Abstract
Cultured Schwann cells were treated with 5.6 mM and 50 mM glucose alternating every 8 hours to simulate intermittent high glucose. The present study analyzed the neuroprotective effects of 1, 10 and 100 μM ginsenoside Rb1 on oxidative damage and apoptosis in Schwann cells induced by intermittent high glucose. Flow cytometry demonstrated that ginsenoside Rb1 reduced intermittent high glucose-mediated reactive oxygen species production. Enzyme linked immunosorbent assay showed that 8-hydroxy-2-deoxy guanosine levels in Schwann cells decreased following ginsenoside Rb1 treatment. Quantitative real-time reverse transcription-PCR and western blot assay results revealed that ginsenoside Rb1 inhibited intermittent high glucose-upregulated Bax expression, but antagonized intermittent high glucose-downregulated Bcl-2 expression in Schwann cells. These effects were most pronounced with 100 μM ginsenoside Rb1. These results indicate that ginsenoside Rb1 inhibits intermittent high glucose-induced oxidative stress and apoptosis in Schwann cells.
Keywords: ginsenoside Rb1, diabetic peripheral neuropathy, Schwann cells, oxidative stress, apoptosis, glucose fluctuation, Chinese herb, neuroprotection, neural regeneration
Research Highlights
-
(1)
Ginsenoside Rb1 reduces intermittent high glucose-induced reactive oxygen species production in Schwann cells in a dose-dependent manner.
-
(2)
Ginsenoside Rb1 decreases 8-hydroxy-2-deoxy guanosine levels in intermittent high glucose-treated Schwann cells in a dose-dependent manner.
-
(3)
Ginsenoside Rb1 upregulates Bcl-2 expression and downregulates Bax expression in intermittent high glucose-treated Schwann cells in a dose-dependent manner.
INTRODUCTION
Diabetic peripheral neuropathy is one of the most common and troublesome microvascular complications of diabetes; however, its pathogenesis remains unclear. Studies have shown that hyperglycemia, either constant or intermittent, can stimulate high levels of reactive oxygen species production in mitochondria, trigger apoptosis, and lead to neuronal injury[1,2,3]. Inhibition of reactive oxygen species production and mitigation of their function may block both the initiation and progression of neuropathy[4,5].
Ginseng is a well-known and widely used traditional Chinese herb. Most pharmacological actions of ginseng are attributed to ginsenosides, which are major components extracted from different species of ginseng. In recent years, accumulating evidence has shown that ginsenoside Rb1 (Figure 1), as an important active compound, exerts protective actions against the occurrence of oxidative stress in diabetic complications[6,7]. Therefore, we conjectured that ginsenoside Rb1 might relieve diabetic neuropathy by reducing intracellular reactive oxygen species production.
Figure 1.
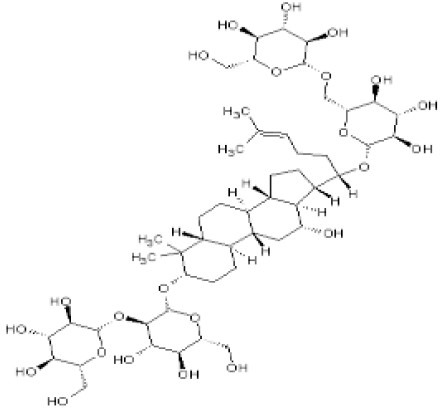
Chemical structure of ginsenoside Rb1.
Schwann cells play an important role in the pathogenesis and development of peripheral neuropathy[5]. Schwann cells are susceptible to hyperglycemia because they take up glucose through the insulin-independent glucose transporter[8,9]. As a support cell for neuronal regeneration and function, Schwann cells might be a natural target for the treatment of diabetic peripheral neuropathy since their defects are reversible[10].
It is now recognized that glucose fluctuation may be more dangerous than constant high glucose[11,12,13]. However, the precise mechanism underlying the impact of intermittent high glucose on Schwann cells is unclear, and whether ginsenoside Rb1 can protect against this process remains to be investigated. This study was aimed at exploring the effects of intermittent high glucose on oxidative stress and Schwann cell apoptosis, and how ginsenoside Rb1 treatment could protect against injury in vitro.
RESULTS
Morphological observation of Schwann cells
Light microscopy revealed the typical bipolar morphology of cultured Schwann cells (Figure 2). Because S-100 protein is often used as a Schwann cell marker, immunocytochemistry with an anti-S-100 antibody was used to assess cell purity. Only second and third passage Schwann cells with a purity of ≥ 95% were used in this study to avoid age-dependent cellular modifications.
Figure 2.

Morphological observation of Schwann cells showing typical bipolar morphology (inverted microscope, × 200).
Ginsenoside Rb1 inhibits intermittent high glucose-induced reactive oxygen species production in Schwann cells in vitro
Cultured Schwann cells were treated in duplicate with 5.6 mM glucose as control, with 5.6 and 50 mM glucose alternating every 8 hours as intermittent high glucose[14], or with intermittent high glucose in the presence of 1, 10 or 100 μM ginsenoside Rb1 for 48 hours as treatment. The intracellular reactive oxygen species content was determined by flow cytometry. The relative level of reactive oxygen species was increased 2.86-fold in the intermittent high glucose group relative to control (P < 0.01), and it was decreased by 36.7% (P < 0.01), 24.2% (P < 0.05) and 10.8% in the 100, 10 and 1 μM ginsenoside Rb1-treated Schwann cells subjected to intermittent high glucose, respectively (Figure 3). Clearly, ginsenoside Rb1 inhibited intermittent high glucose-induced overproduction of reactive oxygen species and oxidative stress in Schwann cells in a dose-dependent manner.
Figure 3.
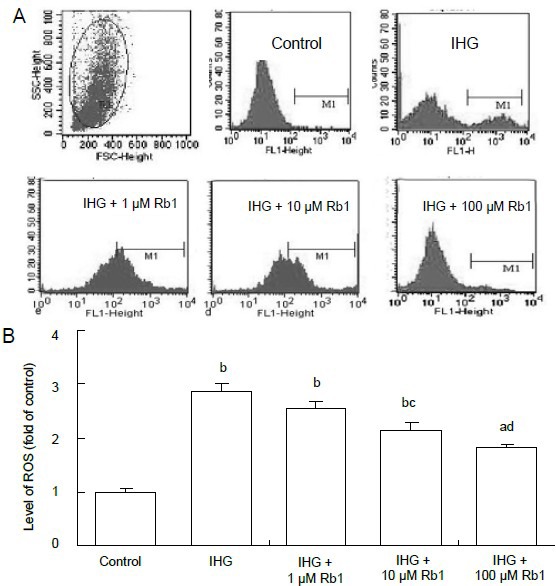
Effect of ginsenoside Rb1 on the relative levels of intracellular reactive oxygen species (ROS) in Schwann cells treated with intermittent high glucose.
ROS were measured by flow cytometry, and fluorescence intensity was analyzed. (A) Representative flow cytometric images. (B) Fluorescence intensity analysis. The value of the control cells was designated as 1.
Data are expressed as mean ± SEM for each group of cells based on three separate experiments. aP < 0.05, bP < 0.01, vs. control group; cP < 0.05, dP < 0.01, vs. intermittent high glucose (IHG) group (one-way analysis of variance followed by Student-Newman-Keuls test).
Ginsenoside Rb1 mitigates the intermittent high glucose-induced increase in 8-hydroxy-2-deoxy guanosine levels in Schwann cells
Oxidative stress can damage DNA, resulting in apoptosis. The oxidized nucleoside 8-hydroxy-2-deoxy guanosine is a sensitive and specific marker of oxidative damage to DNA[15]. To further determine the effect of intermittent high glucose on the induction of oxidative stress, the levels of DNA-related 8-hydroxy-2-deoxy guanosine in the different groups of Schwann cells were detected by enzyme linked immunosorbent assay (ELISA). Our data show that the concentration of 8-hydroxy-2-deoxy guanosine in the intermittent high glucose group was significantly higher than in the control group (P < 0.01), and it was decreased by 32.6% (P < 0.01), 25.1% (P < 0.05) and 11.4% (P < 0.05) when treated with 100, 10 and 1 μM ginsenoside Rb1, respectively (Figure 4). Therefore, ginsenoside Rb1 inhibits intermittent high glucose-related oxidative stress-mediated DNA damage in Schwann cells in a dose-dependent manner.
Figure 4.
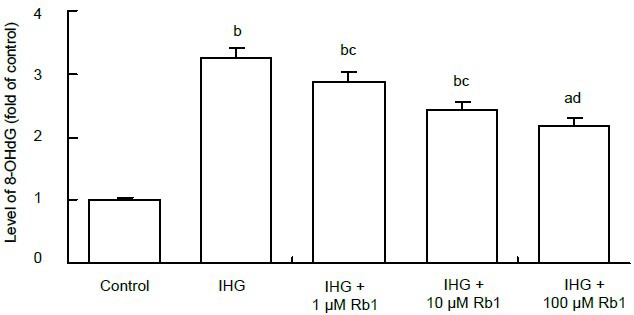
Effect of ginsenoside Rb1 on the relative levels of 8-hydroxy-2-deoxy guanosine (8-OHdG) in Schwann cells treated with intermittent high glucose (IHG).
The concentrations of 8-OHdG were determined by enzyme linked immunosorbent assay. The value of the control cells was designated as 1.
Data are expressed as mean ± SEM for each group of cells based on three separate experiments. aP < 0.05, bP < 0.01, vs. control group; cP < 0.05, dP < 0.01, vs. IHG group (one-way analysis of variance followed by Student-Newman-Keuls test).
Effects of ginsenoside Rb1 on intermittent high glucose-related changes in Bax and Bcl-2 expressions in Schwann cells
To understand the mechanisms underlying the role of ginsenoside Rb1 in intermittent high glucose-related Schwann cell apoptosis, the relative levels of anti-apoptotic Bcl-2 and pro-apoptotic Bax mRNA transcripts were determined by quantitative real-time reverse transcription-PCR (Figures 5A, B). Intermittent high glucose significantly enhanced transcription of the Bax gene, but reduced transcription of the Bcl-2 gene, compared with the control group (P < 0.01). Treatment with Rb1 significantly attenuated the effect of intermittent high glucose on transcription of these genes (P < 0.05 or P < 0.01). Similar effects on Bax and Bcl-2 expression were observed at the protein level in different groups of cells using western blot assay (Figures 5C–E). These two independent lines of evidence demonstrate that ginsenoside Rb1 inhibits intermittent high glucose- mediated changes in Bax and Bcl-2 expression in Schwann cells in vitro.
Figure 5.
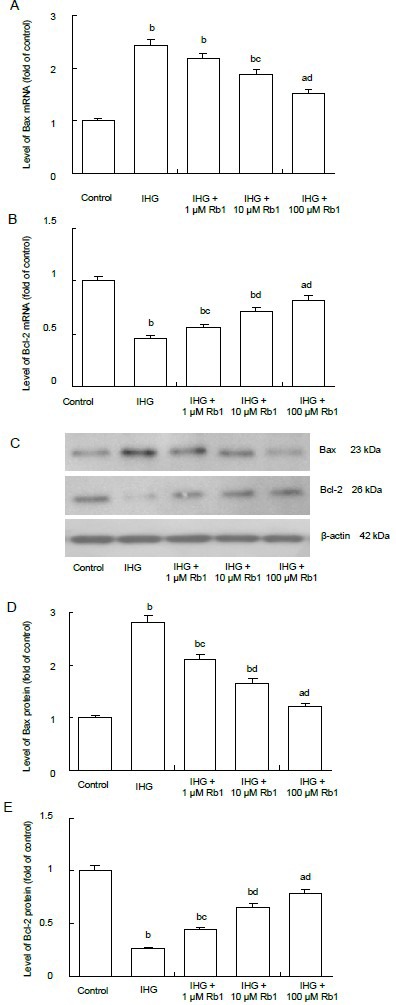
Effect of ginsenoside Rb1 on mRNA and protein expressions of Bax and Bcl-2 in Schwann cells treated with intermittent high glucose.
mRNA and protein expression levels were determined by quantitative real-time reverse transcription-PCR (A, B) and western blot assays (C–E), respectively. GAPDH (for mRNA) and β-actin (for protein) was used as reference.
Data are expressed as mean ± SEM for each group of cells based on three separate experiments, and the value of the control cells was designated as 1. aP < 0.05, bP < 0.01, vs. control group; cP < 0.05, dP < 0.01, vs. intermittent high glucose (IHG) group (one-way analysis of variance followed by Student-Newman-Keuls test).
DISCUSSION
At present, there are few studies on intermittent high glucose-induced oxidative stress and apoptosis in Schwann cells. This study has documented, to our knowledge for the first time, beneficial effects of ginsenoside Rb1 on Schwann cells. We found that cultured Schwann cells exposed to intermittent high glucose exhibited increased apoptosis and oxidative stress damage, suggesting that glucose fluctuations may induce neuropathy and oxidative stress may play a crucial role in this process. Furthermore, ginsenoside Rb1 exerted its protective effects in a dose-dependent manner and inhibited reactive oxygen species production. These findings are important from a clinical perspective and may help broaden our understanding of diabetic peripheral neuropathy.
Traditionally, high-glucose-induced injury has been studied in chronic models. Previous studies mostly focused on the toxic effects of constant high glucose; however, glucose fluctuation is also common and important in patients with diabetes. It is now recognized that “hyperglycemic spikes” may play a direct and significant role in the pathogenesis of diabetic complications, and intermittent high glucose is also associated with the development of Schwann cell injury and diabetic peripheral neuropathy[13,16]. In this study, we proposed a cellular experimental model in which primary cultures of Schwann cells were exposed to conditions that partly mimic glucose fluctuations in vivo in diabetic patients.
To produce hyperglycemic injury of Schwann cells, 30 to 150 mM glucose were used in individual experiments[17,18,19,20]. Our previous results also showed that incubation with 50 mM glucose for 48 hours exerts detrimental effects and significantly inhibits proliferation of Schwann cells[14]. Therefore, we chose 50 mM glucose as the appropriate concentration for high glucose in this study.
Accumulating evidence shows that mitochondria are the main source of endogenous reactive oxygen species in most mammalian cell types[21]. As the hyperglycemic cell metabolizes more glucose, increased turnover of mitochondrial energy-generating complexes occurs, increasing the production of free radicals[22]. In this study, we selected 8-hydroxy-2-deoxy guanosine as a marker to evaluate levels of oxidative stress. In agreement with previous studies[12,23], our study shows that high glucose, and especially intermittent high glucose, induces high levels of reactive oxygen species production, which leads to increased formation of 8-hydroxy-2-deoxy guanosine. Given the crucial role of Schwann cells in the functional integrity and regeneration of nerves, the potent oxidative stress-related cytotoxicity of intermittent high glucose supports the concept that it is a particularly serious risk factor for the development of diabetic complications, including diabetic peripheral neuropathy.
In China, traditional herbs have been widely used for the treatment of diabetes and its complications for thousands of years. Ginsenoside Rb1 is one of the most abundant and bioactive components in Ginseng, which has been considered to have multiple pharmacological activities in vivo and in vitro[24,25,26]. Nevertheless, there is insufficient information on its protective properties in cellular models of diabetic peripheral neuropathy. In this study, we found that ginsenoside Rb1 significantly inhibits intermittent high glucose-stimulated reactive oxygen species production and reduces DNA damage, as shown by reduced 8-hydroxy-2-deoxy guanosine formation, in Schwann cells, in a dose-dependent manner. These data indicate that treatment with ginsenoside Rb1 can reduce oxidative stress in cell models and may be of benefit to patients with diabetic peripheral neuropathy. Given that mitochondrial oxidative stress contributes to the pathogenesis of many chronic diseases, our findings suggest that ginsenoside Rb1 could also be used in the treatment of these chronic diseases.
One of the consequences of cellular responses to hyperglycemia-related stresses is apoptosis[27,28]. Reactive oxygen species are well-known initiators of apoptosis in many cell types[29,30]. Overproduction of reactive oxygen species can upregulate the expression of proapoptotic factors and downregulate the expression of antiapoptotic factors, such as Bax/Bcl-2[31,32]. In the present study, we found that intermittent high glucose significantly upregulates Bax expression, but downregulates Bcl-2 expression in Schwann cells. Furthermore, ginsenoside Rb1 inhibits intermittent high glucose-upregulated Bax expression, but antagonizes intermittent high glucose-downregulated Bcl-2 expression in Schwann cells. The protective effects of ginsenoside Rb1 on apoptotic pathway-related events induced by intermittent high glucose are at least partly mediated by its ability to inhibit the production of reactive oxygen species in Schwann cells. However, further studies are still needed to clarify this process and to characterize the regulatory pathways that are compromised. Taken together, the present study demonstrates that the protective effects of ginsenoside Rb1 on Schwann cells under intermittent hyperglycemic conditions are due to its ability to inhibit oxidative stress and apoptosis, and suggests that ginsenoside Rb1 may be useful in preventing diabetic peripheral neuropathy and other demyelinating diseases characterized by Schwann cell loss and/or abnormalities. Given that reactive oxygen species are considered a causal link between hyperglycemia and numerous metabolic abnormalities in the development of diabetic complications[33,34,35], we hypothesize that ginsenoside Rb1 may be of great use in the prevention and treatment of other diabetic complications as well.
MATERIALS AND METHODS
Design
A cytological, comparative, observational study.
Time and setting
Experiments were performed at the Department of Endocrinology, General Hospital of Chinese PLA, China from September 2010 to September 2011.
Materials
Neonatal Sprague-Dawley rats were obtained from Weitong Lihua Experimental Animal Center (license No. SCXK(Jing)2007-0001). All protocols were conducted in accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, formulated by the Ministry of Science and Technology of China[36].
Methods
Schwann cell culture
Schwann cells were obtained from the sciatic nerves of newborn Sprague-Dawley rats (3 days old) under aseptic conditions as previous described[19]. Cultured cells were identified as Schwann cells by their morphology and immunohistochemistry using anti-S-100 antibody (1:100 dilution; Boster, Wuhan, China). Only second and third passage Schwann cells with a purity of ≥ 95% were used in this study to avoid age-dependent cellular modifications.
Intermittent high glucose induction and ginsenoside Rb1 treatment
Cultured Schwann cells were treated in duplicate with 5.6 mM glucose (Sigma-Aldrich, St. Louis, MO, USA) as the control, with 5.6 and 50 mM glucose alternating every 8 hours as intermittent high glucose[19], or with intermittent high glucose in the presence of 1, 10 or 100 μM Rb1 (purity 98%, eBioscience Ltd, Shanghai, China) for 48 hours as treatment.
Measurement of intracellular reactive oxygen species
The Schwann cells in different culture conditions were harvested and treated with 10 μM 2-7-dichlorofluorescin diacetate (DCFH-DA, Molecular Probes, Eugene, OR, USA) at 37°C for 60 minutes in the dark. The fluorescence intensity was analyzed by flow cytometry (Becton-Dickinson Co, Rutherford, NJ, USA). The reactive oxygen species values were calculated as the relative levels to unlabeled control.
Detection of 8-hydroxy-2-deoxy guanosine concentration
Schwann cells in different culture conditions were harvested and DNA was isolated using a genomic DNA extraction kit (Generay biotechnology, Shanghai, China). The amount of 8-hydroxy-2-deoxy guanosine was determined using a competitive ELISA kit (StressMarq Biosciences, Victoria, Canada), according to the manufacturer's instructions.
Quantitative real-time reverse transcription-PCR
Total RNA was extracted from each group of Schwann cells using Trizol (Invitrogen, Grand Island, NY, USA), and RNA (1 μg) was reverse-transcribed into cDNA using a cDNA synthesis kit (Invitrogen), according to the manufacturer's protocols. The relative level of each mRNA transcript to GAPDH was determined by quantitative real-time reverse transcription-PCR using the SYBR pre-mixed system (Invitrogen) and specific primers (Life Technologies, Shanghai, China). The sequences of the primers were as follows: forward, 5’-GCG TCA ACA GGG AGA TGT CA-3’ and reverse, 5’-GGT ATG CAC CCA GAG TGA TG-3’ for Bcl-2 (225 bp); forward, 5’-GGC GAA TTG GAG ATG AAC TG-3’ and reverse, 5’-GAT CAG CTC GGG CAC TTT AG-3’ for Bax (209 bp); forward, 5’-TGT CTC CTG CGA CTT CAA CAG-3’ and reverse, 5’-GAG GCC ATG TAG GCC ATG AG-3’ for GAPDH (256 bp). The PCR reactions were performed in triplicate at 95°C for 2 minutes and subjected to 40 cycles of 95°C for 20 seconds, 58°C for 20 seconds and 72°C for 25 seconds. The relative levels of each gene mRNA transcript to GAPDH were analyzed using the 2−ΔΔCt method.
Western blot analysis
The relative levels of Bcl-2 and Bax expression were determined by western blot assay. Briefly, Schwann cells in different culture conditions were harvested and lysed. The cell lysates (50 μg/lane) were separated by SDS-PAGE and transferred onto polyvinylidene fluoride membranes (Millpore, Billerica, MA, USA). The membranes were blocked with 5% fat-free milk and incubated with 1:200 diluted mouse monoclonal anti-rat Bcl-2, Bax and β-actin (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at 4°C for 10 hours. The bound antibodies were detected by incubation with 1:5 000 donkey anti-mouse IgG-horseradish peroxidase conjugated secondary antibody (Santa Cruz Biotechnology) for 2 hours at room temperature, then visualized with enhanced chemiluminescence detection reagents (Applygen Technologies, Beijing, China). The relative levels of each protein to β-actin were determined by densitometric analysis using Image J (National Institutes of Health, Rockville, Maryland, USA).
Statistical analysis
All data were expressed as mean ± SEM. Dfferences between groups were analyzed by one-way analysis of variance followed by Student-Newman-Keuls test, using SPSS 13.0 software (SPSS, Chicago, IL, USA). A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This study was supported by the Postdoctoral Science Foundation of China, No. 20090461435.
Conflicts of interest: None declared.
Ethical approval: The study was approved by the Animal Ethics Committee of General Hospital of Chinese PLA, China.
(Edited by Tu QY, Bai H/Qiu Y/Wang L)
REFERENCES
- [1].Edwards JL, Vincent AM, Cheng HT, et al. Diabetic neuropathy: mechanisms to management. Pharmacol Ther. 2008;120(1):1–34. doi: 10.1016/j.pharmthera.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Figueroa-Romero C, Sadidi M, Feldman EL. Mechanisms of disease: the oxidative stress theory of diabetic neuropathy. Rev Endocr Metab Disord. 2008;9(4):301–314. doi: 10.1007/s11154-008-9104-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Vincent AM, McLean LL, Backus C, et al. Short-term hyperglycemia produces oxidative damage and apoptosis in neurons. FASEB J. 2005;196(6):638–640. doi: 10.1096/fj.04-2513fje. [DOI] [PubMed] [Google Scholar]
- [4].Habib AA, Brannagan TH., 3rd Therapeutic strategies for diabetic neuropathy. Curr Neurol Neurosci Rep. 2010;10(2):92–100. doi: 10.1007/s11910-010-0093-7. [DOI] [PubMed] [Google Scholar]
- [5].Vincent AM, Callaghan BC, Smith AL, et al. Diabetic neuropathy: cellular mechanisms as therapeutic targets. Nat Rev Neurol. 2011;7(10):573–583. doi: 10.1038/nrneurol.2011.137. [DOI] [PubMed] [Google Scholar]
- [6].Xiong Y, Shen L, Liu KJ, et al. Antiobesity and antihyperglycemic effects of ginsenoside Rb1 in rats. Diabetes. 2010;59(10):2505–2512. doi: 10.2337/db10-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Wu Y, Xia ZY, Dou J, et al. Protective effect of ginsenoside Rb1 against myocardial ischemia/reperfusion injury in treptozotocin-induced diabetic rats. Mol Biol Rep. 2011;38(7):4327–4335. doi: 10.1007/s11033-010-0558-4. [DOI] [PubMed] [Google Scholar]
- [8].Muona P, Jaakkola S, Salonen V, et al. Expression of glucose transporter 1 in adult and developing human peripheral nerve. Diabetologia. 1993;36(2):133–140. doi: 10.1007/BF00400694. [DOI] [PubMed] [Google Scholar]
- [9].Magnani P, Cherian PV, Gould GW, et al. Glucose transporters in rat peripheral nerve: paranodal expression of GLUT1 and GLUT3. Metabolism. 1996;45(12):1466–1473. doi: 10.1016/s0026-0495(96)90174-2. [DOI] [PubMed] [Google Scholar]
- [10].Gumy LF, Bampton ET, Tolkovsky AM. Hyperglycaemia inhibits Schwann cell proliferation and migration and restricts regeneration of axons and Schwann cells from adult murine DRG. Mol Cell Neurosci. 2008;37(2):298–311. doi: 10.1016/j.mcn.2007.10.004. [DOI] [PubMed] [Google Scholar]
- [11].Shi XL, Ren YZ, Wu J. Intermittent high glucose enhances apoptosis in INS-1 cells. Exp Diabetes Res. 2011;2011:754673. doi: 10.1155/2011/754673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Piconi L, Quagliaro L, Assaloni R, et al. Constant and intermittent high glucose enhances endothelial cell apoptosis through mitochondrial superoxide overproduction. Diabetes Metab Res Rev. 2006;22(3):198–203. doi: 10.1002/dmrr.613. [DOI] [PubMed] [Google Scholar]
- [13].Horváth EM, Benko R, Kiss L, et al. Rapid ‘glycaemic swings’ induce nitrosative stress, activate poly(ADP-ribose) polymerase and impair endothelial function in a rat model of diabetes mellitus. Diabetologia. 2009;52(5):952–961. doi: 10.1007/s00125-009-1304-0. [DOI] [PubMed] [Google Scholar]
- [14].Qu L, Liang XC, Zhang H, et al. Effect of Jinmaitong serum on the proliferation of rat Schwann cells cultured in high glucose medium. Chin J Integr Med. 2008;14(4):293–297. doi: 10.1007/s11655-008-0293-z. [DOI] [PubMed] [Google Scholar]
- [15].Wu LL, Chiou CC, Chang PY, et al. Urinary 8-OHdG: a marker of oxidative stress to DNA and a risk factor for cancer, atherosclerosis and diabetics. Clin Chim Acta. 2004;339(1-2):1–9. doi: 10.1016/j.cccn.2003.09.010. [DOI] [PubMed] [Google Scholar]
- [16].Sumner CJ, Sheth S, Griffin JW, et al. The spectrum of neuropathy in diabetes and impaired glucose tolerance. Neurology. 2003;60(1):108–111. doi: 10.1212/wnl.60.1.108. [DOI] [PubMed] [Google Scholar]
- [17].Delaney CL, Russell JW, Cheng HL, et al. Insulin-like growth factor-I and over-expression of Bcl-xL prevent glucose-mediated apoptosis in Schwann cells. J Neuropathol Exp Neurol. 2001;60(2):147–160. doi: 10.1093/jnen/60.2.147. [DOI] [PubMed] [Google Scholar]
- [18].Suzuki T, Sekido H, Kato N, et al. Neurotrophin-3-induced production of nerve growth factor is suppressed in Schwann cells exposed to high glucose: involvement of the polyol pathway. J Neurochem. 2004;91(6):1430–1438. doi: 10.1111/j.1471-4159.2004.02824.x. [DOI] [PubMed] [Google Scholar]
- [19].Sango K, Suzuki T, Yanagisawa H, et al. High glucose-induced activation of the polyol pathway and changes of gene expression profiles in immortalized adult mouse Schwann cells IMS32. J Neurochem. 2006;98(2):446–458. doi: 10.1111/j.1471-4159.2006.03885.x. [DOI] [PubMed] [Google Scholar]
- [20].Min S, Jian-Bo L, Hong-Man Z, et al. Neuritin is expressed in Schwann cells and down-regulated in apoptotic Schwann cells under hyperglycemia. Nutr Neurosci. doi: 10.1179/1476830512Y.0000000022. in press. [DOI] [PubMed] [Google Scholar]
- [21].Lee HC, Wei YH. Oxidative stress, mitochondrial DNA mutation, and apoptosis in aging. Exp Biol Med (Maywood) 2007;232(5):592–606. [PubMed] [Google Scholar]
- [22].Leinninger GM, Edwards JL, Lipshaw MJ, et al. Mechanisms of disease: mitochondria as new therapeutic targets in diabetic neuropathy. Nat Clin Pract Neurol. 2006;2(11):620–628. doi: 10.1038/ncpneuro0320. [DOI] [PubMed] [Google Scholar]
- [23].Quagliaro L, Piconi L, Assaloni R, et al. Intermittent high glucose enhances apoptosis related to oxidative stress in human umbilical vein endothelial cells. Diabetes. 2003;52(11):2795–2804. doi: 10.2337/diabetes.52.11.2795. [DOI] [PubMed] [Google Scholar]
- [24].Zhu J, Jiang Y, Wu L, et al. Suppression of local inflammation contributes to the neuroprotective effect of ginsenoside Rb1 in rats with cerebral ischemia. Neuroscience. 2012;202:342–351. doi: 10.1016/j.neuroscience.2011.11.070. [DOI] [PubMed] [Google Scholar]
- [25].Lo YT, Tsai YH, Wu SJ, et al. Ginsenoside Rb1 inhibits cell activation and liver fibrosis in rat hepatic stellate cells. J Med Food. 2011;14(10):1135–1143. doi: 10.1089/jmf.2010.1485. [DOI] [PubMed] [Google Scholar]
- [26].Wang Z, Li M, Wu WK, et al. Ginsenoside Rb1 preconditioning protects against myocardial infarction after regional ischemia and reperfusion by activation of phosphatidylinositol-3-kinase signal transduction. Cardiovasc Drugs Ther. 2008;22(6):443–452. doi: 10.1007/s10557-008-6129-4. [DOI] [PubMed] [Google Scholar]
- [27].Kamboj SS, Vasishta RK, Sandhir R. N-acetylcysteine inhibits hyperglycemia- induced oxidative stress and apoptosis markers in diabetic neuropathy. J Neurochem. 2010;112(1):77–91. doi: 10.1111/j.1471-4159.2009.06435.x. [DOI] [PubMed] [Google Scholar]
- [28].Malhotra A, Vashistha H, Yadav VS, et al. Inhibition of p66ShcA redox activity in cardiac muscle cells attenuates hyperglycemia-induced oxidative stress and apoptosis. Am J Physiol Heart Circ Physiol. 2009;296(2):380–388. doi: 10.1152/ajpheart.00225.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Allen DA, Harwood S, Varagunam M, et al. High glucose-induced oxidative stress causes apoptosis in proximal tubular epithelial cells and is mediated by multiple caspases. FASEB J. 2003;17(8):908–910. doi: 10.1096/fj.02-0130fje. [DOI] [PubMed] [Google Scholar]
- [30].Le Bras M, Clément MV, Pervaiz S, et al. Reactive oxygen species and the mitochondrial signaling pathway of cell death. Histol Histopathol. 2005;20(1):205–219. doi: 10.14670/HH-20.205. [DOI] [PubMed] [Google Scholar]
- [31].Mehmeti I, Lenzen S, Lortz S. Modulation of Bcl-2-related protein expression in pancreatic beta cells by pro-inflammatory cytokines and its dependence on the antioxidative defense status. Mol Cell Endocrinol. 2011;332(1):88–96. doi: 10.1016/j.mce.2010.09.017. [DOI] [PubMed] [Google Scholar]
- [32].Azad N, Iyer A, Vallyathan V, et al. Role of oxidative/nitrosative stress-mediated Bcl-2 regulation in apoptosis and malignant transformation. Ann N Y Acad Sci. 2010;1203:1–6. doi: 10.1111/j.1749-6632.2010.05608.x. [DOI] [PubMed] [Google Scholar]
- [33].Folli F, Corradi D, Fanti P, et al. The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: avenues for a mechanistic-based therapeutic approach. Curr Diabetes Rev. 2011;7(5):313–324. doi: 10.2174/157339911797415585. [DOI] [PubMed] [Google Scholar]
- [34].Karasu C. Glycoxidative stress and cardiovascular complications in experimentally-induced diabetes: effects of antioxidant treatment. Open Cardiovasc Med J. 2010;4:240–256. doi: 10.2174/1874192401004010240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615–1625. doi: 10.2337/diabetes.54.6.1615. [DOI] [PubMed] [Google Scholar]
- [36].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]


