Abstract
Cerebral neuroinflammation models were established by injecting 10 μg lipopolysaccharide into the hippocampus of male Sprague-Dawley rats. The rats were treated with an intraperitoneal injection of 120, 90, or 60 mg/kg oxymatrine daily for three days prior to the lipopolysaccharide injection. Twenty-four hours after model induction, the hippocampus was analyzed by real-time quantitative PCR, and the cerebral cortex was analyzed by enzyme-linked immunosorbent assay and western blot assay. The results of the enzyme-linked immunosorbent assay and the real-time quantitative PCR showed that the secretion and mRNA expression of the pro-inflammatory cytokines interleukin-1β and tumor necrosis factor-α were significantly decreased in the hippocampus and cerebral cortex of model rats treated with oxymatrine. Western blot assay and real-time quantitative PCR analysis indicated that toll-like receptor 4 mRNA and protein expression were significantly decreased in the groups receiving different doses of oxymatrine. Additionally, 120 and 90 mg/kg oxymatrine were shown to reduce protein levels of nuclear factor-κB p65 in the nucleus and of phosphorylated IκBα in the cytoplasm of brain cells, as detected by western blot assay. Experimental findings indicate that oxymatrine may inhibit neuroinflammation in rat brain via downregulating the expression of molecules in the toll-like receptor 4/nuclear factor-κB signaling pathway.
Keywords: oxymatrine, neuroinflammation, toll-like receptor 4, nuclear factor-κB, signaling pathway, inflammatory factors, lipopolysaccharide, hippocampus, cerebral cortex, neural regeneration
Research Highlights
-
(1)
Cerebral neuroinflammation models were established by injecting lipopolysaccharide into the hippocampus of male rats.
-
(2)
Oxymatrine inhibits the expression of interleukin-1β and tumor necrosis factor-α mRNA in the hippocampus and the secretion of interleukin-1β and tumor necrosis factor-α in the cerebral cortex of model rats.
-
(3)
Oxymatrine decreases toll-like receptor 4 mRNA expression in the hippocampus and protein expression in the cerebral cortex.
-
(4)
Oxymatrine reduces protein levels of nuclear factor-κB p65 in the nucleus and of phosphorylated IκBα in the cytoplasm of brain cells of model rats.
-
(5)
Oxymatrine may inhibit neuroinflammation in rat brains via the toll-like receptor 4/nuclear factor-κB signaling pathway.
INTRODUCTION
Neuroinflammation is a complex response to brain injury. Memory deficits have been observed following many neuroinflammatory insults including infection, traumatic brain injury, Alzheimer's disease and others. Emerging evidence indicates that neuroinflammation within the central nervous system is a classical feature of ischemia, neurodegenerative disease, infections and trauma, and may often contribute to neuronal damage[1]. Cumulative evidence suggests that toll-like receptor 4 plays an important role in initiating the neuroinflammation related to Alzheimer's disease, Huntington's disease and Parkinson's disease[2]. All toll-like receptors activate common signaling pathways: the nuclear factor-κB transcription factors and the mitogen-activated protein kinases. One of the most important downstream molecules in the toll-like receptor signaling pathway, nuclear factor-κB, is a transcriptional factor required for the gene expression of many pro-inflammatory cytokines, such as interleukin-1β, tumor necrosis factor-α, interleukin-6 and intercellular adhesion molecule-1[3].
Oxymatrine, also called kushenin, has a tetracyclic quinolizine structure. Its molecular formula is C15H24N2O2 (Figure 1).
Figure 1.
Chemical structure of oxymatrine.
It is an alkaloid extracted from Sophora flavescens Ait (a traditional Chinese medicine). Oxymatrine has anti-inflammatory, anti-apoptotic, anti-tumor, anti-viral and anti-arrhythmic effects[4,5,6]. Liu et al m[4] found that oxymatrine can reduce the volume of infarct induced by permanent middle cerebral artery occlusion, especially at a dose of 120 mg/kg. Toll-like receptors are a group of pattern recognition receptors that are attracting increasing attention in the field of infectious and non-pathogenic inflammatory injury. Toll-like receptors in the central nervous system are not only involved in innate immunity, but also participate in and regulate inflammatory and neurodegenerative diseases. Nuclear factor-κB is downstream of the toll-like receptor signaling pathway, and its expression can lead to a cascading release of a variety of inflammatory factors, resulting in injury.
The aim of the present study was to determine whether oxymatrine could attenuate the lipopolysaccharide-induced activation of the toll-like receptor 4/nuclear factor-κB signaling pathway in the rat brain. We hypothesized that the effect of oxymatrine on modulating the toll-like receptor 4/nuclear factor-κB signaling pathway could be a mechanism by which oxymatrine inhibits the induction of neuroinflammation.
RESULTS
Quantitative analysis of experimental animals
Thirty rats were randomly divided into five groups (n = 6 for each group): sham-operated group, lipopolysaccharide group, high-dose (rats with lipopolysaccharide and 120 mg/kg oxymatrine treatment), medium-dose (rats with lipopolysaccharide and 90 mg/kg oxymatrine treatment) and low-dose (rats with lipopolysaccharide and 60 mg/kg oxymatrine treatment) oxymatrine groups. All 30 rats were involved in the final analysis.
Oxymatrine inhibits the secretion and mRNA expression of interleukin-1β and tumor necrosis factor-α in the hippocampus and cerebral cortex of rats
Twenty-four hours after surgery, the levels of interleukin-1β and tumor necrosis factor-α mRNA in hippocampus of each group were analyzed by real-time quantitative PCR. Results showed that expression was significantly higher in the lipopolysaccharide group than in the sham-operated group (P < 0.01), and was significantly lower in the oxymatrine groups than in the lipopolysaccharide group (P < 0.01), especially the high-dose and medium-dose oxymatrine groups (Figure 2A). Meanwhile, the expression of interleukin-1β and tumor necrosis factor-α in the cerebral cortex of each group was tested by enzyme-linked immunosorbent assay, and the results were consistent with those of the real-time quantitative PCR (Figure 2B).
Figure 2.
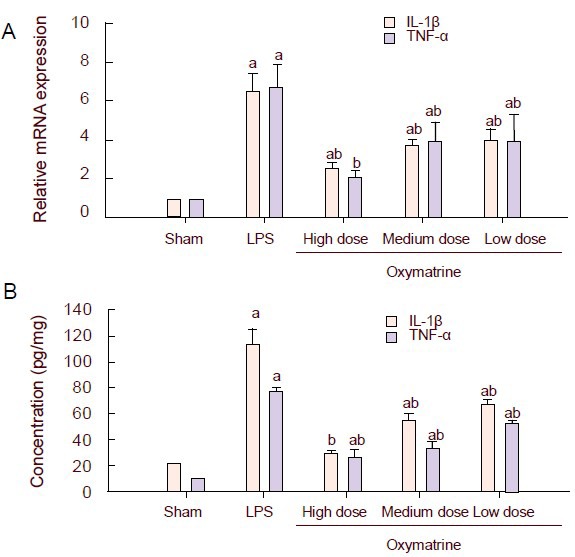
Effect of different doses of oxymatrine on lipopolysaccharide (LPS)-induced production of interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF-α).
(A) The mRNA expression of the pro-inflammatory cytokines IL-1β and TNF-α in the hippocampus was examined by real-time quantitative PCR. Data were plotted as relative quantity (RQ, RQ=2-ΔΔCT). The expression of the target gene in the sham-operated group was standardized to one, and the RQ values of the other groups represent the fold changes compared with the sham-operated group.
(B) The release of IL-1β and TNF-α in the cerebral cortex was examined by enzyme-linked immunosorbent assay.
Data are represented as mean ± SD; there are three rats in each group. aP < 0.01, vs. sham-operated (Sham) group; bP < 0.01, vs. LPS group, one-way analysis of variance followed by a Scheffé test.
Oxymatrine decreases the expression of toll-like receptor 4 mRNA and protein in the hippocampus and cerebral cortex of rats
Twenty-four hours after surgery, the levels of toll-like receptor 4 mRNA in the hippocampus of each group were analyzed by real-time quantitative PCR. Results showed that expression was significantly increased in the lipopolysaccharide group compared with the sham-operated oxymatrine group (P < 0.01), and was significantly decreased in the oxymatrine groups compared with the lipopolysaccharide group, especially in the high-dose oxymatrine group (P < 0.01; Figure 3A). Meanwhile, the protein expression of toll-like receptor 4 in the cerebral cortex was analyzed by western blot (Figures 3B, C). The expression was significantly increased in the lipopolysaccharide group (P < 0.05). Only the high-dose and medium-dose oxymatrine groups had significant differences in toll-like receptor 4 expression compared with the lipopolysaccharide group (P < 0.05).
Figure 3.
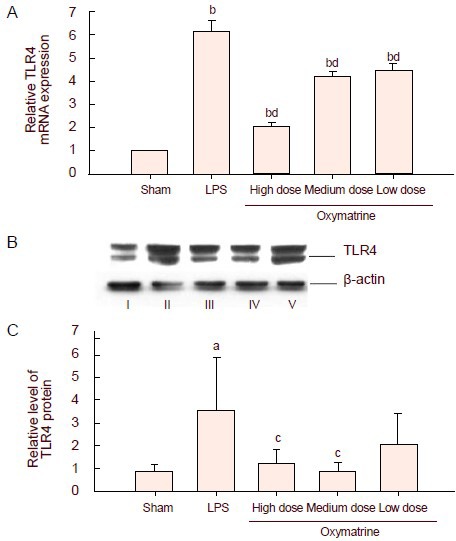
Effect of different doses of oxymatrine on lipopolysaccharide (LPS)-induced production of toll-like receptor 4 (TLR4) mRNA and protein.
(A) The mRNA expression of TLR4 in the hippocampus was tested by real-time quantitative PCR. Data were plotted as relative quantity (RQ, RQ=2-ΔΔCT). The expression of the target gene in the sham-operated group was standardized to one, and the RQ values of the other groups represent the fold changes compared with the sham-operated group.
(B) Representative western blot analysis of TLR4 protein (89-kDa) in the cerebral cortex of the different groups. β-actin (43-kDa) was used as a control. I–V: Sham, LPS, high, medium, low dose oxymatrine groups, respectively.
(C) Graph showing changes in the levels of TLR 4 in the different groups.
Data are expressed as mean ± SD (A, RQ; B, ratio of the absorbance value of TLR 4 to that of β-actin), there are three rats in each group. aP < 0.05, bP < 0.01, vs. sham-operated (Sham) group; cP < 0.05, dP < 0.01, vs. LPS group, by one-way analysis of variance followed by a Scheffé test.
Oxymatrine decreases levels of nuclear factor-κB p65 in the nucleus and of phosphorylated IκBα in the cytoplasm
The protein levels of nuclear factor-κB p65 and of phosphorylated IκBα in the cerebral cortex were analyzed by western blot assay (Figure 4). Results showed that the protein levels of nuclear factor-κB p65 and of phosphorylated IκBα in the cerebral cortex were significantly increased in the lipopolysaccharide group (P < 0.01). The levels were significantly decreased in the high-dose and medium-dose oxymatrine groups compared with the lipopolysaccharide group (P < 0.05). 90 mg/kg oxymatrine significantly down-regulated the protein expression of nuclear factor-κB p65, while 120 mg/kg oxymatrine led to a significant reduction in the levels of phosphorylated IκBα in the cerebral cortex (P < 0.05).
Figure 4.
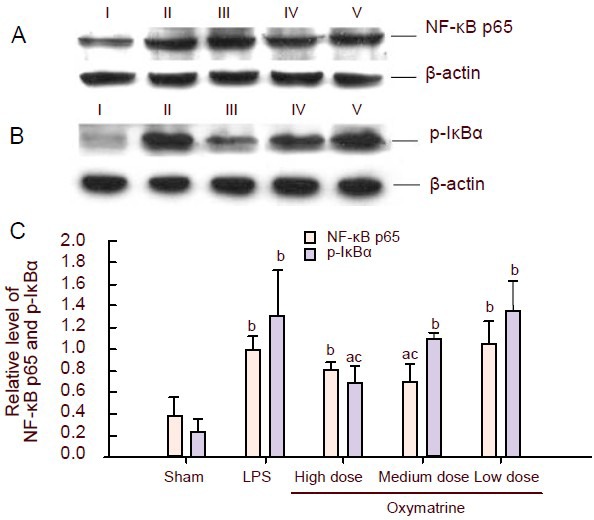
Effect of different doses of oxymatrine on lipopolysaccharide(LPS)-induced production of nuclear factor-κB (NF-κB) p65 in nucleus and phosphorylated IκBα (p-IκBα) in cytoplasm.
(A) NF-κB p65 protein (65-kDa) levels in the cerebral cortex of rat brains were detected by western blot assay. β-actin (43-kDa) was used as an internal control.
(B) p-IκBα protein (41-kDa) levels in the cerebral cortex of rat brains were detected by western blot analysis. β-actin (43-kDa) was used as an internal control. I–V: Sham, LPS, high, medium, low dose oxymatrine groups, respectively.
(C) Graph showing changes in the levels of NF-κB p65 in the nucleus and p-IκBα in the cytoplasm in the different groups. Data are expressed as mean ± SD (ratio of the absorbance value of NF-κB p65/p-IκBα to that of β-actin); there are three rats in each group. aP < 0.05, bP < 0.01, vs. sham-operated (Sham) group; cP < 0.05, vs. LPS group, one-way analysis of variance followed by a Scheffé test.
DISCUSSION
The initiation of neuroinflammation may participate in secondary brain lesions, while a delayed inflammatory response may, on the contrary, be beneficial and reparative[7]. Thus, the treatment of acute neuroinflammation is seen as a potential target for neuroprotective strategies. The concept is emerging that the balance between pro-inflammatory cytokines and anti-inflammatory cytokines affects the outcome of certain diseases such as neurodegenerative disorders[8]. Lipopolysaccharide, a bacterial endotoxin, is known to activate microglia and astrocytes in brain, causing the release of pro-inflammatory cytokines such as interleukin-1β and tumor necrosis factor-α[9].
Recent reports suggest that microglia activated by lipopolysaccharide release pro-inflammatory cytokines and contribute to neurodegenerative processes[10]. With in-depth study of Alzheimer's disease and the hypothesis of inflammatory pathogenesis in recent years, some scholars have also successfully established animal models of Alzheimer's disease with lipopolysaccharide[11,12]. Therefore, in the present study, lipopolysaccharide was used to induce acute neuroinflammation in rat brain. After model establishment we examined changes of interleukin-1β and tumor necrosis factor-α in the rats’ brains. The levels of interleukin-1β and tumor necrosis factor-α mRNA in the hippocampus and the protein expression of interleukin-1β and tumor necrosis factor-α in the cerebral cortex were tested by real-time PCR and western blot, respectively. Results showed that they were both increased in the lipopolysaccharide group.
A variety of toll-like receptors has been identified in human cells and the brains of some other species. Several studies have suggested that toll-like receptor 4 is critical for lipopolysaccharide-induced neurodegeneration in the central nervous system[13]. Toll-like receptor 4 seems to initiate inflammation following neurodegeneration. During preliminary experiments in our study, we found that hippocampal neurons generally express mRNA for toll-like receptors 1–9. Among these toll-like receptors, the levels of toll-like receptor 4 were the highest (published in another article, currently in press). To answer the question of whether toll-like receptor 4 can be activated by lipopolysaccharide in this model, we observed changes in the expression levels of toll-like receptor 4 mRNA and protein. The levels of toll-like receptor 4 mRNA in the hippocampus and the protein expression of toll-like receptor 4 in the cortex were increased in the lipopolysaccharide group. Thus, toll-like receptor 4 in hippocampal and cortex neurons can be activated by lipopolysaccharide, and we assume that lipopolysaccharide combines with a lipopolysaccharide binding protein to activate membrane toll-like receptor 4 in hippocampal and cortex neurons.
What we then wished to know was what the downstream signaling is after the activation of toll-like receptor 4 in hippocampal and cortex neurons treated with lipopolysaccharide. Transcription factor nuclear factor-κB was found in 1986 by Sen and Baltimore[14], and the family has five members, namely p65 (Rel-A), Rel-B, c-Rel (cytoplasmic Rel), p50 and p52. Nuclear factor-κB serves as a critical regulator of cytokine production, lymphocyte activation, and proliferation as a homodimer or heterodimer[15,16]. In its inactive state, it is present as a dimer consisting of p65 and p50 subunits and binds the inhibitor IκB. Nuclear factor-κB localizes to the cytoplasm. Stimulating IκB kinase, which phosphorylates IκB, may lead to the dissociation and translocation into the nucleus of nuclear factor-κB and the activation of target genes[17,18]. An important function of nuclear factor-κB in acute inflammation is its ability to regulate the promoters of a variety of genes whose products, such as interleukin-1β, tumor necrosis factor-α, interleukin-6, intercellular adhesion molecule-1 and acute phase proteins, are critical to inflammatory processes[19,20]. The inhibition of nuclear factor-κB activation by corticosteroid hormones, antioxidants, protease inhibitors and other compounds may provide a pharmacological basis for interference with pathological inflammatory conditions[21]. The toll-like receptor-mediated intracellular signaling pathways converge to activate nuclear factor-κB, which induces the transcription of a series of cytokine/ chemokine genes that are involved in the initiation or regulation of the inflammatory response[2]. We detected the expression of phosphorylated IκBα in the cytoplasm and nuclear factor-κB p65 in the nucleus by western blot assay. In our present study, lipopolysaccharide significantly increased the levels of phosphorylated IκBα in the cytoplasm and nuclear factor-κB p65 in the nucleus. These results indicate that lipopolysaccharide may induce neuroinflammation through the toll-like receptor 4/nuclear factor-κB signaling pathway.
The anti-inflammatory effects of oxymatrine have attracted attention over the past few years[4,22]. A recent report demonstrated that oxymatrine can act on nonspecific inflammatory reactions. It exerts its anti-inflammatory effect directly on inflammatory cells[23]. In our preliminary experiments, oxymatrine was used for intraperitoneal injection at a dose of 90 mg/kg, in accordance with the clinical therapeutic dosage. We found that inflammatory cytokines are significantly decreased with this dose of oxymatrine. Thus, we used 60, 90 and 120 mg/kg of oxymatrine in this study. Meanwhile, as previously described[4,24], lipopolysaccharide was used to establish a neuroinflammatory model and pro-inflammatory cytokines were significantly increased 24 hours after injection. We thus collected the brains 24 hours after injection of lipopolysaccharide. In our study, administration of oxymatrine (especially in the high- and medium-dose groups) was sufficient to reduce the expression of interleukin-1β, tumor necrosis factor-α, nuclear factor-κB, phosphorylated IκB and toll-like receptor 4. These results indicate that the down-regulation of the toll-like receptor 4/nuclear factor-κB signaling pathway by oxymatrine is a potential anti-inflammatory mechanism in the brain. We suggest that oxymatrine may be a novel, effective therapeutic drug for the treatment of neuroinflammation in the brain.
MATERIALS AND METHODS
Design
A randomized, controlled trial.
Time and setting
The experiments were performed at the Department of Pathophysiology, Medical School of Nantong University, China, from June 2010 to November 2011.
Materials
Thirty clean three-month-old male Sprague-Dawley rats weighing 250 g were provided by the Experimental Animal Center of Nantong University, Jiangsu Province, China (license No. SYXK (Su) 2007-0021). All experimental protocols were in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[25].
Methods
Lipopolysaccharide microinjection
Lipopolysaccharide was injected intracerebrally using a stereotaxic apparatus (Stoelting Co., Wheat Ln., Wood Dale, IL, USA), according to Sharma and Gupta[26]. Rats were anesthetized with chloral hydrate (300 mg/kg, intraperitoneal; Chemical Plant of Xiling, Shantou, Guangdong Province, China). The head was positioned in a stereotaxic frame and a midline sagittal incision was made in the scalp. Burr holes were drilled on both sides of the skull using the following coordinates[27]: 3.0 mm posterior to bregma, 2.0 mm lateral to the bregma, 3.0 mm beneath the surface of the skull. Lipopolysaccharide (prepared from E.coli, serotype 055:B5; Sigma, St. Louis, MO, USA) diluted in normal saline or plain normal saline was chronically infused into the hippocampus (0.5 μL/min, total 5 μL, 10 μg). The syringe was slowly withdrawn from the brain 5 minutes later. The incisions were sutured and the rats were injected with 1 × 105 units of penicillin intramuscularly to prevent infection. After that the animals were returned to their cages and carefully tended antibiotics.
Oxymatrine administration
Oxymatrine (Baoji F.S. Biological Development Co. Ltd., Baoji, Shaanxi Province, China) with a purity of 98.5% was dissolved in saline. For the high-, medium- and low-dose oxymatrine groups, oxymatrinewas administered once daily by intraperitoneal injection at doses of 120, 90 and 60 mg/kg[28], respectively, for three days before surgery. In the sham-operated and lipopolysaccharide groups, an equal volume normal saline was administered in the same manner.
Sampling of rat brains
Rats were sacrificed 24 hours after lipopolysaccharide injection. The brain was carefully removed from each rat and kept over a glass plate placed on ice for 15 minutes and then dissected into different regions: cerebral cortex and hippocampus.
Enzyme-linked immunosorbent assay
The levels of interleukin-1β and tumor necrosis factor-α in the cerebral cortex were measured 24 hours after surgery using a commercially available enzyme-linked immunosorbent assay kit (Groundwork Biotechnology Diagnosticate, Santiago, CA, USA) following the manufacturer's protocol. Absorbance was read at 450 nm using a microtiter plate reader (BIO-TEK, Winooski, VT, USA) within 30 minutes of finishing the assay. The standard curve was generated by plotting the averageabsorbance (450 nm) obtained for each of the three standard concentrations on the vertical (Y) axis versus the corresponding concentration on the horizontal (X) axis.
Real-time quantitative PCR analysis
Total RNA was isolated from the hippocampus 24 hours after surgery using Trizol reagent (Invitrogen, Carlsbad, CA, USA). 2 μg of total RNA was reverse transcribed into cDNA following the reverse transcription protocol (Fermentas, Burlington, Canada). Real-time PCR amplification was carried out with a StepOnePlus™ Real-Time PCR System (ABI, Carlsbad, CA, USA). 1 μL of cDNA was replicated in a 10 μL PCR reaction system including 5 μL SYBR Green/ROX qPCR Master Mix (Fermentas), 1 μL of both forward and reverse primers and 3 μL nuclease-free water. cDNA was replaced with nuclease-free water in negative control.
The cycle profile was as follows: an initial step at 95°C for 10 minutes, 40 cycles at 95°C for 15 seconds, 56°C (toll-like receptor 4) or 54°C (interleukin-1β and tumor necrosis factor-α) for 30 seconds, and 72°C for 30 seconds. A melting curve analysis was carried out after amplification to verify the accuracy of the amplicon. The following primers were used:
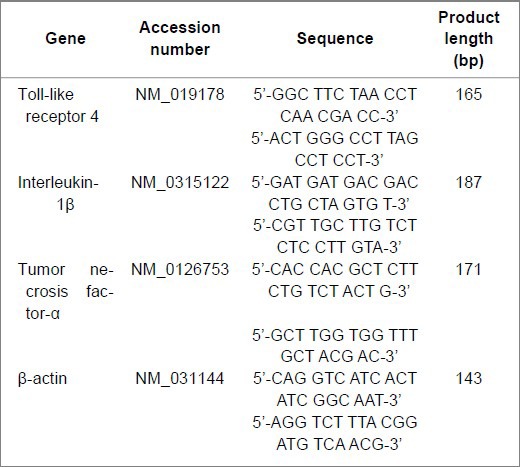
The amplification efficiency of the target and internal control genes was verified to be equal or close to one before designing a new assay. The relative quantification of target genes is shown as the relative quantity value.
Western blot analysis
Total protein, cytoplasmic protein and nuclear protein were extracted from the cerebral cortex 24 hours after surgery with a protein extraction kit (Beyotime Institute of Biotechnology, Beijing, China) and stored in a −80°C freezer (Sanyo, Tokyo, Japan) until use. The protein concentrations were determined with a BCA protein concentration determination kit (Beyotime Institute of Biotechnology). Western blot analysis was performed using previously described protocols[29]. Briefly, samples were separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis, transferred to PVDF membranes, and blocked with 5% non-fat dried milk (skim milk powder; Guangming, Shanghai, China) in TBST (10 mM Tris-HCl, 150 mM NaCl and 0.1% Tween-20, pH 7.5) at room temperature. After washing three times with TBST buffer, the membranes were probed with a rabbit polyclonal antibody against toll-like receptor 4 (1:200; Santa Cruz, Santa Cruz, CA, USA), a rabbit polyclonal antibody against nuclear factor-κB p65 (1:1 000; Kangchen Bio-Tech, Shanghai, China), a mouse monoclonal antibody against phosphorylated IκBα (1:1 000; Kangchen Bio-Tech) and a mouse monoclonal antibody against β-actin overnight at 4°C. Membranes were then incubated with the horseradish peroxidase-tagged secondary antibodies goat anti-rabbit IgG and goat anti-mouse IgG (1:5 000; Kangchen Bio-Tech), and visualized with the enhanced chemiluminescence plus detection system (Millipore, Billerica, MA, USA) followed by autoradiography. β-actin was used as an internal control. Quantity One software (Bio-Rad, Hercules, CA, USA) was used to analyze the absorbance of each band. The ratio between the absorbance of the proteins of interest and the internal control of the same sample was calculated, and expressed as relative content.
Statistical analysis
Data are expressed as mean ± SD. Statistical analysis was performed using SPSS 15.0 for Windows (SPSS, Chicago, IL, USA). The data were analyzed by a one-way analysis of variance followed by a Scheffé test. P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by a project of the Priority Academic Program Development of Jiangsu Higher Education Institutions; Applied Research and Technology Plan of Nantong City, No. k2010036; 2011 Jiangsu Graduated Students’ Research and Innovation Program, No. CX2211-0640; Nantong University Graduated Students’ Technological and Innovative Program, No. YKC11033; and Students’ Practice Innovative Training Project of Nantong University.
Conflicts of interest: None declared.
Ethical approval: All procedures involving animals were approved by the Institutional Animal Care and Use Committee of Nantong University in China.
(Edited by Chen JX, Huang SJ/Yang Y/Wang L)
REFERENCES
- [1].Amor S, Puentes F, Baker D, et al. Inflammation in neurodegenerative diseases. Immunology. 2010;129(2):154–169. doi: 10.1111/j.1365-2567.2009.03225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lee SJ, Lee S. Toll-like receptors and inflammation in the CNS. Curr Drug Targets Inflamm Allergy. 2002;1(2):181–191. doi: 10.2174/1568010023344698. [DOI] [PubMed] [Google Scholar]
- [3].Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16(1):3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- [4].Liu Y, Zhang XJ, Yang CH, et al. Oxymatrine protects rat brains against permanent focal ischemia and downregulates NF-kappaB expression. Brain Res. 2009;1268:174–180. doi: 10.1016/j.brainres.2009.02.069. [DOI] [PubMed] [Google Scholar]
- [5].Cao CX, Yang QW, Lv FL, et al. Reduced cerebral ischemia-reperfusion injury in Toll-like receptor 4 deficient mice. Biochem Biophys Res Commun. 2007;353(2):509–514. doi: 10.1016/j.bbrc.2006.12.057. [DOI] [PubMed] [Google Scholar]
- [6].Lu LG, Zeng MD, Mao YM, et al. Oxymatrine therapy for chronic hepatitis B: a randomized double-blind and placebo-controlled multi-center trial. World J Gastroenterol. 2003;9(11):2480–2483. doi: 10.3748/wjg.v9.i11.2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Leker RR, Shohami E. Cerebral ischemia and trauma-different etiologies yet similar mechanisms: neuroprotective opportunities. Brain Res Brain Res Rev. 2002;39(1):55–73. doi: 10.1016/s0165-0173(02)00157-1. [DOI] [PubMed] [Google Scholar]
- [8].O’Shea JJ, Ma A, Lipsky P. Cytokines and autoimmunity. Nat Rev Immunol. 2002;2(1):37–45. doi: 10.1038/nri702. [DOI] [PubMed] [Google Scholar]
- [9].Quan N, Sundar SK, Weiss JM. Induction of interleukin-1 in various brain regions after peripheral and central injections of lipopolysaccharide. J Neuroimmunol. 1994;49(1-2):125–134. doi: 10.1016/0165-5728(94)90188-0. [DOI] [PubMed] [Google Scholar]
- [10].Tanaka S, Ide M, Shibutani T, et al. Lipopolysaccharide- induced microglial activation induces learning and memory deficits without neuronal cell death in rats. J Neurosci Res. 2006;83(4):557–566. doi: 10.1002/jnr.20752. [DOI] [PubMed] [Google Scholar]
- [11].Cui CA, Jin DQ, Hwang YK, et al. Macelignan attenuates LPS-induced inflammation and reduces LPS-induced spatial learning impairments in rats. Neurosci Lett. 2008;448(1):110–114. doi: 10.1016/j.neulet.2008.10.035. [DOI] [PubMed] [Google Scholar]
- [12].Herber DL, Mercer M, Roth LM, et al. Microglial activation is required for Abeta clearance after intracranial injection of lipopolysaccharide in APP transgenic mice. J Neuroimmune Pharmacol. 2007;2(2):222–231. doi: 10.1007/s11481-007-9069-z. [DOI] [PubMed] [Google Scholar]
- [13].Lehnardt S, Massillon L, Follett P, et al. Activation of innate immunity in the CNS triggers neurodegeneration through a Toll-like receptor 4-dependent pathway. Proc Natl Acad Sci U S A. 2003;100(14):8514–8519. doi: 10.1073/pnas.1432609100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Sen R, Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- [15].Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25(51):6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- [16].Karin M, Greten FR. NF-kappaB: linking inflammation and immunity to cancer development and progression. Nat Rev Immunol. 2005;5(10):749–759. doi: 10.1038/nri1703. [DOI] [PubMed] [Google Scholar]
- [17].Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol. 2002;2(10):725–734. doi: 10.1038/nri910. [DOI] [PubMed] [Google Scholar]
- [18].Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109(Suppl):S81–96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- [19].Baeuerle PA, Baltimore D. NF-kappa B: ten years after. Cell. 1996;87(1):13–20. doi: 10.1016/s0092-8674(00)81318-5. [DOI] [PubMed] [Google Scholar]
- [20].Lucas PC, McAllister-Lucas LM, Nunez G. NF-kappaB signaling in lymphocytes: a new cast of characters. J Cell Sci. 2004;117(Pt 1):31–39. doi: 10.1242/jcs.00904. [DOI] [PubMed] [Google Scholar]
- [21].Beauparlant P, Hiscott J. Biological and biochemical inhibitors of the NF-kappa B/Rel proteins and cytokine synthesis. Cytokine Growth Factor Rev. 1996;7(2):175–190. doi: 10.1016/1359-6101(96)00020-2. [DOI] [PubMed] [Google Scholar]
- [22].Hong-Li S, Lei L, Lei S, et al. Cardioprotective effects and underlying mechanisms of oxymatrine against Ischemic myocardial injuries of rats. Phytother Res. 2008;22(7):985–989. doi: 10.1002/ptr.2452. [DOI] [PubMed] [Google Scholar]
- [23].Liu F, Liu J, Chen X, et al. 5. Vol. 31. Yixue Ban: Jilin Daxue Xuebao; 2005. Effect of oxymatrine on inflammation and its mechanism; pp. 728–730. [Google Scholar]
- [24].Chen G, Zhang S, Shi J, et al. Simvastatin reduces secondary brain injury caused by cortical contusion in rats: possible involvement of TLR4/NF-kappaB pathway. Exp Neurol. 2009;216(2):398–406. doi: 10.1016/j.expneurol.2008.12.019. [DOI] [PubMed] [Google Scholar]
- [25].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [26].Sharma M, Gupta YK. Intracerebroventricular injection of streptozotocin in rats produces both oxidative stress in the brain and cognitive impairment. Life Sci. 2001;68(9):1021–1029. doi: 10.1016/s0024-3205(00)01005-5. [DOI] [PubMed] [Google Scholar]
- [27].Paxions G, Watson C. Beijing: People's Medical Publishing House; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- [28].Fan H, Li L, Zhang X, et al. Oxymatrine downregulates TLR4, TLR2, MyD88, and NF-kappaB and protects rat brains against focal ischemia. Mediators Inflamm. 2009;2009:704706. doi: 10.1155/2009/704706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Li X, Kong H, Wu W, et al. Aquaporin-4 maintains ependymal integrity in adult mice. Neuroscience. 2009;162(1):67–77. doi: 10.1016/j.neuroscience.2009.04.044. [DOI] [PubMed] [Google Scholar]


