Abstract
A diabetes mellitus model was established through single intraperitoneal injection of streptozotocin into rats. Seven days later, model rats were intraperitoneally administered zinc protoporphyrin, a heme oxygenase-1 inducer, and cobalt protoporphyrin, a heme oxygenase-1 inhibitor, once every two days, for 5 successive weeks. After administration, the paw withdrawal mechanical threshold of diabetic mellitus rats significantly decreased, the myelin sheath of the sciatic nerve thickened or showed vacuole defects, the number of spinal dorsal horn neurons reduced, some neurons degenerated and were necrotic, and heme oxygenase-1 was visible in the cytoplasm of spinal dorsal horn neurons. Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling demonstrated that the number of apoptotic neurons increased, which could be inhibited by cobalt protoporphyrin, however, zinc protoporphyrin led to an opposite effect. Our experimental findings indicate that heme oxygenase-1 attenuates neuropathic pain in diabetic mellitus rats through amelioration of peripheral neuropathy and inhibition of spinal dorsal horn neuron apoptosis.
Keywords: diabetes mellitus, neuropathic pain, heme oxygenase-1, cobalt protoporphyrin, zinc protoporphyrin, neurons, apoptosis, peripheral nerve injury, regeneration, neural regeneration
Research Highlights
-
(1)
In diabetic rats with neuropathic pain, the number of apoptotic spinal dorsal horn neurons increased, indicating that apoptosis of neurons is closely related to neuropathic pain.
-
(2)
Pathological changes were observed in diabetic rats with neuropathic pain, and mechanical hyperalgesia was positively correlated with peripheral nerve damage.
-
(3)
Heme oxygenase-1 can attenuate neuropathic pain in diabetic rats.
Abbreviations
HO, heme oxygenase; CoPP, cobalt protoporphyrin; ZnPP, zinc protoporphyrin
INTRODUCTION
The most common manifestation of diabetic neuropathy is distal symmetrical sensorimotor polyneuropathy and autonomic neuropathies. Along the disease course, almost 50–60% of diabetic patients develop peripheral diabetic polyneuropathy[1,2]. Many patients may complain of aberrant sensations presenting as persistent spontaneous pain, burning, tingling, allodynia and hyperalgesia. Diabetic neuropathic pain is typically worse at night, causing considerable interference in sleep and enjoyment of life[3].
There may be multiple etiologies that account for diabetic neuropathic pain. The contribution of hyperglycemia to the development and progression of diabeticneuropathic pain is now beyond dispute. Hyperglycemia leads to overload of the electron transport chain causing oxidative stress. The combination of oxidative stress and hyperglycemia activate the detrimental polyol[4] and protein kinase C pathways[5], and the production of advanced glycation end products[6], which result in a redox imbalance, gene expression disturbances, and further oxidative stress. This oxidative stress, which is associated with the development of apoptosis in neurons and supporting glial cells, leads to nervous system damage in diabetic patients[7,8]. Several morphological studies have reported the occurrence of neuronal apoptosis in the spinal dorsal horn following peripheral nerve damage[9,10]. In addition, excitotoxic death of inhibitory interneurons within laminas I–III of the spinal dorsal horn is thought to contribute to neuropathic pain[11,12]. In diabetes with ongoing damage, peripheral and central sensitization continues, presenting a lowered activation threshold, abnormal spontaneous activity, and increased response to a given stimulus[13].
Unfortunately, to date, besides tight glycemic control, a feasible treatment for diabetic neuropathic pain is not available. Therapies for diabetic neuropathic pain may be divided into treatments that target the underlying pathogenetic mechanisms and those aiming to relieve symptoms[14]. Tight glycemic control can delay the onset and slow the progression of diabetic neuropathies[15,16], but continued tight glycemic control is quite a challenge in most cases. As oxidative stress may play an important role in the pathogenesis of diabetic neuropathy, new medications have emerged in recent years, such as aldose reductase inhibitors, protein kinase C-β inhibitor, and antioxidant like α-lipoic acid[17,18,19]. These medications may provide protection through reduction of oxidative stress-related damage in diabetic neuropathy[20,21,22]. However, classical antioxidants have failed to show convincing beneficial effects. Therefore, there is a need to identify antioxidants that will be effective in attenuating diabetic neuropathic pain.
Heme oxygenase (HO) enzymatically degrades heme to free iron, which is bound to the heavy chain ferritin, carbon monoxide and biliverdin[23]. There are two isoforms of HO. HO-2 is a constitutive enzyme, whereas HO-1, the inducible form, which occurs at low expression levels in a majority of cells and tissues, is markedly upregulated upon exposure to its substrate heme and other oxidative stress stimuli[24]. HO-1 is markedly up-regulated by a variety of stressful stimuli, as well as by heme or certain metalloporphyrins, such as cobalt protoporphyrin (CoPP); but it can be inhibited by zinc protoporphyrin (ZnPP)[25]. The end-products of heme breakdown are potent antioxidants and anti-inflammatory molecules[26], and are able to modulate cell proliferation and cell death[27]. Therefore, the aims of this study are to investigate the effect of HO-1 on streptozotocin-induced neuropathic pain in rats and to explore the possible mechanisms involved.
RESULTS
Quantitative analysis of experimental animals
At the beginning of the study, 32 rats were randomly divided into 2 groups: streptozotocin-treated model rats (n = 24) and control rats (n = 8). After diabetes mellitus (DM) induction with streptozotocin, 24 DM rats were randomly divided into three groups containing eight animals each: DM, DM + ZnPP, DM + CoPP. A total of 32 rats were included in the final analysis.
Effect of HO-1 on diabetes-induced mechanical hyperalgesia
The paw withdrawal test was carried out to quantify the nociception thresholds[28]. At baseline, no significant difference was observed among the four groups. On day 7 after diabetes induction, the paw withdrawal mechanical threshold of diabetic rats was dramatically decreased (P < 0.01), indicating the presence of diabetes-induced allodynia. The threshold values were significantly lower than the control group from day 7 to day 42 (P < 0.01). The paw withdrawal mechanical threshold of the DM + CoPP group was significantly improved after 1 week treatment (P < 0.05). While in the DM + ZnPP group, the threshold values decreased after 1 week treatment (P < 0.05). The control group did not show alterations in withdrawal threshold (Figure 1).
Figure 1.
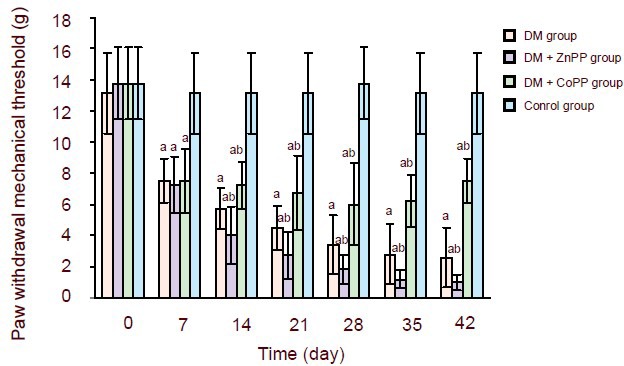
Time-course of the effect of heme oxygenase-1 on paw withdrawal mechanical threshold (g) in streptozotocin-induced diabetic rats.
Treatments started at 7 days. Data are expressed as mean ± SD of 8 rats in each group. Comparisons of values among groups were made using one-way analysis of variance followed by the q-test. aP < 0.01, vs. control group; bP < 0.05, vs. DM group.
DM: Diabetes mellitus; Copp: cobalt protoporphyrin; Znpp: zinc protoporphyrin.
Effect of HO-1 on hyperglycemia-induced structural changes in the sciatic nerve
Transmission electron microscopy of the sciatic nerve from diabetic rats revealed demyelination and Wallerian degeneration (Figure 2A). Administration of CoPP to diabetic rats alleviated pathological damage to the sciatic nerve (Figure 2C), while treatment with ZnPP in diabetic rats enhanced damage (Figure 2B). Sciatic nerve sections from the control group revealed normal morphology (Figure 2D).
Figure 2.
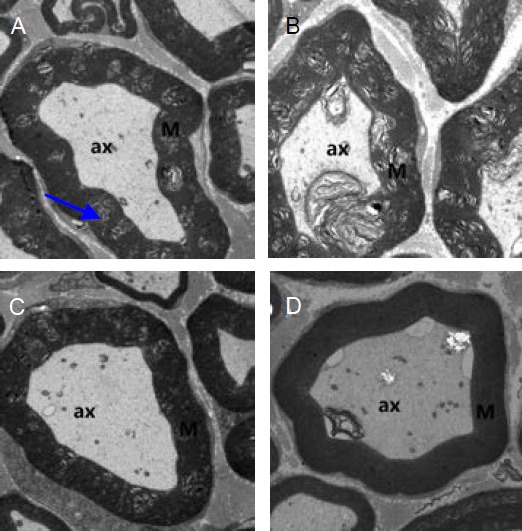
Transmission electron microscopy of the sciatic nerve from diabetic rats treated with ZnPP or CoPP (× 1 700).
Compared with the DM group (A), damage was more evident in the DM + ZnPP group (B), while it was less severe in the DM + CoPP group (C). (D) Sciatic nerve sections from the control group did not change.
The sciatic nerve from diabetic rats revealed demyelination (arrow). ax: Axons; M: myelination; DM: diabetes mellitus; CoPP: cobalt protoporphyrin; ZnPP: zinc protoporphyrin.
Expression of HO-1 in the spinal dorsal horn
Immunohistochemistry staining revealed HO-1 expression, which manifested as yellow or brown staining, was low in the control group. The expression of HO-1 in the spinal dorsal horn of the DM group was lower than that of the DM + CoPP group (P < 0.01), but higher than that of the DM + ZnPP group (P < 0.01; Figure 3).
Figure 3.
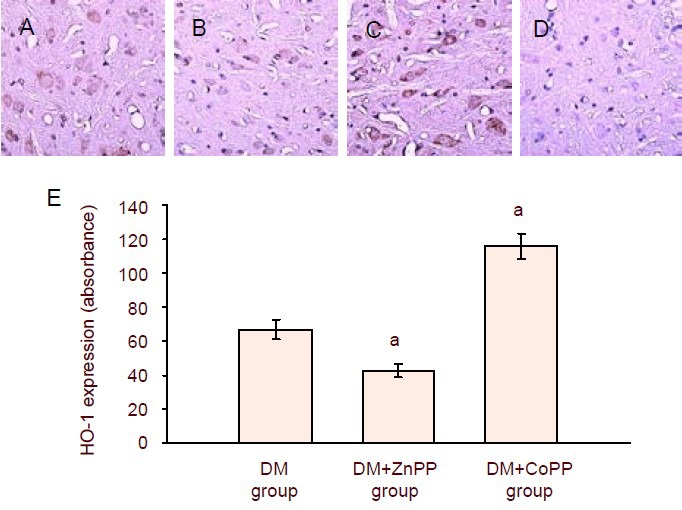
Immunohistochemistry staining for HO-1 expression in the spinal dorsal horn from diabetic rats treated with ZnPP or CoPP.
(A–D) Sections were viewed at a magnification of 200 ×. Cells with yellow or brown granules in the cytoplasm were taken as positive staining. (A) DM group; (B) DM + ZnPP group; (C) DM + CoPP group; (D) control group.
(E) Quantification of HO-1 expression in the spinal dorsal horn. Data are expressed as mean ± SD of 8 rats in each group. Values were compared among groups using one-way analysis of variance followed by the q-test. aP < 0.01, vs. DM group.
HO: Heme oxygenase; DM: diabetes mellitus; CoPP: cobalt protoporphyrin; ZnPP: zinc protoporphyrin
Apoptosis in the spinal dorsal horn
Terminal deoxynucleotidyl transferase-mediated dUTP-biotin nick end labeling (TUNEL)-positive cells were seldom seen in the spinal dorsal horn of control rats. In the DM group, the incidence of TUNEL-positive cells was markedly increased. Compared with the DM group, treatment with CoPP in diabetic rats caused a significant decrease in the number of TUNEL-positive cells in the spinal dorsal horn, while treatment with ZnPP led to an opposite result (Figure 4).
Figure 4.
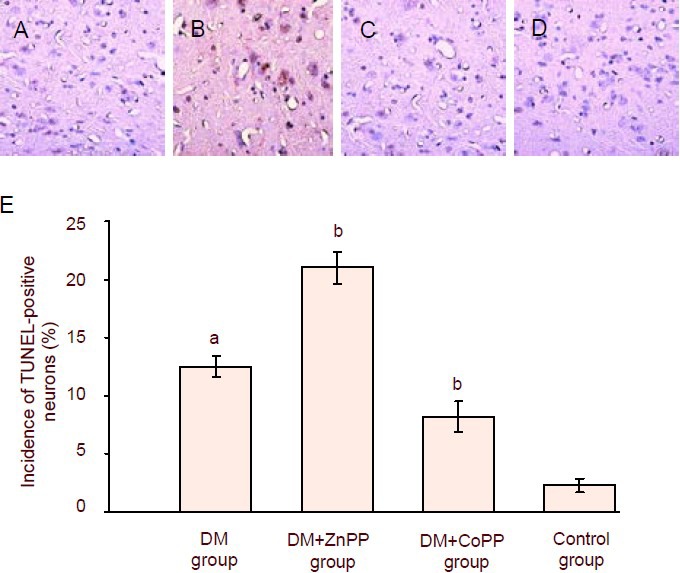
TUNEL-positive cells in the spinal dorsal horn from diabetic rats treated with ZnPP or CoPP.
(A–D) TUNEL-positive profiles in the spinal dorsal horn of rats 42 days after streptozotocin injection (× 200). Diabe-tes induced a marked increase in the incidence of TUNEL-positive cells in the DM group (A), compared with the control group (D). CoPP treatment in diabetic rats caused a reduction in the number of TUNEL-positive cells (C). The opposite effect was observed after ZnPP treat-ment (B). TUNEL-positive cells appeared brown.
(E) Quantification of TUNEL-positive neurons in the spinal dorsal horn. Data are expressed as mean ± SD of 8 rats in each group. Comparisons of values among groups were made using one-way analysis of variance, followed by the q-test. aP < 0.01, vs. control group; bP < 0.01, vs. DM group.
DM: Diabetes mellitus; CoPP: cobalt protoporphyrin; ZnPP: zinc protoporphyrin; TUNEL: terminal deoxynucleo-tidyl transferase-mediated dUTP-biotin nick end labeling.
DISCUSSION
In our study, administration of streptozotocin induced experimental type-1 DM in rats, with severe hyperglycemia. In diabetic rats, the paw withdrawal threshold was significantly lower than that in control animals, indicating development of mechanical hyperalgesia. This is in accordance with the observations that streptozotocin-induced diabetic animals showed mechanical hyperalgesia when the paw was exposed to noxious stimuli[29,30]. At the end of the study (day 42), mechanical hyperalgesia were observed in 100% of DM rats. In addition, mechanical hyperalgesia became worse with the time course of the disease.
Our results show the basal expression of HO-1 was very low in the spinal dorsal horn of control rats, but was markedly increased in diabetic rats. It has been reported that the products of HO-mediated heme degradation regulated important biological processes, including oxidative stress and apoptosis[31], and that HO-1 was a potent cytoprotective enzyme. In spinal dorsal horn neurons of diabetic rats, the expression of HO-1 was significantly induced, which may indicate the presentation of hyperglycemia-induced oxidative stress.
After CoPP treatment, an inducer of HO-1, the DM + CoPP group showed an increase in HO-1 expression and slight mechanical hyperalgesia when compared to the DM group, while the DM+ ZnPP group exerted a decrease in HO-1 expression and more severe mechanical hyperalgesia. Therefore, in diabetic rats representing neuropathic pain, HO-1 can attenuate the onset of hyperalgesia to mechanical stimuli.
Mounting evidence suggests that the hyperglycemic environment overloads the metabolic capacity of mitochondria, producing oxidative stress[32]. This oxidative stress leads to mitochondrial damage, which contributes to neuronal damage[33,34]. In our study, abnormalities emerged in the sciatic nerves of diabetic rats, including demyelination and Wallerian degeneration, as previously described[35]. Compared with the DM group, pathologic alterations to the sciatic nerves in CoPP-supplemented diabetic rats were not pronounced coinciding with some slight mechanical hyperalgesia. However, the DM + ZnPP group showed significant nerve damage and more severe mechanical hyperalgesia. Therefore, this study suggests that, in diabetic neuropathic pain, mechanical hyperalgesia positively correlates with peripheral nerve damage, and HO-1 can attenuate nerve damage as well as mechanical hyperalgesia.
Because mitochondria play an important role in metabolism, oxidative stress, and apoptosis, hyperglycemia-induced mitochondrial damage can cause apoptosis of neurons[7]. In experimental models of neuropathic pain, the apoptosis of inhibitory GABAergic interneurons in the spinal dorsal horn decreases inhibitory GABAergic input, which may activate dorsal horn neurons[36,37]. In turn, it has been recognized that the hyperactivity of dorsal horn neurons in the spinal cord (central sensitization) may contribute to the mechanism of diabetic neuropathic pain[38]. In the present study, we observed that TUNEL-positive apoptotic spinal dorsal horn neurons were increased in diabetic rats with neuropathic pain. We also found that when the expression of HO-1 was elevated after CoPP treatment, the apoptosis of spinal dorsal horn neurons was prevented. In contrast, the number of apoptotic neurons increased while the expression of HO-1 was inhibited. Although the mechanism by which HO-1 prevents neuron apoptosis and the type of neurons affected remain unknown, it is likely that HO-1 exerts its neuroprotective effect by inhibiting programmed cell death in spinal dorsal horn neurons. As it has been shown in our experimental study, when compared with the DM group, the DM + CoPP group exerted slight mechanical hyperalgesia while the DM + ZnPP group resulted in more severe hyperalgesia, suggesting that the apoptosis of spinal dorsal horn neurons is closely related to neuropathic pain.
In summary, our data indicates that HO-1 ameliorates hyperglycemia-induced mechanical hyperalgesia, and attenuates oxidative stress-mediated initiation of neuron apoptosis and nerve damage, which may be responsible for diabetic neuropathic pain. These findings suggest that HO-1 may be beneficial for chronic diabetics exhibiting neuropathic pain.
MATERIALS AND METHODS
Design
A randomized, controlled, animal experiment.
Time and setting
The experiment was performed at the Center for Laboratory Animals, Renmin Hospital of Wuhan University, China, between August 2010 and March 2011.
Materials
Male Sprague-Dawley rats of a specific pathogen free level, weighing 225–250 g, aged 2 months on arrival at the housing room, were provided by the Laboratory Animal Center of Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China (certification No. 00010941). The Guidelines for the International Association for the Study of Pain for Pain Research in Animals were followed[39].
Methods
Induction of DM model
After an overnight fast, a single intraperitoneal injection of streptozotocin (Sigma-Aldrich Branch, Shanghai, China) that was freshly dissolved in 0.1 M citrate buffer (pH 4.6) was administered at a dose of 65 mg/kg[40]. Three days later, blood glucose was measured with the glucose pilot (Aventir Biotech, California, CA, USA) using a drop of blood obtained from the tail vein, in non-fasting animals. Streptozotocin-treated rats with a blood glucose concentration higher than 16.7 mM were considered diabetic, and were recruited into the study.
Intraperitoneal injection of ZnPP and CoPP
Seven days after streptozotocin injection, rats in the DM + ZnPP treated group and DM + CoPP treated group were intraperitoneally injected with ZnPP and CoPP, respectively, every other day at a dose of 10 μmoL/kg for 5 weeks. At the same time, the control and DM groups were given PBS (0.1 M, pH 7.4).
Hindpaw withdrawal threshold test
Before treatment and 7, 14, 21, 28, 35 and 42 days after streptozotocin treatment, tactile allodynia was assessed by measuring the hindpaw withdrawal threshold to the application of a calibrated series of 6 Von Frey filaments (bending forces of 2, 4, 6, 8, 10 and 15 g; North Coast Medical, Canada), using a modification of the up-down method[28]. Rats were placed on a metal mesh floor covered with a plexiglas chamber (20 cm × 20 cm × 25 cm), 15–30 minutes before the beginning of the tests, in a quiet room. After the acclimatization period, the rats were quiet and showed no exploratory behavior. Von Frey filaments were applied from underneath the metal mesh floor to the paws, and the test started with the filament that had the lowest force (2 g). The filament was applied perpendicularly to the mid-plantar surface of the paw, with sufficient force to cause the filament to buckle slightly. Brisk withdrawal or paw flinching was considered a positive response. Each filament was applied five stimulations to each paw (6–8 seconds per stimulation; an inter stimulus interval of 1 minute). A minimum recording of 5 positive responses (50%) out of 10 stimulations for both paws was considered the threshold (in grams). If the application of a filament induced less than 5 withdrawals out of 10 stimulations, then the next graded filament of increasing weight was used. The 15 g filament was selected as the upper limit for testing, because stiffer filaments tended to raise the entire limb rather than to buckle, substantially changing the nature of the stimulus.
Sampling
On day 43 after streptozotocin injection, rats were anesthetized under deep anesthesia. Rats were perfused transcardially with 100 mL heparin-prepared saline, followed by 300 mL of 4% (w/v) paraformaldehyde in PBS (pH 7.4). Lumbar spinal cords (L4-6) were removed and post-fixed by immersion in 4% (w/v) paraformaldehyde (4°C) for at least 24 hours, then dehydrated in graded ethanol and embedded in paraffin. Serial transverse sections (5 µm thickness) of the lumbar segments of the spinal cord were sliced. Sections were chosen for TUNEL and immunohistochemical staining, so as to detect apoptotic cells and the expression levels of HO-1, respectively.
Ultrastructure of sciatic nerves using electron microscopy
Sciatic nerves were quickly removed, fixed by immersion in 2.5% (w/v) glutaraldehyde overnight at 4°C and post-fixed in 1% (w/v) osmium tetroxide, embedded in epoxy resin, and used for electron microscope observations. Ultrathin sections (50 nm) were counterstained with uranyl acetate and lead citrate, and examined with a Tecnai G2 20 transmission electron microscope (FEI Inc., Eindhoven, Netherlands).
Immunohistochemical detection of HO-1 expression in spinal dorsal horn neurons
Immunohistochemical analysis was used to detect the expression of HO-1[41]. Paraffin-embedded cord sections were dewaxed in xylene, and hydrated through a series of graded ethanols, and double-distilled water. Following this, sections were incubated in 3% (v/v) H2O2 for 10 minutes to prevent a reaction with endogenous peroxidases. Antigen retrieval was carried out using microwave heating (80°C, 30 minutes × three times). Sections were blocked with goat serum for 30 minutes at 37°C and incubated with rabbit anti-rat HO-1 monoclonal antibody (1:1 000; Beijing Zhongshan Biotechnology Co., Ltd., Beijing, China) at 4°C overnight. After rinsing with PBS, the sections were incubated with horseradish peroxidase-labeled goat anti-rabbit IgG monoclonal antibody (1:500; Beijing Zhongshan Biotechnology Co., Ltd.) for 30 minutes and then placed in a horseradish peroxidase complex solution for 30 minutes at 37°C. Peroxidase activity was revealed by steeping the sections in a mixture containing 0.05% (w/v) 3-3’-diaminobenzidine (Beijing Zhongshan Biotechnology Co., Ltd.) and 0.03% (v/v) H2O2 for 5 minutes. Sections were then air-dried, dehydrated and coverslipped. Images of the sections were captured using a computer-assisted image analyzer system (Olympus, Tokyo, Japan). Application of control serum instead of the primary antibody on other sections of the same spinal cord samples provided a negative control for each stain. The integrated absorbance of the signal for HO-1 was analyzed with Image Pro-Plus 6.0 software (Media Cybernetics, Silver Spring, MD, USA).
TUNEL for apoptotic spinal dorsal horn neurons
We used an apoptosis detection kit (Lot No. 1-684-817, Roche Applied Science, Penzberg, USA) to detect apoptotic cells in spinal dorsal horn[42]. Paraffin-embedded cord sections were dewaxed in xylene, and hydrated through a series of graded ethanols, and double-distilled water. Following this, the sections were incubated with 20 μg/mL proteinase K in freshly prepared 0.1 M Tris buffer (pH 8.0) at 37°C for 15 minutes to strip off nuclear proteins. After rinsing in PBS twice for 10 minutes, the area around the sample was dried, and the sections were incubated with TUNEL reaction mixture overnight at 4°C in a humidified atmosphere in the dark. The slides were rinsed three times in PBS to stop the reaction, and incubated with an anti-fluorescein-peroxidase-conjugate (Converter- peroxidase) in a humidified chamber for 30 minutes at 37°C. The sections were colorized with diaminobenzidine substrate working solution, and were then counterstained with hematoxylin. Sections were rinsed with distilled water, hydrated with graded ethanol, and vitrified with xylene, and embedded in paraffin. TUNEL-positive cells were identified by light microscopic (Olympus, Tokyo, Japan) examination. Positive neural cells in five non consecutive sections, randomly selected from each lumbar spinal cord, were quantified by an experimenter blinded to the treatment.
Statistical analysis
Statistical analysis was performed using SPSS 13.0 software (SPSS, Chicago, IL, USA). Results were expressed as mean ± SD. Multiple groups were compared using one-way analysis of variance followed by the q-test. Differences were considered statistically significant at the level of P < 0.05.
Acknowledgments
We would like to thank Dong Xia and his staff from the Laboratory of Wuhan University School of Basic Medical Sciences, for their technical support.
Footnotes
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Animal Ethics Committee of International Association for the Study of Pain.
(Edited by Zhang CM, Zhang L/Yang Y/Song LP)
REFERENCES
- [1].Vinik AI, Park TS, Stansberry KB, et al. Diabetic neuropathies. Diabetologia. 2000;43(8):957–973. doi: 10.1007/s001250051477. [DOI] [PubMed] [Google Scholar]
- [2].Ziegler D, Rathmann W, Dickhaus T, et al. Prevalence of polyneuropathy in pre-diabetes and diabetes is associated with abdominal obesity and macroangiopathy: the MONICA/KORA Augsburg Surveys S2 and S3. Diabetes Care. 2008;31(3):464–469. doi: 10.2337/dc07-1796. [DOI] [PubMed] [Google Scholar]
- [3].Abbott CA, Malik RA, van Ross ER, et al. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U. K. Diabetes Care. 2011;34(10):2220–2224. doi: 10.2337/dc11-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Yagihashi S, Yamagishi SI, Wada Ri R, et al. Neuropathy in diabetic mice overexpressing human aldose reductase and effects of aldose reductase inhibitor. Brain. 2001;124(Pt 12):2448–2458. doi: 10.1093/brain/124.12.2448. [DOI] [PubMed] [Google Scholar]
- [5].Geraldes P, King GL. Activation of protein kinase C isoforms and its impact on diabetic complications. Circ Res. 2010;106(8):1319–1331. doi: 10.1161/CIRCRESAHA.110.217117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Goh SY, Cooper ME. Clinical review: The role of advanced glycation end products in progression and complications of diabetes. J Clin Endocrinol Metab. 2008;93(4):1143–1152. doi: 10.1210/jc.2007-1817. [DOI] [PubMed] [Google Scholar]
- [7].Vincent AM, Brownlee M, Russell JW. Oxidative stress and programmed cell death in diabetic neuropathy. Ann N Y Acad Sci. 2002;959:368–383. doi: 10.1111/j.1749-6632.2002.tb02108.x. [DOI] [PubMed] [Google Scholar]
- [8].Russell JW, Golovoy D, Vincent AM, et al. High glucose- induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 2002;16(13):1738–1748. doi: 10.1096/fj.01-1027com. [DOI] [PubMed] [Google Scholar]
- [9].Moore KA, Kohno T, Karchewski LA, et al. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22(15):6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Niederberger E, Kühlein H, Geisslinger G. Update on the pathobiology of neuropathic pain. Expert Rev Proteomics. 2008;5(6):799–818. doi: 10.1586/14789450.5.6.799. [DOI] [PubMed] [Google Scholar]
- [11].Kawamura T, Akira T, Watanabe M, et al. Prostaglandin E1 prevents apoptotic cell death in superficial dorsal horn of rat spinal cord. Neuropharmacology. 1997;36(8):1023–1030. doi: 10.1016/s0028-3908(97)00096-8. [DOI] [PubMed] [Google Scholar]
- [12].Whiteside GT, Munglani R. Cell death in the superficial dorsal horn in a model of neuropathic pain. J Neurosci Res. 2001;64(2):168–173. doi: 10.1002/jnr.1062. [DOI] [PubMed] [Google Scholar]
- [13].Baron R. Neuropathic pain: a clinical perspective. Handb Exp Pharmacol. 2009;(194):3–30. doi: 10.1007/978-3-540-79090-7_1. [DOI] [PubMed] [Google Scholar]
- [14].Ziegler D. Painful diabetic neuropathy: treatment and future aspects. Diabetes Metab Res Rev. 2008;24(Suppl 1):S52–57. doi: 10.1002/dmrr.817. [DOI] [PubMed] [Google Scholar]
- [15].The Diabetes Control and Complications Trial Research Group. The effect of intensive diabetes therapy on the development and progression of neuropathy. Ann Intern Med. 1995;122(8):561–568. doi: 10.7326/0003-4819-122-8-199504150-00001. [DOI] [PubMed] [Google Scholar]
- [16].UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33) Lancet. 1998;352(9131):837–853. [PubMed] [Google Scholar]
- [17].Balkis Budin S, Othman F, Louis SR, et al. Effect of alpha lipoic acid on oxidative stress and vascular wall of diabetic rats. Rom J Morphol Embryol. 2009;50(1):23–30. [PubMed] [Google Scholar]
- [18].Jin HY, Joung SJ, Park JH, et al. The effect of alpha-lipoic acid on symptoms and skin blood flow in diabetic neuropathy. Diabet Med. 2007;24(9):1034–1038. doi: 10.1111/j.1464-5491.2007.02179.x. [DOI] [PubMed] [Google Scholar]
- [19].Vallianou N, Evangelopoulos A, Koutalas P. Alpha-lipoic Acid and diabetic neuropathy. Rev Diabet Stud. 2009;6(4):230–236. doi: 10.1900/RDS.2009.6.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Pop-Busui R, Sima A, Stevens M. Diabetic neuropathy and oxidative stress. Diabetes Metab Res Rev. 2006;22(4):257–273. doi: 10.1002/dmrr.625. [DOI] [PubMed] [Google Scholar]
- [21].Ziegler D, Sohr CG, Nourooz-Zadeh J, et al. Oxidative stress and antioxidant defense in relation to the severity of diabetic polyneuropathy and cardiovascular autonomic neuropathy. Diabetes Care. 2004;27(9):2178–2183. doi: 10.2337/diacare.27.9.2178. [DOI] [PubMed] [Google Scholar]
- [22].Figueroa-Romero C, Sadidi M, Feldman EL. Mechanisms of disease: the oxidative stress theory of diabetic neuropathy. Rev Endocr Metab Disord. 2008;9(4):301–314. doi: 10.1007/s11154-008-9104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Tenhunen R, Marver HS, Schmid R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc Natl Acad Sci U S A. 1968;61(2):748–755. doi: 10.1073/pnas.61.2.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Applegate LA, Luscher P, Tyrrell RM. Induction of heme oxygenase: a general response to oxidant stress in cultured mammalian cells. Cancer Res. 1991;51(3):974–978. [PubMed] [Google Scholar]
- [25].Drummond GS. Control of heme metabolism by synthetic metalloporphyrins. Ann N Y Acad Sci. 1987;514:87–95. doi: 10.1111/j.1749-6632.1987.tb48764.x. [DOI] [PubMed] [Google Scholar]
- [26].Kirkby KA, Adin CA. Products of heme oxygenase and their potential therapeutic applications. Am J Physiol Renal Physiol. 2006;290(3):F563–571. doi: 10.1152/ajprenal.00220.2005. [DOI] [PubMed] [Google Scholar]
- [27].Morse D, Lin L, Choi AM, et al. Heme oxygenase-1, a critical arbitrator of cell death pathways in lung injury and disease. Free Radic Biol Med. 2009;47(1):1–12. doi: 10.1016/j.freeradbiomed.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Chaplan SR, Bach FW, Pogrel JW, et al. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- [29].Courteix C, Eschalier A, Lavarenne J. Streptozocin- induced diabetic rats: behavioural evidence for a model of chronic pain. Pain. 1993;53(1):81–88. doi: 10.1016/0304-3959(93)90059-X. [DOI] [PubMed] [Google Scholar]
- [30].Fox A, Eastwood C, Gentry C, et al. Critical evaluation of the streptozotocin model of painful diabetic neuropathy in the rat. Pain. 1999;81(3):307–316. doi: 10.1016/S0304-3959(99)00024-X. [DOI] [PubMed] [Google Scholar]
- [31].Dulak J, Deshane J, Jozkowicz A, et al. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation. 2008;117(2):231–241. doi: 10.1161/CIRCULATIONAHA.107.698316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414(6865):813–820. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- [33].Edwards JL, Vincent AM, Cheng HT, et al. Diabetic neuropathy: mechanisms to management. Pharmacol Ther. 2008;120(1):1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shakher J, Stevens MJ. Update on the management of diabetic polyneuropathies. Diabetes Metab Syndr Obes. 2011;4:289–305. doi: 10.2147/DMSO.S11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Behse F, Buchthal F, Carlsen F. Nerve biopsy and conduction studies in diabetic neuropathy. J Neurol Neurosurg Psychiatry. 1977;40(11):1072–1082. doi: 10.1136/jnnp.40.11.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sugimoto T, Bennett GJ, Kajander KC. Transsynaptic degeneration in the superficial dorsal horn after sciatic nerve injury: effects of a chronic constriction injury, transection, and strychnine. Pain. 1990;42(2):205–213. doi: 10.1016/0304-3959(90)91164-E. [DOI] [PubMed] [Google Scholar]
- [37].de Novellis V, Siniscalco D, Galderisi U, et al. Blockade of glutamate mGlu5 receptors in a rat model of neuropathic pain prevents early over-expression of pro-apoptotic genes and morphological changes in dorsal horn lamina II. Neuropharmacology. 2004;46(4):468–479. doi: 10.1016/j.neuropharm.2003.10.014. [DOI] [PubMed] [Google Scholar]
- [38].Chen SR, Pan HL. Hypersensitivity of spinothalamic tract neurons associated with diabetic neuropathic pain in rats. J Neurophysiol. 2002;87(6):2726–2733. doi: 10.1152/jn.2002.87.6.2726. [DOI] [PubMed] [Google Scholar]
- [39].Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16(2):109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- [40].Suthagar E, Soudamani S, Yuvaraj S, et al. Effects of streptozotocin (STZ)-induced diabetes and insulin replacement on rat ventral prostate. Biomed Pharmacother. 2009;63(1):43–50. doi: 10.1016/j.biopha.2008.01.002. [DOI] [PubMed] [Google Scholar]
- [41].Wang F, Duan ZJ, Sun YJ. Influence of heme oxygenase-1 expression on immune liver fibrosis induced by cobalt protoporphyrin in rats. World J Gastroenterol. 2009;15(24):3009–3014. doi: 10.3748/wjg.15.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Zhang HP, Yuan LB, Zhao RN, et al. Isoflurane preconditioning induces neuroprotection by attenuating ubiquitin-conjugated protein aggregation in a mouse model of transient global cerebral ischemia. Anesth Analg. 2010;111(2):506–514. doi: 10.1213/ANE.0b013e3181e45519. [DOI] [PubMed] [Google Scholar]


