compared with the fibrin group. For histological evaluation, tissue-engineered nerves demonstrated a significant increase in the number of regenerating nerve fibers and thickness of the myelin sheath compared with tissue-engineered nerves using fibrin seeing matrix. These histological results are consistent with the electrophysiological results, which showed that the platelet-rich plasma group displayed superior functionality in both compound muscle action potential and nerve conduction velocity. In addition, for retrograde dye Fluoro-gold labeling, the platelet-rich plasma group demonstrated increased Fluoro-gold- positive neurons in the spinal cord anterior horn and dorsal root ganglion. The interpretation of these observations is that the platelet-rich plasma gel may play a dual role. First, the fibrin network serves as the three-dimensional extracellular matrix for incorporation of regenerative cells. Second, growth factors and active proteins may act as a catalyst for accelerating the healing process of the regenerating nerve fibers by improving the molecular environment surrounding them.
In conclusion, tissue-engineered nerves, using autologous platelet-rich plasma gel as a delivery system for Schwann cell-like cells in poly(lactic-co-glycolic acid) conduits, demonstrated good efficacy in repairing 10 mm injury of sciatic nerves in rabbits. This also indicates that they possess excellent biodegradability, biocompatibility and bioactivity. Our study offers experimental basis for the application of autologous platelet-rich plasma gel as a seeding matrix to construct tissue-engineered nerves. Additional benefits of using autologous platelet-rich plasma also include its safety, cost effectiveness and availability in an easy-to-develop manner. We therefore believe that autologous platelet-rich plasma gel will have a promising application for nerve tissue engineering.
MATERIALS AND METHODS
Design
A randomized controlled animal experiment.
Time and setting
All experiments were performed at the Central Laboratory of the Affiliated Hospital Medical College, Qingdao University, China, between October 2009 and August 2011.
Materials
A total of 16 healthy, clean, 10 month-old, male New Zealand white rabbits, each weighing approximately 3.0 kg, were provided by the Laboratory Animal Center of Qingdao University (license No. SCXK (Lu) 2010-0017). All experimental procedures were in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[24].
Methods
Expansion and differentiation of mesenchymal stem cells into Schwann cell-like cells
Mesenchymal stem cells samples were obtained from iliac bone marrow aspirates of 16 male New Zealand white rabbits. Mesenchymal stem cells isolation and expansion were performed as reported previously[25]. Third-passage mesenchymal stem cells were differentiated into Schwann cell-like cells according to a previously established protocol[26]. Schwann cell-like cell characteristics were investigated by immunocytochemical analyses with the antibody to S-100 (Sigma, St. Louis, MO, USA). S-100 is a marker for the differentiation of mesenchymal stem cells into the Schwann cell phenotype (Figure 5)[27].
Figure 5.
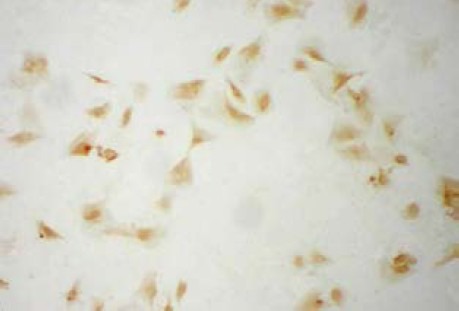
Expression of S-100 protein in Schwann cell-like cells (immunocytochemical staining, light microscope, × 100).
More than 95% of Schwann cell-like cells stained brown (S-100 protein).
Autologous platelet-rich plasma preparation
Autologous platelet-rich plasma was prepared using a previously described technique[28]. Briefly, 10 mL of Peripheral blood was withdrawn from each New Zealand rabbit which served an mesenchymal stem cell donor. Peripheral blood was initially centrifuged at 2 400 r/min for 10 minutes to separate the platelet-rich plasma and platelet-poor plasma portions from the red blood cell fraction. The platelet-rich plasma and platelet-poor plasma portions were centrifuged again at 3 600 r/min for 15 minutes to separate the platelet-rich plasma from the platelet-poor plasma (supplementary Figure 1 online). Platelet counts were then conducted for each donor. A mean value of peripheral blood platelet counts yielded 203 ± 41 × 103 platelets/μL, and the platelet-rich plasma platelet count was 1 083 ± 107 × 103 platelets/μL. The platelet-rich plasma was activated just before application with a 10% calcium chloride solution and 5 000 units of bovine thrombin (Tissucol-Duo, Baxter, Germany) to form a gel.
Construction of tissue-engineered nerve filled with Schwann cell-like cells
Schwann cell-like cells were resuspended in activated autologous platelet-rich plasma at a density of 1 × 106 cells/mL. The suspension was introduced with a micropipette into the 10 mm long poly(lactic-co-glycolic acid) conduit (Institute of Polymer Science and Engineering of Qingdao University, Qingdao, Shandong Province, China) before it became sticky (platelet-rich plasma group). Controls were established using fibrin (fibrin glue, Tisseel Kit VH 1.0, Baxter SA, Switzerland) as a seeding matrix to construct tissue-engineered nerves with Schwann cell-like cells at the same density (fibrin group).
Grafting procedure for bridging the defected sciatic nerves
Surgical implantation was carried out using an operating microscope (Zeiss, Oberkochen, Germany). The left sciatic nerves were bluntly separated along the spatium intermusculare of the left hind leg and a 10 mm section was removed. The tissue-engineered nerve was applied to the injury to bridge the gap and fixation of the nerve ends was carried out with a single epineural nylon suture (9/0 Prolen; Ethicon, Cincinnati, Ohio, USA). Tension was avoided and atraumatic handling and correct rotational alignment were employed throughout all procedures.
Histological evaluation
Specimens were harvested at the midpoint of the regenerated nerve 12 weeks postoperatively and were fixed with 2.5% glutaraldehyde. They were then embedded in Epon resin using a standard method, cut cross-sectionally to a thickness of 50 μm, and stained with toluidine blue for light microscopy. For transmission electron microscopy (JEM-2000EX, Jeol, Japan), the specimens were cut into 70 nm thick slices. The number and diameters of the myelinated fibers and thickness of myelin sheath were evaluated with an image analysis system (IBAS; Contron, Erching, Germany).
Electrophysiological examination
Sciatic nerves were exposed just before the specimens were harvested 12 weeks after implantation. Electrophysiological examination was performed with a Nicolet Viking Quest System (Nicolet Biomedical Inc., Madison, WI, USA). The nerve potential amplitude and nerve conduction velocity were detected.
Retrograde labeling with Fluoro-gold dye
Retrograde Fluoro-gold dye (Biotium, Hayward, CA, USA) was used to trace the injured peripheral axons. Sciatic nerves, including the bridged sections, were exposed 10 weeks postoperatively. Fluoro-gold, in 5% physical saline, was injected in a total volume of 1 μL at four sites of the sciatic nerve under the 10 mm injury distal to the implant. A microsampler (Bio-Rad, Hercules, CA, USA) was used for the injection; after retaining the microsampler inside the injection sites for a few seconds, it was retrieved slowly and the surgical opening was closed. Two weeks later, retrograde-labeled neurons in the dorsal root ganglia and spinal cord ventral horn were assessed by measuring the average number of Fluoro-gold-positive neurons under an ultraviolet illuminator (Boteck, Chongqing, China; 40 × magnification for 20 fields).
Statistical analysis
Results are expressed as mean ± SD. Statistical analysis to compare results between groups was carried out using an independent-sample t-test with SPSS 11.0 software (SPSS, Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by the High Education Development Foundation of Shandong Province, No. J11LF22.
Conflicts of interest: None declared.
Ethical approval: This protocol was approved by the Animal Ethics Committee of Qingdao University in China.
Supplementary information: Supplementary data associated with this article can be found in the online version, by visiting www.nrronline.org.
(Edited by Pan DL, Tang JS/Qiu Y/Song LP)
REFERENCES
- [1].Lundborg G. A 25-year perspective of peripheral nerve surgery: evolving neuroscientific concepts and clinical significance. J Hand Surg Am. 2000;25(3):391–414. doi: 10.1053/jhsu.2000.4165. [DOI] [PubMed] [Google Scholar]
- [2].Siemionow M, Sonmez E. Nerve allograft transplantation: a review. J Reconstr Microsurg. 2007;23(8):511–520. doi: 10.1055/s-2007-1022694. [DOI] [PubMed] [Google Scholar]
- [3].Reyes O, Sosa I, Kuffler DP. Promoting neurological recovery following a traumatic peripheral nerve injury. P R Health Sci J. 2005;24(3):215–223. [PubMed] [Google Scholar]
- [4].Tohill M, Terenghi G. Stem-cell plasticity and therapy for injuries of the peripheral nervous system. Biotechnol Appl Biochem. 2004;40(Pt 1):17–24. doi: 10.1042/BA20030173. [DOI] [PubMed] [Google Scholar]
- [5].Fansa H, Keilhoff G. Comparison of different biogenic matrices seeded with cultured Schwann cells for bridging peripheral nerve defects. Neurol Res. 2004;26(2):167–173. doi: 10.1179/016164104225013842. [DOI] [PubMed] [Google Scholar]
- [6].Dezawa M, Takahashi I, Esaki M, et al. Sciatic nerve regeneration in rats induced by transplantation of in vitro differentiated bone-marrow stromal cells. Eur J Neurosci. 2001;14(11):1771–1776. doi: 10.1046/j.0953-816x.2001.01814.x. [DOI] [PubMed] [Google Scholar]
- [7].Hadlock T, Sundback C, Hunter D, et al. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 2000;6(2):119–127. doi: 10.1089/107632700320748. [DOI] [PubMed] [Google Scholar]
- [8].Kalbermatten DF, Kingham PJ, Mahay D, et al. Fibrin matrix for suspension of regenerative cells in an artificial nerve conduit. J Plast Reconstr Aesthet Surg. 2008;61(6):669–675. doi: 10.1016/j.bjps.2007.12.015. [DOI] [PubMed] [Google Scholar]
- [9].Ding T, Luo ZJ, Zheng Y, et al. Rapid repair and regeneration of damaged rabbit sciatic nerves by tissue-engineered scaffold made from nano-silver and collagen type I. Injury. 2010;41(5):522–527. doi: 10.1016/j.injury.2009.04.003. [DOI] [PubMed] [Google Scholar]
- [10].Chen MB, Zhang F, Lineaweaver WC. Luminal fillers in nerve conduits for peripheral nerve repair. Ann Plast Surg. 2006;57(4):462–471. doi: 10.1097/01.sap.0000237577.07219.b6. [DOI] [PubMed] [Google Scholar]
- [11].Marx RE. Platelet-rich plasma: evidence to support its use. J Oral Maxillofac Surg. 2004;62(4):489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- [12].Farrag TY, Lehar M, Verhaegen P, et al. Effect of platelet rich plasma and fibrin sealant on facial nerve regeneration in a rat model. Laryngoscope. 2007;117(1):157–165. doi: 10.1097/01.mlg.0000249726.98801.77. [DOI] [PubMed] [Google Scholar]
- [13].Elgazzar RF, Mutabagani MA, Abdelaal SE, et al. Platelet rich plasma may enhance peripheral nerve regeneration after cyanoacrylate reanastomosis: a controlled blind study on rats. Int J Oral Maxillofac Surg. 2008;37(8):748–755. doi: 10.1016/j.ijom.2008.05.010. [DOI] [PubMed] [Google Scholar]
- [14].Albala DM, Lawson JH. Recent clinical and investigational applications of fibrin sealant in selected surgical specialties. J Am Coll Surg. 2006;202(4):685–697. doi: 10.1016/j.jamcollsurg.2005.11.027. [DOI] [PubMed] [Google Scholar]
- [15].Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: state of the art. Tissue Eng Part B Rev. 2008;14(1):61–86. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- [16].Bensaïd W, Triffitt JT, Blanchat C, et al. A biodegradable fibrin scaffold for mesenchymal stem cell transplantation. Biomaterials. 2003;24(14):2497–2502. doi: 10.1016/s0142-9612(02)00618-x. [DOI] [PubMed] [Google Scholar]
- [17].Boivin A, Pineau I, Barrette B, et al. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J Neurosci. 2007;27(46):12565–12576. doi: 10.1523/JNEUROSCI.3027-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hall H. Modified fibrin hydrogel matrices: both 3D-scaffolds and local and controlled release systems to stimulate angiogenesis. Curr Pharm Des. 2007;13(35):3597–3607. doi: 10.2174/138161207782794158. [DOI] [PubMed] [Google Scholar]
- [19].Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30(7):209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- [20].Sameem M, Wood TJ, Bain JR. A systematic review on the use of fibrin glue for peripheral nerve repair. Plast Reconstr Surg. 2011;127(6):2381–2390. doi: 10.1097/PRS.0b013e3182131cf5. [DOI] [PubMed] [Google Scholar]
- [21].Choi BH, Han SG, Kim SH, et al. Autologous fibrin glue in peripheral nerve regeneration in vivo. Microsurgery. 2005;25(6):495–499. doi: 10.1002/micr.20154. [DOI] [PubMed] [Google Scholar]
- [22].Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83(3):835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- [23].Lucarelli E, Beccheroni A, Donati D, et al. Platelet-derived growth factors enhance proliferation of human stromal stem cells. Biomaterials. 2003;24(18):3095–3100. doi: 10.1016/s0142-9612(03)00114-5. [DOI] [PubMed] [Google Scholar]
- [24].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [25].Frank O, Heim M, Jakob M, et al. Real-time quantitative RT-PCR analysis of human bone marrow stromal cells during osteogenic differentiation in vitro. J Cell Biochem. 2002;85(4):737–746. doi: 10.1002/jcb.10174. [DOI] [PubMed] [Google Scholar]
- [26].Brohlin M, Mahay D, Novikov LN, et al. Characterization of human mesenchymal stem cells following differentiation into Schwann cell-like cells. Neurosci Res. 2009;64(1):41–49. doi: 10.1016/j.neures.2009.01.010. [DOI] [PubMed] [Google Scholar]
- [27].Caddick J, Kingham PJ, Gardiner NJ, et al. Phenotypic and functional characteristics of mesenchymal stem cells differentiated along a Schwann cell lineage. Glia. 2006;54(8):840–849. doi: 10.1002/glia.20421. [DOI] [PubMed] [Google Scholar]
- [28].Okuda K, Kawase T, Momose M, et al. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J Periodontol. 2003;74(6):849–857. doi: 10.1902/jop.2003.74.6.849. [DOI] [PubMed] [Google Scholar]


