Abstract
Studies have shown that pre-moxibustion protects the gastric mucosa by up-regulating the expression of heat shock protein 70. However, the signaling pathway underlying this effect remains unclear. Rats were intragastrically administered absolute alcohol, causing obvious lesion of the gastric mucosa. Following pre-moxibustion at Zusanli (ST36) for 8 days, the ulcer index decreased to different degrees. The results of an enzyme linked immunosorbent assay and western blotting showed significant upregulation of heat shock protein 70 expression in the gastric mucosa and serum. None out of transection of the spinal cord, damage to the nucleus of the solitary tract, neurotomy of the vagal nerve and neurotomy of the common peroneal nerve affected the decrease in ulcer index or the increase in heat shock protein 70 expression in serum after pre-moxibustion at Zusanli, and heat shock protein 70 expression was obviously decreased in the gastric mucosa. These findings suggest that pre-moxibustion at Zusanli can protect the gastric mucosa against lesioning, and that the mechanism underlying this effect involves its induction of heat shock protein 70 expression. Neural pathways participate in the regulatory effects of moxibustion on heat shock protein 70 expression in the gastric mucosa.
Keywords: pre-moxibustion, transection of neural pathway, Zusanli (ST36), gastric mucosal lesion, heat shock proteins-70, ulcer index, traditional Chinese medicine, neural regeneration
Research Highlights
-
(1)
Moxibustion stimulation transformed into bioinformation related to outcomes activated the protective potential of the body through various pathways, and protected the gastric mucosa against lesion.
-
(2)
This study explored the correlation between endogenous protective information and neural pathways by studying the influence of the afferent pathways of damaged nerves on the protective effects of moxibustion.
INTRODUCTION
Chemical and physical factors, microorganism infection, bacterial toxins, and oral drugs can cause acute inflammatory reaction of the gastric mucosa, lead to gastric mucosal lesions. After formation of gastric mucosal lesions, cell proliferation occurs at multiple areas around the bottom and cervical parts of the gastric gland[1,2,3].
Among members of the heat shock protein family, heat shock protein 70 is involved in the stress response, and is important for protection of the gastric mucosa[4,5,6]. Nisitani et al[7] first showed that cultured gastric mucosal cells can resist excoriation and acute necrosis, caused by ethanol of lethal concentration, after heat shock treatment, which induces heat shock protein expression. Heat shock protein 70 is strongly expressed in post-transplanted alvine mucosal epithelium of the small intestine and inflammatory cells; therefore, heat shock protein 70 is considered to play an important role in the regeneration of the mucous membrane[8]. The inducement of heat shock protein in rat stomach occurs in proportion to the protection of the gastric mucosa[9]. Tsukimi et al[10] reported that heat shock protein 70 expression in the ulcer margin was gradually increased during ulcer healing. Overexpression of heat shock protein 70 in the stomach seems to protect against gastric ulcers through its cytoprotective effects on the gastric mucosa by increasing mucosal blood flow[11]. Accumulation of heat shock protein 70 can accelerate the restoration of the gastric mucosa through regulating apoptosis and rebuilding cytoarchitecture[12]. Therefore, heat shock protein 70 is considered to play an important role in the recovery from gastric mucosal lesions[13,14,15,16].
Pre-moxibustion is named “Ni-jiu” in Chinese. The Chinese term means using the method of pre-moxibustion to guard against disease. It is a traditional Chinese medicine treatment that aims to excite the Qi in channels, to promote immunity and irritability, and to prevent sickness and alleviate the injury caused by illness using moxibustion when disease has not occurred or the illness is minor. Modern traditional Chinese medicine experts and practitioners have studied the effects of and mechanisms underlying pre-moxibustion warming and invigorating of the spleen and stomach from various angles. Pre-moxibustion to prevent gastric mucosal lesions is an effective naturopathy, which has been used from ancient to modern times. The modern theory of stress considers that pre-moxibustion can arouse body potentials, and that it can also arouse and regulate intrinsic resistance against diseases. In these processes, the irritated responsiveness can mobilize an intrinsic prevention mechanism, part of the defense reflex[17]. The study of the effects of pre-moxibustion on gastric mucosal lesions and the underlying mechanisms has become a research hotspot in the field of research into the mechanisms by which moxibustion prevents sickness[18,19,20].
The nervus peroneus communis, spinal cord, nucleus of the solitary tract and vagus nerve are all involved in transducing signals from the splanchnic nerves[21,22,23]. In this study, neurotomy of these parts of the rats’ central and peripheral nervous systems was performed; then, we carried out pre-moxibustion at acupoint Zusanli (ST36). After acute gastric mucosal lesion models were made, endogenous heat shock protein 70 in the gastric mucosa and serum was examined. Using this method, the relationship between the effect of pre-moxibustion on gastric mucosal lesions and neural regulation was explored.
RESULTS
Quantitative analysis of experimental animals
A total of 70 Sprague-Dawley rats were randomly divided into seven groups: control, model, moxibustion, moxibustion + transection of spinal cord, moxibustion + damaged nucleus of the solitary tract, moxibustion + vagotomy and moxibustion + neurotomy of the common peroneal nerve. Neurotomy of the spinal cord, nucleus of solitary tract, vagus, and common peroneal nerve were performed in rats from the last four groups before pre-moxibustion at acupoint Zusanli was carried out for 8 days. Acute gastric mucosal lesion models were made in all groups except the control group. All 70 rats were involved in the final analysis.
Changes in gastric mucosal lesions following transection of various neural pathways and moxibustion pretreatment
As shown in Table 1, considerable damage appeared in the gastric mucosa of animals in the model group. Damage in the gastric mucosa of animals in the moxibustion, moxibustion + transection of spinal cord, moxibustion + damaged nucleus of solitary tract, moxibustion + vagotomy and moxibustion + neurotomy of common peroneal nerve groups was decreased compared with that in the model group. The amounts of damage in the gastric mucosa in the moxibustion + transection of spinal cord, moxibustion + damaged nucleus of the solitary tract, moxibustion + vagotomy, and moxibustion + neurotomy of the common peroneal nerve groups were greater than that in the moxibustion group.
Table 1.
Ulcer index and heat shock protein 70 (HSP70) expression in serum and the gastric mucosa
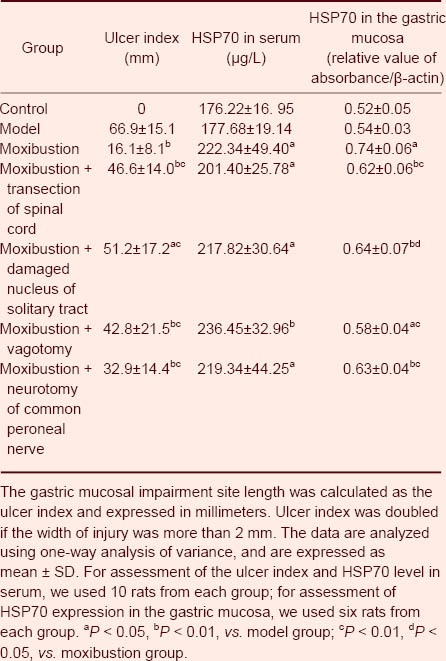
Heat shock protein 70 expression in the serum and gastric mucosa following transection of various neural pathways
There was no difference in the level of expression of heat shock protein 70 in the gastric mucosa between the model and control groups. The level of expression of heat shock protein 70 in the serum and gastric mucosa of animals in the moxibustion, moxibustion + transection of the spinal cord, moxibustion + damaged nucleus of the solitary tract, moxibustion + vagotomy and moxibustion + neurotomy of the common peroneal nerve groups was significantly greater than the levels in the model group. The level of heat shock protein 70 expression in the serum of operation groups (moxibustion + transection of the spinal cord, moxibustion + damaged nucleus of the solitary tract, moxibustion + vagotomy, and moxibustion + neurotomy of the common peroneal nerve group) was not significantly different from that in the moxibustion group, but the level of heat shock protein 70 expression in the gastric mucosa of operation groups was lower than that in the moxibustion group (Table 1, Figure 1).
Figure 1.
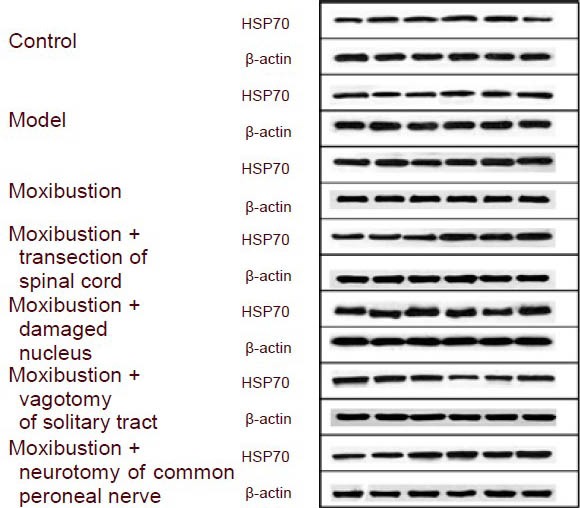
Heat shock protein 70 (HSP70) expression in the gastric mucosa (Western blot: HSP70, 70 kDa; β-actin, 43 kDa).
DISCUSSION
A previous study showed that moxibustion at Zusanli and Liangmen (ST21) has a protective effect on the gastromucosa in stressed rats with gastric ulcers, and that this effect is strongly associated with its actions in promoting the synthesis of tumor growth factor-α and proliferation of gastromucosal cells, suppressing apoptosis of gastric mucosa cells and upregulating heat shock protein 70 mRNA expression[24]. Moxibustion at Zusanli and Liangmen can protect the gastric mucosa through inducing high expression of heat shock protein 70 protein and mRNA, with relative specificity of the acupoints[20].
Moxibustion can protect the gastric mucosa from oxidative injury by inducing heat shock protein 70 expression with relative specificity of acupoints[25]. Moxibustion may up-regulate heat shock protein 70 expression in the gastric mucosa, and then act on targets associated with the mitochondrial signal transduction pathway to inhibit gastric mucosal cell apoptosis and protect the gastric mucosa. This protective effect is acupoint specific to some extent[26]. While heat shock protein 70 expression in the gastric mucosa was up-regulated by treatment with pre-moxibustion, cell apoptosis was suppressed in rats with acute gastric mucosal injuries. However, after heat shock protein 70 expression was blocked by quercetin, the effect of pre-moxibustion on apoptosis was canceled. This study confirmed that pre-moxibustion has a protective effect on the gastric mucosa, and that the effect is correlated with up-regulation of heat shock protein 70 expression[18].
Furthermore, moxibustion at Zusanli and Liangmen has been shown to increase heat shock protein 70 expression in organs and heat shock protein 70 mRNA levels in the rat gastric mucosa, which may be one of the mechanisms by which it promotes health[27]. In summary, some studies have suggested that the mechanism underlying the effect of pre-moxibustion on gastric mucosal lesions may occur through neural regulation, but the precise pathways involved remain unclear.
In this study, neurotomies on selected nerve tracts in rats were carried out, and the preventive effect of pre-moxibustion at acupoint Zusanli on formation of gastric mucosal lesions as well as the expression of an endogenous protective protein in serum and the gastric mucosa were observed. We aimed to explore the mechanism underlying the effect of pre-moxibustion at acupoint Zusanli in preventing gastric mucosal lesions, and determine if the pathways involved are correlated to neural regulation or humoral coordination. Based on the changes in the ulcer index, pre-moxibustion at acupoint Zusanli has an obvious preventive effect on gastric mucosal lesions, and this effect can be interfered by neurotomy. Pre-moxibustion at acupoint Zusanli can promote heat shock protein 70 expression in serum and the gastric mucosa. The key point is that heat shock protein 70 expression in the gastric mucosa was interfered by neurotomy, but that in serum was not. This result shows that pre-moxibustion at acupoint Zusanli has good healing efficacy on gastric mucosal lesions, and that the underlying mechanism involves induction of heat shock protein 70 expression. Nerve tracts are clearly involved in the mechanism of action, so neural regulation is related to the effect of moxibustion.
MATERIALS AND METHODS
Design
A randomized controlled animal experiment.
Time and setting
This experiment was conducted in the Key Laboratory of Biological Information Analysis for Acupuncture and Moxibustion, Hunan University of Chinese Medicine, China in 2011.
Materials
A total of 70 specific pathogen-free, healthy, Sprague Dawley rats aged 3–4 months and weighing 220–250 g (35 females and 35 males) were provided by the Animal Scientific Center of Hunan University of Chinese Medicine. Rats were kept under the following conditions: 20–25°C, humidity of 60–70%, 12-hour light/dark cycles. Experimentss were performed in direct accordance with the Guidance Suggestions for the Care and Use of Laboratory Animals, issued by the Ministry of Science and Technology of China[28].
Methods
Transection of spinal cord
After a 24-hour fast, rats were given intraperitoneal anesthesia (20% urethane, 0.6 mL/100 g), and then secured on an animal operating table in the prone position. The spinal segment was determined by counting the ribs. After disinfection, the skin was opened with a scalpel in the middle of the rat's back (T8-12). Spinous processes and both sides of the neural scute (T9-11) were exposed. The neural scute at T10 was removed using bone shears. Spinal cord was exposed and upraised lightly with forceps. A spinal dura mater incision was made using a scalpel, and a crosscut incision was made on the spinal cord; 2 mm spinal cord was removed. Subsequently, the rat wagged its tail or twitched its leg muscles, or uroclepsia appeared. After the operation, hemostatic sutures were placed, and penicillin (160 000 U/d) was given by intramuscular injection for 7 days. The rat's bladder was emptied by massage twice a day until its urinary reflex recovered[29,30,31].
Damage to the nucleus of the solitary tract
After a 24-hour fast, rats were intraperitoneally anesthetized with 20% urethane, 0.6 mL/100 g, and then secured on an animal operating table in the prone position. After disinfection, under sterile conditions, with reference to the Rat Brain Stereotaxic Coordinates[32], craniectomy (1 mm2) was performed 13–14 mm back from the bregma, and then 1 mm left and right using a dental drill and straight blade. An insulative electrode (diameter of 0.35 mm, exposed tip length 0.5 mm) was inserted into the animal's brain (coordinate: anterior posterior 13.2 mm, lateral to bregma 0.9 mm, below the dorsal surface 7.7–7.8 mm). Both sides of the nucleus of the solitary tract were damaged by anode direct current electricity using a BL-410 Biology Signal System (Taimeng Electronics, Sichuan Province, China) (anode connected to the electrode; cathode connected to the operative incision; parameters: continuous anode direct current, 1 mA, 50 Hz; band width: 0.5 ms, 1 minute). After surgery, hemostatic sutures were placed, and penicillin (160 000 U/d) was intramuscularly injected for 7 days[33,34]. Animal brains were obtained, and sections were taken for hematoxylin-eosin staining to check the damage to the nucleus of the solitary tract using a binocular microscope (XS-200, Jiangnan Photic Instrument Factory, Nanjing, China) and a MoticTeK 3.1 image collection system (Xiamen, China) (Figure 2).
Figure 2.
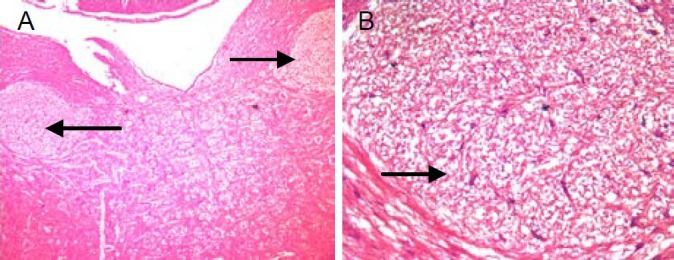
Hematoxylin-eosin stain section of rat brain. Arrow marks point out the damage in the focuses of nucleus of solitary tract and nerve conduction bunch, which were newborn after damage appeared.
(A) × 40; (B) × 100.
Subphrenic vagotomy
After a 24-hour fast, rats were intraperitoneally anesthetized with 20% urethane, 0.6 mL/100 g, and then secured on an animal operating table in the supine position. After disinfection, the abdominal cavity was opened along the median line. Both anterior and posterior branches of the vagal nerve were cut off at the surface of the esophagus, 5 mm up from cardia. Hemostatic sutures were placed, and penicillin (160 000 U/d) was intramuscularly injected for 7 days[35].
Neurotomy of the common peroneal nerve
After a 24-hour fast, rats were intraperitoneally anesthetized with 20% urethane, 0.6 mL/100 g, and then secured on an animal operating table in the prone position. After disinfection, the skin of the rat back thigh (both sides) was opened with a scalpel. The biceps muscle of the thigh was dissected, and then the common peroneal nerve was identified and cut using ophthalmic scissors. Hemostatic sutures were placed, and penicillin (160 000 U/d) was intramuscularly injected for 7 days[36].
Method of pre-moxibustion
After operations, animals were given postoperative care for about 2 days. Rats were secured on a board. After removal of hair, an insulation fabric with a 3-mm hole in it, was pasted onto Zusanli, on both sides, about 5 mm under the capitulum fibulae[37]. Moxa rolls (Hanyi-Moxi Limited Liability Company, Nanyang, China) were placed 0.5 cm above rat skin and were lit to carry out moxibustion treatment at Zusanli for about 30 minutes. The temperature of the skin was controlled at 42°C. This treatment was carried out once a day for 8 days.
Preparation of gastric mucosal lesion models by absolute alcohol
Approximately one day after pre-moxibustion (24-hour fast), rats were given absolute alcohol (99%, 0.6 mL/ 100 g) using a stomach tube to make a gastric mucosal lesion model; rats in control, model and moxibustion groups were given physiological saline (0.6 mL/100 g) as a control[38].
Detection of ulcer index
At 24 hours after model establishment, the stomachs of rats were cut off from the pylorus to the cardia along the greater gastric curvature, cleaned gently with physiological saline, flattened on filter paper, and then observed under a magnifying glass (× 10). According to the method of Guth et al[39], about 24 hours after the last pre-moxibustion, the gastric mucosal impairment site length was calculated as an ulcer index and expressed in millimeters. The ulcer index was doubled if the width of injury was more than 2 mm.
Assessment of heat shock protein 70 expression in the gastric mucosa by western blotting
Heat shock protein 70 expression in the gastric mucosa was assessed by western blotting. Gastric mucosa tissue samples (0.25 g) were solubilized in radioimmunoprecipitation assay buffer (Pierce) for 30 minutes on ice, and then equal volumes of reducing SDS loading buffer (× 5) were added and boiled at 98°C for 5 minutes. Total protein samples (10 μg protein/lane) were separated by SDS-PAGE on 10% polyacrylamide gels alongside the PageRuler™ Plus Prestained Protein Ladder (Peking Liuyi), and electrophoretically transferred onto polyvinylidene difluoride membranes. Following overnight blocking with 5% non-fat dry milk and 0.05% Tween-20 (Sigma) in PBS containing 5% bovine serum albumin at 4°C, the blots were probed with rabbit anti- heat shock protein 70 polyclonal antibody (0.2 μg/mL) (Proteintech Group, Chicago, IL, USA). After a 1-hour incubation at room temperature, the blots were then incubated with horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1: 3 000; Proteintech Group) for 1 hour at room temperature. The corresponding bands were developed, and exposed to X-ray film. A Chemidoc EQ system with Quantity One software (Bio-Rad, Alfred Nobel Drive Hercules, USA) was used to determine the absorbance of protein bands.
Assessment of heat shock protein 70 expression in serum by enzyme-linked immunosorbent assay
Expression of heat shock protein 70 in serum (blood collected from the abdominal aorta) was checked by enzyme-linked immunosorbent assay[40]. These assays were performed by the Changsha Well Bio-tech Company, China
Statistical analysis
Data were analyzed using SPSS 16.0 software (SPSS, Chicago, IL, USA) and one-way analysis of variance, and are expressed as mean ± SD. A value of P < 0.05 was considered statistically significant.
Footnotes
Funding: This project was funded by the National Basic Research Program of China (973 Program), No. 2009CB522904; the National Natural Science Foundation of China, No. 81173326 and 30973802; the Hunan Provincial Scientific Project, No. 2011SK3095; and the Changsha Scientific Key Project, No. K1005020-31.
Conflicts of interest: None declared.
Ethical approval: Experimental procedures were approved by the Animal Ethics Committee of the Laboratory Animal Center, Hunan University of Chinese Medicine, China.
(Edited by Liang FR, Wu HG/Qiu Y/Wang L)
REFERENCES
- [1].Takeuchi K, Kato S, Amagase K. Prostaglandin EP receptors involved in modulating gastrointestinal mucosal integrity. J Pharmacol Sci. 2010;114(3):248–261. doi: 10.1254/jphs.10r06cr. [DOI] [PubMed] [Google Scholar]
- [2].Takeuchi K. Prostaglandin EP receptors and their roles in mucosal protection and ulcer healing in the gastrointestinal tract. Adv Clin Chem. 2010;51:121–144. doi: 10.1016/s0065-2423(10)51005-9. [DOI] [PubMed] [Google Scholar]
- [3].Mise S, Tonkic A, Pesutic V, et al. The presentation and organization of adaptive cytoprotection in the rat stomach, duodenum, and colon. Dedicated to André Robert the founder of the concept of cytoprotection and adaptive cytoprotection. Med Sci Monit. 2006;12(4):BR146–153. [PubMed] [Google Scholar]
- [4].Ritosa F. A new pulffing pattern induced by temperature shock and DNP in Drasophila. Experientia. 1962;18(12):571–573. [Google Scholar]
- [5].Pawlowska Z, Baranska P, Jerczynska H, et al. Heat shock proteins and other components of cellular machinery for protein synthesis are up-regulated in vascular endothelial cell growth factor-activated human endothelial cells. Proteomics. 2005;5(5):1217–1227. doi: 10.1002/pmic.200400983. [DOI] [PubMed] [Google Scholar]
- [6].Welch WJ. Mammalian stress response: cell physiology, structure/function of stress proteins, and implications for medicine and disease. Physiol Rev. 1992;72(4):1063–1081. doi: 10.1152/physrev.1992.72.4.1063. [DOI] [PubMed] [Google Scholar]
- [7].Nisitani I, Ueda N. The antioxidative substance in the products of burned moxa. J Jpn Society Acupuncture. 1988;38(1):29. [Google Scholar]
- [8].Moriyama H, Noguchi T, Takeno S, et al. Analysis of mucosal regeneration and the expression profile of heat shock protein 70 in the isolated small bowel segment. Wound Repair Regen. 2002;10(5):320–327. doi: 10.1046/j.1524-475x.2002.10508.x. [DOI] [PubMed] [Google Scholar]
- [9].Itoh YH, Noguchi R. Pre-treatment with mild whole-body heating prevents gastric ulcer induced by restraint and water-immersion stress in rats. Int J Hyperthermia. 2000;16(2):183–191. doi: 10.1080/026567300285376. [DOI] [PubMed] [Google Scholar]
- [10].Tsukimi Y, Nakai H, Itoh S, et al. Involvement of heat shock proteins in the healing of acetic acid-induced gastric ulcers in rats. J Physiol Pharmacol. 2001;52(3):391–406. [PubMed] [Google Scholar]
- [11].Shichijo K, Ihara M, Matsuu M, et al. Overexpression of heat shock protein 70 in stomach of stress-induced gastric ulcer-resistant rats. Dig Dis Sci. 2003;48(2):340–348. doi: 10.1023/a:1021939829515. [DOI] [PubMed] [Google Scholar]
- [12].Garrido C, Gurbuxani S, Ravagnan L, et al. Heat shock proteins: endogenous modulators of apoptotic cell death. Biochem Biophys Res Commun. 2001;286(3):433–442. doi: 10.1006/bbrc.2001.5427. [DOI] [PubMed] [Google Scholar]
- [13].Du J. Nanjing: Nanjing Medical University; 2003. Effect of heat shock protein 70 (HSP70) on the adaptive cytoprotection of gastric mucosa in chronic alcohol drinking rats. [Google Scholar]
- [14].Niu P, Liu L, Gong Z, et al. Overexpressed heat shock protein 70 protects cells against DNA damage caused by ultraviolet C in a dose-dependent manner. Cell Stress Chaperones. 2006;11(2):162–169. doi: 10.1379/CSC-175R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Nakada J, Matsura T, Okazaki N, et al. Oral administration of geranylgeranylacetone improves survival rate in a rat endotoxin shock model: administration timing and heat shock protein 70 induction. Shock. 2005;24(5):482–487. doi: 10.1097/01.shk.0000180980.63247.a9. [DOI] [PubMed] [Google Scholar]
- [16].Dixon MF, Genta RM, Yardley JH, et al. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am J Surg Pathol. 1996;20(10):1161–1181. doi: 10.1097/00000478-199610000-00001. [DOI] [PubMed] [Google Scholar]
- [17].Sun ZR, Wang YM. Infection of HSP70 by preventive moxibustion on ischemia reperfusion rats. Zhonghua Zhongyiyao Xuekan. 2008;26(7):1382–1383. [Google Scholar]
- [18].Yi SX, Yu J, Chang XR, et al. Effect of Quercetin blocking HSP70 expression on pre-moxibustion suppressing gastric mucosal cell apoptosis. Zhongguo Zhongyiyao Xinxi Zazhi. 2009;16(10):30–33. [Google Scholar]
- [19].Yi SX, Peng Y, Chang XR, et al. Effect of pre-moxibustion on proliferation and restoring of gastric mucosa in rats with acute gastric ulcer. Shijie Zhongxiyi Jiehe Zazhi. 2007;2(1):21–24. [Google Scholar]
- [20].Chang XR, Peng N, Yi SX, et al. Effects of moxibustion on expression of HSP70 protein and mRNA in stress-induced gastric ulcer of rats. Shijie Huaren Xiaohua Zazhi. 2006;14(13):1252–1256. [Google Scholar]
- [21].Qin M, Rao ZR, Wang JJ, et al. Influence of intrathecal injection of fluorocitrate on the protective effect of electroacupuncture on gastric mucosa in high humid heat stress rats. Zhenci Yanjiu. 2007;32(3):158–162. [PubMed] [Google Scholar]
- [22].Li JS, Yan J, He JF. Effects of acupuncturing acupoints Neiguan and Zusanli on neurons discharges of NTS in rats. Hunan Zhongyiyao Daxue Xuebao. 2007;27(3):55–58. [Google Scholar]
- [23].Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117(2):289–296. doi: 10.1172/JCI30555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yi SX, Peng Y, Chang XR, et al. Effect of pre-moxibustion on apoptosis and proliferation of gastric mucosa cells. World J Gastroenterol. 2007;13(15):2174–2178. doi: 10.3748/wjg.v13.i15.2174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chang XR, Peng N, Yi SX, et al. Moxibustion at Zusanli and Liangmen prevents gastric mucosa from oxidation injury through inducing high expression of heat shock protein 70. Shijie Huaren Xiaohua Zazhi. 2006;14(35):3405–3408. [Google Scholar]
- [26].Yi SX, Yu J, Chang XR, et al. Effect of moxibustion- induced up-regulation of HSP70 expression on mitochondrial signal transduction pathway during gastric mucosal cell apoptosis in rats. Shijie Huaren Xiaohua Zazhi. 2008;16(24):2689–2694. [Google Scholar]
- [27].Yu J, Yi SX, Chang XR, et al. Effects of moxibustion on points Zusanli and Liangmen on expression of heat shock protein 70 in different organs of rats. Hunan Zhongyiyao Daxue Xuebao. 2009;29(4):67–69. [Google Scholar]
- [28].The Ministry of Science and Technology of the People's Republic of China. Guidance Suggestions for the Care and Use of Laboratory Animals. 2006 Sep 30; [Google Scholar]
- [29].Yu Q, Xu J. Changes in expression of SP in the dorsal root ganglion following hemisected spinal cord injury in rats. Jiepou Xue Yanjiu. 2008;30(1):8–10. 14. [Google Scholar]
- [30].Yang K, Hu KY, Xu XD, et al. Method of opening the spinal canal to make rat spinal cord damage model. Jiepou Xue Zazhi. 2009;(5):M0004. [Google Scholar]
- [31].Liu M, Wu XP, Tong M. Effect of ultra-early hyperbaric oxygenation on spinal edema and hind limb motor function in rats with complete spinal cord transection. Nan Fang Yi Ke Da Xue Xue Bao. 2009;29(10):2014–2017. [PubMed] [Google Scholar]
- [32].Paxinos G, Watson C. 5th ed. London: Academic Press; 2005. The Rat Brain in Stereotaxic Coordinates. [Google Scholar]
- [33].Huang BL, Zhang B, Yu Z, et al. 1. Vol. 22. Yixue Ban: Xianning Xueyuan Xuebao; 2008. Role of nucleus tractus solitarious in protective effect of electroacupuncture against stress gastric mucosal lesionin rats; pp. 1–3. [Google Scholar]
- [34].Wang SJ, Sun YH, Zhao ZG, et al. Three methods of correcting the target site in the rat brain on stereotaxic coordinates. Hebei Yike Daxue Xuebao. 2001;22(2):75–77. [Google Scholar]
- [35].Mordes JP, el Lozy M, Herrera MG, et al. Effects of vagotomy with and without pyloroplasty on weight and food intake in rats. Am J Physiol. 1979;236(1):R61–66. doi: 10.1152/ajpregu.1979.236.1.R61. [DOI] [PubMed] [Google Scholar]
- [36].Wei YZ, Gu XB, Zhang FM, et al. 6. Vol. 29. Yixue Kexue Ban: Zhongshan Daxue Xuebao; 2008. Tracing of regenerating axons in peroneal nerve neurorrhaphy to tibial nerve in rats; pp. 720–723. [Google Scholar]
- [37].Li ZR. Beijing: China Press of Chinese Traditional Medicine; 2007. Experimental Acupuncture. [Google Scholar]
- [38].Takano H, Satoh M, Shimada A, et al. Cytoprotection by metallothionein against gastroduodenal mucosal injury caused by ethanol in mice. Lab Invest. 2000;80(3):371–377. doi: 10.1038/labinvest.3780041. [DOI] [PubMed] [Google Scholar]
- [39].Guth PH, Aures D, Paulsen G. Topical aspirin plus HCl gastric lesions in the rat. Cytoprotective effect of prostaglandin, cimetidine, and probanthine. Gastroenterology. 1979;76(1):88–93. [PubMed] [Google Scholar]
- [40].Njemini R, Demanet C, Mets T. Comparison of two ELISAs for the determination of Hsp70 in serum. J Immunol Methods. 2005;306(1-2):176–182. doi: 10.1016/j.jim.2005.08.012. [DOI] [PubMed] [Google Scholar]


