Abstract
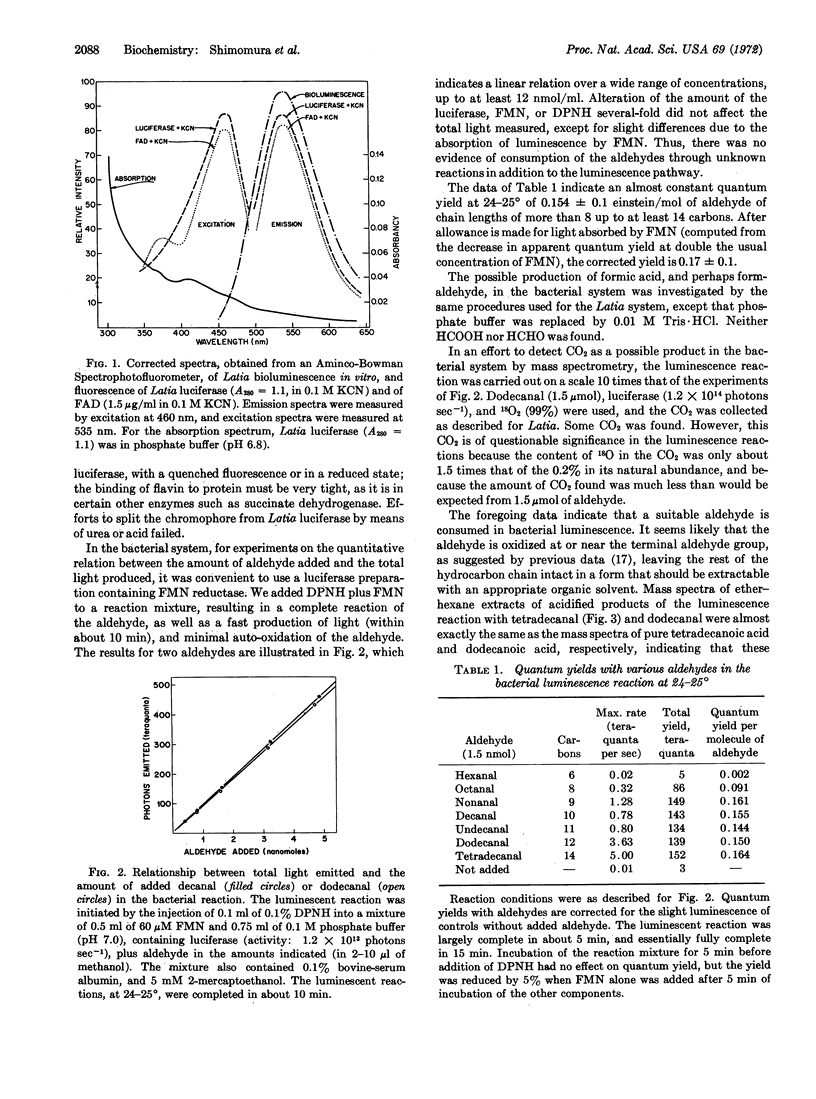
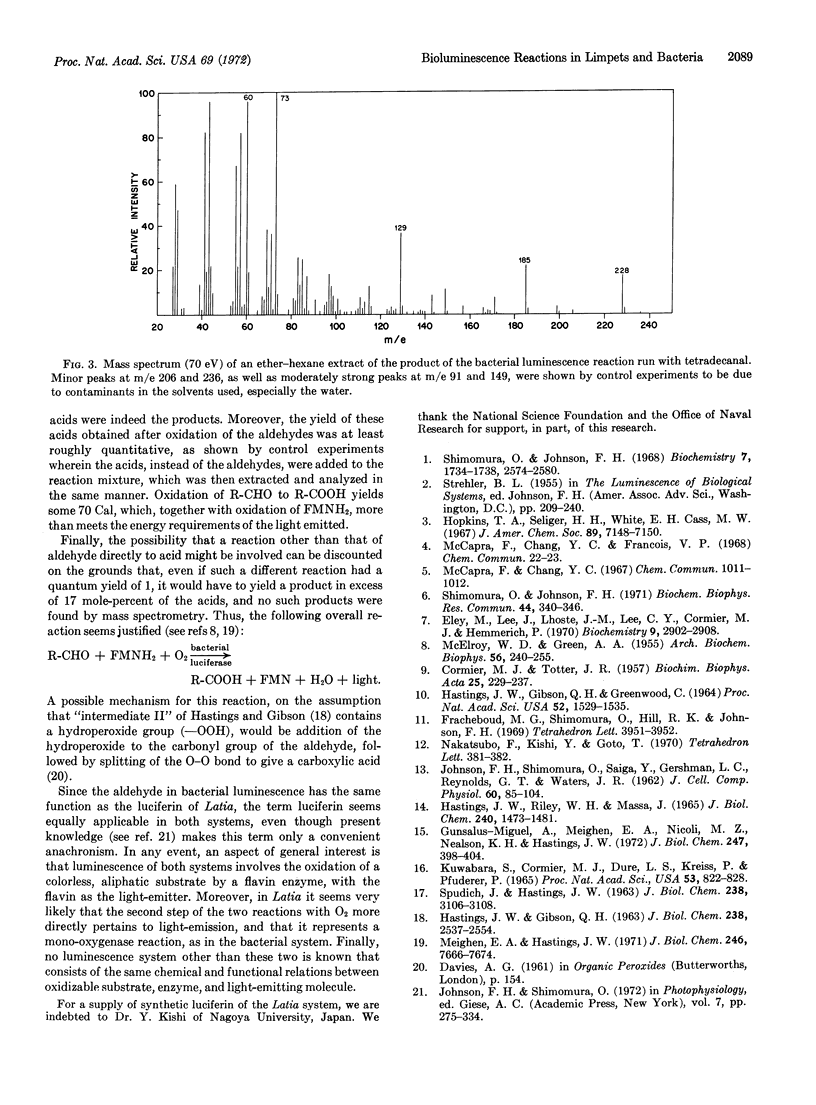
Luminescence in Latia involves a specific flavoprotein enzyme (“luciferase”), which has a tightly bound flavin group constituting the light-emitter. The overall reaction includes oxidation of a specific substrate (“luciferin,” an enol formate derivative of an aliphatic aldehyde), by 2 O2 molecules, in the presence of a “purple protein” cofactor, yielding a ketone, HCOOH, CO2, and light. In Achromobacter, a required aliphatic aldehyde, which is functionally equivalent to Latia luciferin, is oxidized to an acid containing the same hydrocarbon chain as the aldehyde; this reaction proceeds in the presence of bacterial luciferase and reduced flavin mononucleotide with a quantum yield of 0.17 + 0.1 photons per aldehyde molecule that is independent of aldehyde chain length from 9 to at least 14 carbons.
Keywords: Achromobacter fischeri, aldehyde, luciferin, luciferase, flavin, quantum yield
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CORMIER M. J., TOTTER J. R. Quantum efficiency determinations on components of the bacterial luminescence system. Biochim Biophys Acta. 1957 Aug;25(2):229–237. doi: 10.1016/0006-3002(57)90463-8. [DOI] [PubMed] [Google Scholar]
- Eley M., Lee J., Lhoste J. M., Lee C. Y., Cormier M. J., Hemmerich P. Bacterial bioluminescence. Comparisons of bioluminescence emission spectra, the fluorescence of luciferase reaction mixtures, and the fluorescence of flavin cations. Biochemistry. 1970 Jul 7;9(14):2902–2908. doi: 10.1021/bi00816a023. [DOI] [PubMed] [Google Scholar]
- Gunsalus-Miguel A., Meighen E. A., Nicoli M. Z., Nealson K. H., Hastings J. W. Purification and properties of bacterial luciferases. J Biol Chem. 1972 Jan 25;247(2):398–404. [PubMed] [Google Scholar]
- HASTINGS J. W., GIBSON Q. H., GREENWOOD C. ON THE MOLECULAR MECHANISM OF BIOLUMINESCENCE. I. THE ROLE OF LONG-CHAIN ALDEHYDE. Proc Natl Acad Sci U S A. 1964 Dec;52:1529–1535. doi: 10.1073/pnas.52.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HASTINGS J. W., GIBSON Q. H. Intermediates in the bioluminescent oxidation of reduced flavin mononucleotide. J Biol Chem. 1963 Jul;238:2537–2554. [PubMed] [Google Scholar]
- HASTINGS J. W., RILEY W. H., MASSA J. THE PURIFICATION PROPERTIES, AND CHEMILUMINESCENT QUANTUM YIELD OF BACTERIAL LUCIFERASE. J Biol Chem. 1965 Mar;240:1473–1481. [PubMed] [Google Scholar]
- Hopkins T. A., Seliger H. H., White E. H., Cass M. H. The chemiluminescence of firefly luciferin. A model for the bioluminescent reaction and identification of the product excited state. J Am Chem Soc. 1967 Dec 20;89(26):7148–7150. doi: 10.1021/ja01002a076. [DOI] [PubMed] [Google Scholar]
- KUWABARA S., CORMIER M. J., DURE L. S., KREISS P., PFUDERER P. CRYSTALLINE BACTERIAL LUCIFERASE FROM PHOTOBERTERIUM FISCHERI. Proc Natl Acad Sci U S A. 1965 Apr;53:822–828. doi: 10.1073/pnas.53.4.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McELROY W. D., GREEN A. A. Enzymatic properties of bacterial luciferase. Arch Biochem Biophys. 1955 May;56(1):240–255. doi: 10.1016/0003-9861(55)90353-2. [DOI] [PubMed] [Google Scholar]
- Meighen E. A., Hastings J. W. Binding site determination from kinetic data. Reduced flavin mononucleotide binding to bacterial luciferase. J Biol Chem. 1971 Dec 25;246(24):7666–7674. [PubMed] [Google Scholar]
- SPUDICH J., HASTINGS J. W. INHIBITION OF THE BIOLUMINESCENT OXIDATION OF REDUCED FLAVIN MONONUCLEOTIDE BY 2-DECENAL. J Biol Chem. 1963 Sep;238:3106–3108. [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Mechanism of the luminescent oxidation of cypridina luciferin. Biochem Biophys Res Commun. 1971 Jul 16;44(2):340–346. doi: 10.1016/0006-291x(71)90605-x. [DOI] [PubMed] [Google Scholar]
- Shimomura O., Johnson F. H. Purification and properties of the luciferase and of a protein cofactor in the bioluminescence system of Latia neritoides. Biochemistry. 1968 Jul;7(7):2574–2580. doi: 10.1021/bi00847a019. [DOI] [PubMed] [Google Scholar]


