Abstract
Recent studies have shown that tea polyphenols can cross the blood-brain barrier, inhibit apoptosis and play a neuroprotective role against cerebral ischemia. Furthermore, tea polyphenols can decrease DNA damage caused by free radicals. We hypothesized that tea polyphenols repair DNA damage and inhibit neuronal apoptosis during global cerebral ischemia/reperfusion. To test this hypothesis, we employed a rat model of global cerebral ischemia/reperfusion. We demonstrated that intraperitoneal injection of tea polyphenols immediately after reperfusion significantly reduced apoptosis in the hippocampal CA1 region; this effect started 6 hours following reperfusion. Immunohistochemical staining showed that tea polyphenols could reverse the ischemia/reperfusion-induced reduction in the expression of DNA repair proteins, X-ray repair cross-complementing protein 1 and apurinic/apyrimidinic endonuclease/redox factor-1 starting at 2 hours. Both effects lasted at least 72 hours. These experimental findings suggest that tea polyphenols promote DNA damage repair and protect against apoptosis in the brain.
Keywords: global cerebral ischemia/reperfusion, X-ray repair cross-complementing protein 1, apurinic/apyrimidinic endonuclease/redox factor-1, tea polyphenols
Research Highlights
-
(1)
During the early period after global cerebral ischemia/reperfusion, tea polyphenols play an important neuroprotective role by reversing the reduction in the expression of DNA repair proteins X-ray repair cross-complementing protein 1 and apurinic/apyrimidinic endonuclease/redox factor-1.
-
(2)
Tea polyphenols promote DNA damage repair.
-
(3)
Tea polyphenols protect against apoptosis in hippocampal cells.
Abbreviations
XRCC1, X-ray repair cross-complementing protein 1; APE/Ref-1, apurinic/apyrimidinic endonuclease/redox factor-1
INTRODUCTION
Numerous studies have focused on the mechanisms associated with cerebral ischemia/reperfusion injury, including DNA damage/repair and apoptosis[1]. Apoptosis is a highly-regulated, time-dependent progress during cerebral ischemia/reperfusion. The extent of apoptosis increases as reperfusion continues and DNA damage accumulates[2,3]. Furthermore, studies have shown that ischemia impairs DNA repair functions[4,5]. The DNA repair system prevents cell death by repairing damaged DNA, but overwhelming impairment of DNA repair can result in apoptosis[6]. DNA repair, such as base excision repair of DNA single-strand breaks, plays a key role in the maintenance of genomic integrity and the regulation of cell cycle checkpoints following DNA damage[7,8].
The DNA repair protein, X-ray repair cross-complementing protein 1 (XRCC1), plays a critical role in DNA single strand break repair. As an important participant in the base excision repair pathway, XRCC1 up-regulates the activity of DNA ligase III, apurinic/apyrimidinic endonuclease and polynucleotide kinase, and inhibits poly(ADP-ribose) polymerase-1/2 and DNA polymerase β[9]. XRCC1-deficient cells show an increase in cell death and a decrease in single-stranded DNA repair[10]. Previous studies have shown that during brain injury, a reduction in XRCC1 levels at the early stage is an important factor contributing to DNA damage and apoptosis[11].
Apurinic/apyrimidinic endonuclease/redox factor-1 (APE/Ref-1) is a multifunctional enzyme in the base excision repair pathway responsible for repairing apurinic/apyrimidinic sites in DNA, which are generated by oxidative stress after ischemia/reperfusion[12]. During the early stage of cerebral ischemia/reperfusion, free radicals can cause several types of DNA damage, including DNA apurinic/apyrimidinic sites, base damage and single-stranded and double-stranded DNA breaks[13,14]. Vidal et al[15] showed that XRCC1 contributes to apurinic/apyrimidinic site repair by interacting with and stimulating the activity of APE/Ref-1. APE/Ref-1 can recognize and repair apurinic/apyrimidinic sites and base damage, function as a 3’-phosphodiesterase and repair single-stranded and double-stranded DNA breaks[16]. This DNA damage mediated by reactive oxygen species is an important mechanism underlying neuronal DNA damage. APE/Ref-1 is the rate-limiting enzyme in the defense against reactive oxygen species damage[17,18]. Furthermore, APE/Ref-1 also participates in redox regulation by regulating the DNA binding activities of various transcription factors, including activator protein-1 and nuclear factor κB[19]. These transcription factors further contribute to neuronal DNA damage repair and defend the brain against apoptosis.
Tea polyphenols, first isolated from green tea, have a structure similar to glutamate. Recent studies have shown that tea polyphenols can penetrate the blood-brain barrier, inhibit the release of excitatory amino acids, improve microcirculation, reduce apoptosis, interact with inflammatory factors and neurotransmitters, and protect the brain against cerebral ischemia[20,21]. Recent studies have shown that tea polyphenols can effectively suppress spiral ganglion neuron apoptosis by down-regulating the expression of Bax, caspase-3 and caspase-6, and up-regulating the expression of Bcl-2[22,23]. Tea polyphenols can also reduce DNA damage induced by free radicals[23,24,25]. This effect has been attributed to their potent antioxidant and free radical scavenging actions[26]. However, little is known about how tea polyphenols affect DNA damage and neuronal apoptosis during cerebral ischemia/reperfusion.
In this study, we employed a rat model of global cerebral ischemia/reperfusion injury to examine the expression of the DNA repair proteins XRCC1 and APE/Ref-1 in the hippocampal CA1 region during ischemia/reperfusion injury. We then explored the protective role of tea polyphenols in this process. This study contributes to the current understanding of the mechanisms underlying cerebral ischemia/reperfusion injury, and provides insight into how brain damage can be alleviated.
RESULTS
Quantitative analysis of experimental animals
A total of 108 rats were initially included in the study and were randomly divided into three groups: a sham group (SH), an ischemia/reperfusion group (IR) and an ischemia/reperfusion followed by tea polyphenols treatment group (TP). Rats were sacrificed 2, 6, 12, 24, 48 or 72 hours after reperfusion. There were 6 rats per group per time point. All 108 rats were included in the final analysis.
Tea polyphenols reduce apoptosis in the hippocampus induced by cerebral ischemia/ reperfusion injury
We examined apoptosis in the hippocampus using TUNEL staining, and subsequently calculated the apoptosis index (Figure 1). In this experiment, the apoptotic index was used to assess the level of apoptosis. Ischemia/reperfusion significantly increased apoptosis at 6 hours, and the apoptotic level peaked at 48 hours (P < 0.01, vs. SH group). From 6–72 hours, administration of tea polyphenols reduced apoptosis induced by ischemia/reperfusion injury (P < 0.01, vs. IR group).
Figure 1.
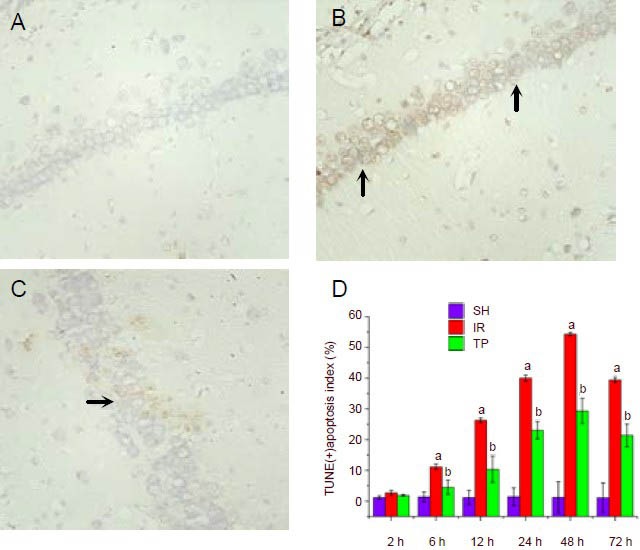
Tea polyphenols reduce apoptosis in the hippocampus induced by cerebral ischemia/reperfusion injury (TUNEL staining, × 400).
Rats were sham treated (SH group), or underwent ischemia/reperfusion (IR group), or underwent ischemia/reperfusion followed by immediate injection of 200 mg/kg tea polyphenols (TP group). Rats were sacrificed at 2, 6, 12, 24, 48 or 72 hours after ischemia/reperfusion, and the apoptotic levels were examined by TUNEL staining. Shown are representative TUNEL staining figures from the hippocampal CA1 region at 48 hours for the SH group (A), IR group (B) and TP group (C).
D shows the percentage of TUNEL-positive apoptotic cells from the three groups at different time points. The hippocampal CA1 region was observed under a light microscope (×400). Five fields of vision were randomly selected to calculate the apoptosis index, defined as the number of apoptotic cells/the total number of cells × 100%.
Arrows indicate TUNEL-positive cells. Under ischemia/reperfusion injury, the apoptotic levels increased significantly at 6 hours and peaked at 48 hours (aP < 0.01, vs. SH group). From 6–72 hours, tea polyphenols administration reduced apoptosis induced by ischemia/reperfusion injury (bP < 0.01, vs. IR group).
Data are presented as mean ± SD, n = 6 per group per time point. Different groups were compared using analysis of variance for two-factor factorial design and least-significant difference t-test.
Tea polyphenols suppress the reduction in XRCC1 expression in the hippocampus induced by cerebral ischemia/reperfusion injury
We next examined the expression of DNA repair protein XRCC1 following ischemia/reperfusion by immunohistochemistry (Figure 2). Under ischemia/ reperfusion injury, the percentage of XRCC1-positive cells decreased significantly from 2–72 hours (P < 0.01, vs. SH group). This reduction could be suppressed by administration of tea polyphenols (P < 0.01, vs. IR group).
Figure 2.
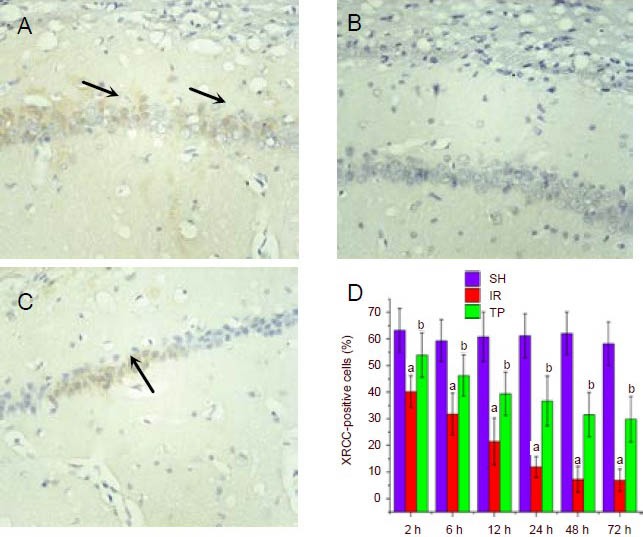
Tea polyphenols suppress the reduction in X-ray repair cross-complementing protein 1 (XRCC1) expression induced by cerebral ischemia/reperfusion injury (immunohistochemistry staining, × 400).
Rats were treated as in Figure 1. Shown are representative XRCC1 immunohistochemistry figures from the hippocampal CA1 region at 48 hours for the SH group (A), IR group (B) and TP group (C). Arrows show XRCC1-positive cells.
D shows the percentage of XRCC1-positive cells from the three groups at different time points. Under ischemia/reperfusion injury, the percentage of XRCC1-positive cells decreased significantly from 2–72 hours (aP < 0.01, vs. SH group). Tea polyphenols suppressed the reduction in XRCC1 expression induced by ischemia/reperfusion injury (bP < 0.01, vs. IR group).
Data are presented as mean ± SD, n = 6 per group per time point. Different groups were compared using analysis of variance for two-factor factorial design and least-significant difference t-test.
SH: Sham treated group; IR: ischemia/reperfusion group; TP: tea polyphenols group.
Tea polyphenols suppress the reduction in APE/Ref-1 expression in the hippocampus induced by cerebral ischemia/reperfusion injury
We further examined the expression of another DNA repair protein, APE/Ref-1, following ischemia/reperfusion by immunohistochemistry (Figure 3). Under ischemia/ reperfusion injury, the percentage of APE/Ref-1-positive cells decreased significantly from 2–72 hours (P < 0.01, vs. SH group). Administration of tea polyphenols suppressed the reduction in APE/Ref-1expression induced by ischemia/reperfusion injury (P < 0.01, vs. IR group).
Figure 3.
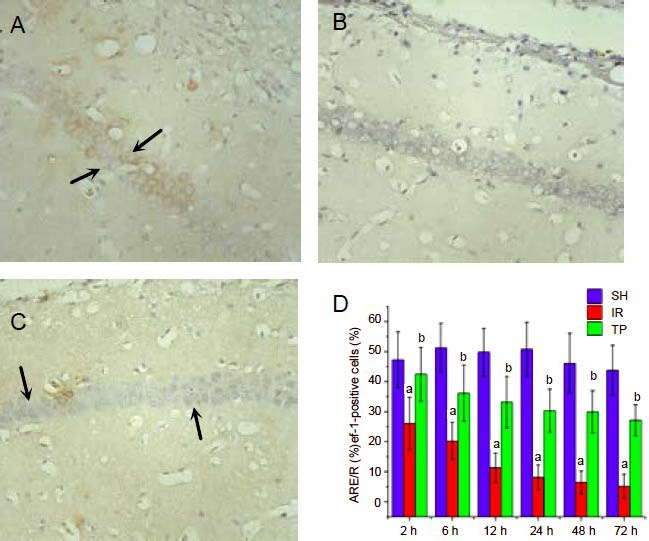
Tea polyphenols suppress the reduction in apurinic/apyrimidinic endonuclease/redox factor-1 (APE/Ref-1) expression induced by cerebral ischemia/reperfusion injury (immunohistochemistry staining, × 400).
Rats were treated as in Figure 1. Shown are representative APE/Ref-1 immunohistochemistry figures from the hippocampal CA1 region at 48 hours for the SH group (A), IR group (B) and TP group (C). Arrows show APE/Ref-1-positive cells.
D shows the percentage of APE/Ref-1-positive cells from the three groups at different time points. Under ischemia/reperfusion injury, the percentage of APE/Ref-1-positive cells decreased significantly from 2- 72 hours (aP < 0.01, vs. SH group). Tea polyphenols suppressed the reduction in APE/Ref-1 expression induced by ischemia/reperfusion injury (bP < 0.01, vs. IR group).
Data are presented as mean ± SD, n = 6 per group per time point. Different groups were compared using analysis of variance for two-factor factorial design and least-significant difference t-test.
SH: Sham treated group; IR: ischemia/reperfusion group; TP: tea polyphenols group.
DISCUSSION
Fujimura et al[27] reported that an early reduction of XRCC1 expression preceded DNA fragmentation in the region supplied by the (occluded) middle cerebral artery, and that this reduction may contribute to DNA damage-induced cell death after transient focal cerebral ischemia in mice.
Our present results indicate that expression of XRCC1 and APE/Ref-1 start to decrease 2 hours after global cerebral ischemia/reperfusion, and remain low for up to 72 hours. Moreover, apoptosis is increased at 6 hours after ischemia/reperfusion, reaching a peak at 48 hours, and showing a subsequent gradual reduction. Combined with results from flow cytometry, we determined that expression of both XRCC1 and APE/Ref-1 negatively correlated with apoptotic rate. Furthermore, the decrease in XRCC1 and APE/Ref-1 expression occurred prior to apoptosis.
These findings provide further evidence that the early reduction of XRCC1 after global cerebral ischemia/reperfusion is associated with the subsequent occurrence of DNA fragmentation. DNA fragmentation is possibly due to a large number of DNA single-strand breaks. The early reduction in XRCC1 protein levels result in reduced DNA single-strand break repair capacity. Consequently, a large number of DNA single-strand breaks accumulate in neurons, leading to DNA fragmentation in the late stage of ischemia/reperfusion, which ultimately results in apoptosis.
Kawase et al[28] demonstrated that APE/Ref-1 decreases after transient g lobal ischemia, and that this reduction precedes apoptosis. Moreover, APE/Ref-1 immunohistochemistry combined with TUNEL staining revealed that all TUNEL-positive CA1 pyramidal neurons were devoid of APE/Ref-1 immunoreactivity. In addition, quantitative analysis revealed that the decrease in the number of APE/Ref-1-immunopositive cells preceded the increase in the number of TUNEL-positive cells. We obtained similar results, suggesting that the loss of APE/Ref-1 and the failure of DNA repair might contribute to neuronal apoptosis in the hippocampal CA1 region a fter global cerebral ischemia.
The exact mechanism by which the reduction of XRCC1 and APE/Ref-1 occurs after ischemia/reperfusion is unclear. A study using the transient focal cerebral ischemia model demonstrated that mice overexpressing the endogenous antioxidant enzyme superoxide dismutase-1 show less reduction of APE/Ref-1 after ischemia/reperfusion compared with wild-type mice[29]. It is well known that reperfusion increases mitochondrial production of superoxide radicals[30]. Therefore, it is conceivable that the mechanism underlying the reduced expression of XRCC1 and APE/Ref-1 is linked to oxidative stress. Further studies of DNA repair using mutant mice deficient in and/or overexpressing SOD are required to clarify this important issue.
Recently, green tea has been reported to have antioxidant properties[31], and many researchers have investigated the cerebral protective activity of tea polyphenols. Previous studies have shown that tea polyphenols protect against DNA damage and reduce neuronal apoptosis after transient cerebral ischemia/reperfusion in gerbils[32,33,34,35].
In our experiment, we employed a rat model of global cerebral ischemia/reperfusion and demonstrated that intraperitoneal injection of tea polyphenols immediately after reperfusion significantly reduces apoptosis in the hippocampal CA1 region. Tea polyphenols suppressed the ischemia/reperfusion-induced reduction in the expression of DNA repair proteins XRCC1 and Ref-1. At 2 hours after cerebral ischemia/reperfusion and intraperitoneal injection of tea polyphenols, XRCC1 and APE/Ref-1 expression were significantly increased in the TP group compared with the IR group, and remained significantly elevated up to 72 hours. These trends are consistent with the changes in the apoptotic levels revealed by TUNEL staining, suggesting that tea polyphenol-induced up-regulation of XRCC1 and Ref-1 may play an important role in reducing apoptosis during ischemia/reperfusion.
The mechanisms underlying the neuroprotective effect of tea polyphenols are not fully understood. Nonetheless, it seems that the potent antioxidant effects of tea polyphenols contribute to its neuroprotective effect against neuronal damage after cerebral ischemia/reperfusion[36]. We propose that oxidative stress decreases XRCC1 and APE/Ref-1 expression in ischemia/reperfusion, and that tea polyphenols exert their antioxidant effects by increasing expression of these critical proteins, which leads to reduced apoptosis in the hippocampal CA1. However, further experiments are required to clarify this concept.
In conclusion, during global cerebral ischemia/ reperfusion, tea polyphenols play an important neuroprotective role by suppressing the reduction in the expression of the DNA repair proteins XRCC1 and APE/Ref-1. This promotes DNA damage repair and protects against apoptosis in the brain. Future studies are required to examine how tea polyphenols affect the expression of XRCC1 and APE/Ref-1.
MATERIALS AND METHODS
Design
A randomized, controlled animal experiment.
Time and setting
The study was performed at Medical College of Xi’an Jiaotong University, China, from March 2009 to January 2010.
Materials
Animals
All the study procedures were in compliance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23). Healthy male Sprague-Dawley rats, aged 55–65 days and weighing 290–310 g, were provided by the Experimental Animal Center of Xi’an Jiaotong University School of Medicine, China (certificate No. SCXK (Shaan) 2007-001). All rats were fasted overnight and had free access to water the night before the operation.
Reagents
Tea polyphenols (powder) were purchased from Shanghai Yuanye Biological Technology Co., Ltd., Shanghai, China; CAS 84650-60-2. Tea polyphenols (25 g) and double distilled water (500 mL) were added into a beaker on a magnetic stirrer and stirred until the tea polyphenols were completely dissolved. This 5% tea polyphenols solution was transferred to a bottle, sealed under sterile conditions, and stored at 4°C.
Methods
Rat model of global cerebral ischemia/reperfusion and drug administration
A Sprague-Dawley rat model of global cerebral ischemia/reperfusion was established using the four-vessel occlusion method as previously described[37]. Briefly, for rats from the IR and TP groups, the carotid artery was occluded using a micro arterial clamp. 5 minutes later, the clamp was loosened to restore cerebral artery blood flow. For rats in the SH group, the bilateral carotid arteries and vertebral arteries were exposed, but the bilateral carotid arteries were not occluded and the vertebral arteries were not cauterized. Immediately after cerebral ischemia/reperfusion, rats received intraperitoneal injection of 4 mL/kg of saline (for the SH and IR groups) or 4 mL/kg (200 mg/kg) of 5% tea polyphenols solution (for the TP group).
Brain tissue preparation
Rats were sacrificed at the indicated time points with heart perfusion of 200 mL of 4% paraformaldehyde. The brain regions that contained the hippocampal CA1 region (1–4 mm posterior to the chiasma opticum) were quickly removed and further fixed for 12 hours in 4% paraformaldehyde at 4°C. Post-fixed brains were embedded in paraffin, followed by preparation of 5-μm thick coronal sections using a microtome.
TUNEL staining
The hippocampal paraffin blocks were dewaxed in xylene and ethanol, digested with Proteinase K (20 mg/L) at room temperature, treated with hydrogen peroxide in methanol, incubated first with terminal deoxynucleotidyl transferase and biotinylated nucleotide (biotin-11-dUTP), then with streptavidin-biotin horseradish peroxidase solution, and finally developed with diaminobenzidine. Cells with dark brown nuclei were apoptotic cells. The hippocampal CA1 region was observed under a light microscope (× 400; B-type biomicroscope; Global Motic Group, Xiamen, China). Five fields were randomly selected to calculate the apoptosis index, defined as the number of apoptotic cells/the total number of cells × 100%.
TUNEL apoptosis detection kit was purchased from Promega Corp., WI, USA. Streptavidin biotin complex (rabbit IgG)-peroxidase kit was from Boster Biotechnology, Wuhan, Hubei Province, China. Diaminobenzidine substrate kit was purchased from Beijing Biosynthesis Biotechnology Co., Ltd., China.
Immunohistochemistry
The hippocampal paraffin blocks were sectioned and processed for immunohistochemistry using the avidin-biotin-peroxidase method[38]. Briefly, sections were dewaxed, treated with hydrogen peroxide in methanol for 10 minutes, washed with triple distilled water for 5 minutes, incubated in PBS (pH 7.4) for 5 minutes, and heated to 95°C for 15–20 minutes. After being cooled down to room temperature, the sections were first blocked with normal goat serum at room temperature for 1 hour, and then incubated with rabbit anti-XRCC1 polyclonal antibody (1:100; Beijing Biosynthesis Biotechnology Co., Ltd.) or rabbit anti-Ref-1 polyclonal antibody (1:150) at 4°C overnight. After three 5-minute washes in PBS, sections were incubated with biotinylated goat anti-rabbit secondary antibody (1:100) at 37°C for 1 hour. After three 5-minute washes in PBS, sections were incubated with streptavidin biotin complex at 37°C for 20 minutes, washed three times with triple distilled water for 5 minutes each, and developed with diaminobenzidine. Cells with brown granules in the cytoplasm were XRCC1- or APE/Ref-1-positive cells. The hippocampal CA1 region was observed under a light microscope (× 400). Five fields were randomly selected to calculate the percentage of positive cells, defined as the number of positive cells/the total number of cells × 100%.
Rabbit anti-XRCC1 polyclonal antibody and rabbit anti-Ref-1 polyclonal antibody were purchased from Beijing Biosynthesis Biotechnology Co., Ltd., China.
Statistical analysis
Data were analyzed using SPSS13.0 software (SPSS, Chicago, IL, USA). Different groups were compared using analysis of variance for two-factor factorial design and least-significant difference t-test. P < 0.05 was considered statistically significant.
Acknowledgments:
We would like to thank Ming Li, from the Department of Anatomy, Medical College of Xi’an Jiaotong University, China, for technical support.
Footnotes
Funding: This study was supported by the National Natural Science Foundation of China, No. 30571790.
Conflicts of interest: None declared.
Ethical approval: This study was approved by the Medical Ethics Committee of Xi’an Jiaotong University, China.
(Edited by Su SW, Li NX/Yang Y/Wang L)
REFERENCES
- [1].Liu ZA, Xu J, Shen XJ, et al. CaMKII antisense oligodeoxynucleotides protect against ischemia-induced neuronal death in the rat hippocampus. J Neurol Sci. 2012;314(1-2):104–110. doi: 10.1016/j.jns.2011.10.012. [DOI] [PubMed] [Google Scholar]
- [2].Doi k. Mechanisms of neurotoxicity induced in the developing brain of mice and rats by DNA-damaging chemicals. J Toxicol Sci. 2011;36(6):695–712. doi: 10.2131/jts.36.695. [DOI] [PubMed] [Google Scholar]
- [3].He KY, Yang SZ, Shen DH, et al. Excision repair cross-complementing 1 expression protects against ischemic injury following middle cerebral artery occlusion in the rat brain. Gene Ther. 2009;16(7):840–848. doi: 10.1038/gt.2009.48. [DOI] [PubMed] [Google Scholar]
- [4].Chopp M, Chan PH, Hsu CY, et al. DNA damage and repair in central nervous system injury: National Institute of Neurological Disorders and Stroke Workshop Summary. Stroke. 1996;27(3):363–369. doi: 10.1161/01.str.27.3.363. [DOI] [PubMed] [Google Scholar]
- [5].Kruman II, Schwartz E, Kruman Y, et al. Suppression of uracil-DNA glycosylase induces neuronal apoptosis. J Biol Chem. 2004;279(42):43952–43960. doi: 10.1074/jbc.M408025200. [DOI] [PubMed] [Google Scholar]
- [6].Gobbel GT, Bellinzona M, Vogt AR, et al. Response of postmitotic neurons to x-irradiation: implications for the role of DNA damage in neuronal apoptosis. J Neurosci. 1998;18(1):147–155. doi: 10.1523/JNEUROSCI.18-01-00147.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Harrison JF, Rinne ML, Kelley MR, et al. Altering DNA base excision repair: use of nuclear and mitochondrial- targeted N-methylpurine DNA glycosylase to sensitize astroglia to chemotherapeutic agents. Glia. 2007;55(14):1416–1425. doi: 10.1002/glia.20556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Houtgraaf JH, Versmissen J, van der Giessen WJ. A concise review of DNA damage checkpoints and repair in mammalian cells. Cardiovasc Revasc Med. 2006;7(3):165–172. doi: 10.1016/j.carrev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- [9].Yang Y, Candelario-Jalil E, Thompson JF, et al. Increased intranuclear matrix metalloproteinase activity in neurons interferes with oxidative DNA repair in focal cerebral ischemia. J. Neurochem. 2010;112(1):134–149. doi: 10.1111/j.1471-4159.2009.06433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Thompson LH, Brookman KW, Jones NJ, et al. Molecular cloning of the human XRCC1 gene, which corrects defective DNA strand break repair and sister chromatid exchange. Mol Cell Biol. 1990;10(2):6160–6171. doi: 10.1128/mcb.10.12.6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Yu SW, Andrabi SA, Wang H, et al. Apoptosis-inducing factor mediates poly (ADP-ribose) (PAR) polymer-induced cell death. Proc Natl Acad Sci U S A. 2006;103(48):18314–18319. doi: 10.1073/pnas.0606528103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kim HW, Cho KJ, Park SC, et al. The adenoviral vector-mediated increase in apurinic/apyrimidinic endonuclease inhibits the induction of neuronal cell death after transient ischemic stroke in mice. Brain Res. 2009;1274:11–18. doi: 10.1016/j.brainres.2009.04.006. [DOI] [PubMed] [Google Scholar]
- [13].Liu D, Croteau DL, Souza-Pinto N, et al. Evidence that OGG1 glycosylase protects neurons against oxidative DNA damage and cell death under ischemic conditions. Cereb Blood Flow Metab. 2011;31(2):680–692. doi: 10.1038/jcbfm.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shimizu S, Saito M, Kinoshita Y, et al. Acute urinary retention and subsequent catheterization cause lipid peroxidation and oxidative DNA damage in the bladder: preventive effect of edaravone, a free-radical scavenger. BJU Int. 2009;104(5):713–717. doi: 10.1111/j.1464-410X.2009.08471.x. [DOI] [PubMed] [Google Scholar]
- [15].Vidal AE, Boiteux S, Hickson AD, et al. XRCC1 coordinates the initial and late stages of DNA abasic site repair through protein-protein interaction. EMBO J. 2001;20(22):6530–6539. doi: 10.1093/emboj/20.22.6530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Huang D, Shenoy A, Cui J, et al. In situ detection of AP sites and DNA strand breaks bearing 3’-phosphate termini in ischemic mouse brain. FASEB J. 2000;14:407–417. doi: 10.1096/fasebj.14.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Xanthoudakis S, Miao G, Wang F, et al. Redox activation of Fos-Jun DNA binding activity is mediated by a DNA repair enzyme. EMBO J. 1992;11(9):3323–3335. doi: 10.1002/j.1460-2075.1992.tb05411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Walker LJ, Robson CN, Black E, et al. Identification of residues in the human DNA repair enzyme HAP1 (Ref-1) that are essential for redox regulation of Jun DNA binding. Mol Cell Biol. 1993;13(9):5370–5376. doi: 10.1128/mcb.13.9.5370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Chang YY, Fujimura M, Morita-Fujimura Y. Neuroprotective effects of an antioxidant in cortical cerebral ischemia: prevention of early reduction of the apurinic/apyrimidinic endonuclease DNA repair enzyme. Neurosci Lett. 1999;277(1):61–64. doi: 10.1016/s0304-3940(99)00799-5. [DOI] [PubMed] [Google Scholar]
- [20].Lee H, Bae JH, Lee SR. Protective effect of green tea polyphenol EGCG against neuronal damage and brain edema after unilateral cerebral ischemia in gerbils. J Neurosci Res. 2004;77(6):892–900. doi: 10.1002/jnr.20193. [DOI] [PubMed] [Google Scholar]
- [21].Zhang S, Liu YH, Zhao Z, et al. Effects of green tea polyphenols on caveolin-1 of microvessel fragments in rats with cerebral ischemia. Neurol Res. 2010;32 (9):963–970. doi: 10.1179/016164110X12700393823570. [DOI] [PubMed] [Google Scholar]
- [22].Khalatbary AR, Tiraihi T, Boroujeni MB, et al. Effects of epigallocatechin gallate on tissue protection and functional recovery after contusive spinal cord injury in rats. Brain Res. 2010;1306:168–175. doi: 10.1016/j.brainres.2009.09.109. [DOI] [PubMed] [Google Scholar]
- [23].Noda Y, Anzai K, Mori A, et al. Hydroxyl and superoxide anion radical scavenging activities os natural source antioxidants using the computerized JES-FR30 ESR spectrometer system. Biochen Mol Biol Int. 1997;42(1):35–44. doi: 10.1080/15216549700202411. [DOI] [PubMed] [Google Scholar]
- [24].Sott BC, Butler J, Halliwell B, et al. Evaluation of the antioxidant actions of ferulic acid and catechins. Free Radic Res. 1993;19(4):241–253. doi: 10.3109/10715769309056512. [DOI] [PubMed] [Google Scholar]
- [25].Tournaire C, Croux S, Maurette MT, et al. Antioxidant activity of flavonoids-efficiency of singdlet oxygen (1-(delta)g) quenching. J Photo Chem Photociol B. 1993;19(3):205–215. doi: 10.1016/1011-1344(93)87086-3. [DOI] [PubMed] [Google Scholar]
- [26].Harvey BS, Musgrave IF, Ohlsson KS, et al. The green tea polyphenol (-)-epigallocatechin-3-gallate inhibits amyloid-β evoked fibril formation and neuronal cell death in vitro. Food Chem. 2011;129(4):1729–1736. [Google Scholar]
- [27].Fujimura M, Morita-Fujimura Y, Sugawara T, et al. Early Decrease of XRCC1, a DNA base excision repair protein, may contribute to dna fragmentation after transient focal cerebral ischemia in mice. Stroke. 1999;30(11):2456–2462. doi: 10.1161/01.str.30.11.2456. [DOI] [PubMed] [Google Scholar]
- [28].Kawase M, Fujimura M, Morita-Fujimura Y, et al. Reduction of apurinic/apyrimidinic endonuclease expression after transient global cerebral ischemia in rats implication of the failure of dna repair in neuronal Apoptosis. Stroke. 1999;30(2):441–448. doi: 10.1161/01.str.30.2.441. [DOI] [PubMed] [Google Scholar]
- [29].Fujimura M, Morita-Fujimura Y, Narasimhan P, et al. Copper-zinc superoxide dismutase prevents the early decrease of apurinic/apyrimidinic endonuclease and subsequent DNA fragmentation after transient focal cerebral ischemia in mice. Stroke. 1999;30(11):2408–2415. doi: 10.1161/01.str.30.11.2408. [DOI] [PubMed] [Google Scholar]
- [30].Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27(6):1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- [31].Mandel SA, Amit T, Weinreb O, et al. Understanding the broad-spectrum neuroprotective action profile of green tea polyphenols in aging and neurodegenerative diseases. J Alzheimers Dis. 2011;25(2):187–208. doi: 10.3233/JAD-2011-101803. [DOI] [PubMed] [Google Scholar]
- [32].Hong JT, Ryu SR, Kim HJ, et al. Protective effect of green tea extract on ischemia/reperfusion-induced brain injury in Mongolian gerbils. Brain Res. 2001;888(1):11–18. doi: 10.1016/s0006-8993(00)02935-8. [DOI] [PubMed] [Google Scholar]
- [33].Choi YT, Jung CH, Lee SR, et al. The green tea polyphenol (-)-epigallocatechin gallate attenuates beta-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sci. 2001;70(5):603–614. doi: 10.1016/s0024-3205(01)01438-2. [DOI] [PubMed] [Google Scholar]
- [34].Lee SR, Suh SI, Kim SP. Protective effects of the green tea polyphenol (-)-epigallocatechin gallate against hippocampal neuronal damage after transient global ischemia in gerbils. Neurosci Lett. 2000;287(3):191–194. doi: 10.1016/s0304-3940(00)01159-9. [DOI] [PubMed] [Google Scholar]
- [35].Katiyar SK, Vaid M, van Steeg H, et al. Green tea polyphenols prevent UV-induced immunosuppression by rapid repair of DNA damage and enhancement of nucleotide excision repair genes. Cancer Prev Res. 2010;3(2):179–189. doi: 10.1158/1940-6207.CAPR-09-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Sutherland BA, Rahman RMA, Appleton I. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. J Nutr Biochem. 2006;17(5):291–306. doi: 10.1016/j.jnutbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- [37].Li C. Beijing: People's Medical Publishing House; 2008. Replication of Animal Models for Human Diseases. [Google Scholar]
- [38].Phanithi PB, Yoshida Y, Santana A, et al. Mild hypothermia mitigates post-ischemic neuronal death following focal cerebral ischemia in rat brain: immunohistochemical study of Fas, caspase-3 and TUNEL. Neuropathology. 2000;20:273–282. doi: 10.1046/j.1440-1789.2000.00346.x. [DOI] [PubMed] [Google Scholar]


