Abstract
Insects are responsible for the transmission of major infectious diseases. Recent advances in insect genomics and transformation technology provide new strategies for the control of insect borne pathogen transmission and insect pest management. One such strategy is the genetic modification of insects with genes that block pathogen development. Another is to suppress insect populations by releasing either sterile males or males carrying female-specific dominant lethal genes into the environment. Although significant progress has been made in the laboratory, further research is needed to extend these approaches to the field. These insect control strategies offer several advantages over conventional insecticide-based strategies. However, the release of genetically modified insects into the environment should proceed with great caution, after ensuring its safety, and acceptance by the target populations.
Keywords: conditional gene expression, insect genetic control, sterile insect technology, transposable element, vector borne disease
Introduction
Insects are efficient vectors for pathogens that cause serious public health problems. Mosquitoes are responsible for the spread of diseases such as malaria, dengue fever, yellow fever and lymphatic filariasis, which cause millions of deaths every year, and morbidity to nearly half of the world’s population. Unfortunately, these diseases are on the rise as a result of changes in public health policy, global warming, insecticide resistance of the vector, drug resistance and genetic changes in pathogens, as well as demographic and societal changes. The prevalence and transmission of these diseases depends upon the attributes and requirements of at least three organisms: the pathogen, the insect vector, and the human host. Disease incidence is grossly disproportionate, with the overwhelming impact being on developing countries in the tropics.
The prevention and control of vector borne diseases in the early 20th century was achieved through the efficient control of vector populations. The best examples are the control of yellow fever in South America during the early 1900s and the worldwide control of malaria in the 1950s through the 1970s (Gubler 1998). Because of these successes, there was considerable optimism that these diseases might even be eradicated using insecticide-based arthropod population control measures and drugs targeting pathogens. However, these expectations were short lived in part because of increasing vector insecticide resistance, harmful effects of certain insecticides on the environment, and lack of resources as well as logistical difficulties associated with implementation of such plans. Clearly, available weapons to fight vector borne diseases are insufficient, and new approaches need to be developed.
The ability to genetically modify insects opens the door to novel techniques for the control of vector populations (Wimmer 2003; Schneider & James 2006). Significant progress has been made in bringing such approaches closer to reality (Moreira et al. 2002a). Here we review the recent advances in insect transformation techniques and various strategies for the genetic control of vector borne diseases and insect population suppression, with emphasis on mosquito vectors. These approaches are also applicable to other disease vectors as well as insects of agricultural importance.
Genetic transformation of insects
Germline transformation results in a stable and heritable integration of a foreign DNA fragment into the insect genome (Atkinson et al. 2001). This is usually accomplished by use of a transposable element. Such elements have been shown to be highly efficient for the transformation of Drosophila as well as other insects (Handler 2001). Trans-posable elements are usually around 3000 bp long and contain one transcriptional unit encoding a transposase flanked by terminal inverted repeats (TIR). The transposase catalyzes the excision and insertion (cut and paste) of the DNA fragment within the TIR (Atkinson et al. 2001). The ability of the transposase to act in trans has enabled the development of a binary vector–helper system in which the TIR flanks the gene or genes of interest, while transposase provided by a separate helper plasmid catalyzes excision and insertion events. Transposase expression from the helper plasmid is regulated by a constitutive (e.g. actin) or an inducible (e.g. hsp70) promoter. A mixture of helper and transfer plasmids is microinjected into the posterior pole of the embryo where the germ cells are formed (Morris 1997; Lobo et al. 2006). Transposase mediates the excision of genes of interest from the transfer plasmid followed by integration into the insect genome. Because the helper plasmid lacks TIR, it is lost during subsequent cell divisions, allowing the inserted gene to remain stably integrated. Thus, the integrated genes are typically non-autonomous transposons that require an exogenous source of transposase to remobilize. Transposable elements such as piggyBac, Hermes, Minos and Mariner have been successfully used for the transformation of non-Drosophilid insects. The Drosophila P-element, the first transposable element to be used in germline transformation, is genus specific and does not work in non-Drosophilid organisms.
piggyBac
The piggyBac element was discovered as an insertion sequence that caused an altered plaque phenotype in Galleri melonellea nuclear polyhedrosis virus, when passaged through cells of the cabbage looper Trichoplusia ni (Fraser et al. 1983). This transposable element inserts specifically at TTAA target sites, and as with all other transposable elements of its class, generates a duplication of the target site upon insertion. piggyBac is 2.5 kb long and possesses 13 bp TIR and flanking sequences. It has been used to efficiently transform several insect species spanning Diptera, Lepidoptera, Coleoptera and Hymenoptera, making it one of the most widely used transposable elements for insect transformation (Atkinson et al. 2001).
Hermes
The Hermes element was discovered in Musca domestica and is a member of the hobo activator Tam3 (hAT) family of transposons (Warren et al. 1994). It was identified after the unexpected excision of a hobo element from a plasmid introduced into the housefly in the absence of hobo transposase. Hermes is 2.7 kb long and contains a 17 bp TIR. Hermes has proven to be an effective transformation vector in six insect species, including mosquito species. In certain organisms, Hermes has been to shown to have the rare property of integrating the whole plasmid into the genome (“illegitimate recombination”) in addition to the canonical transposase-mediated integration (Jasinskiene et al. 2000; Allen et al. 2001).
Minos and Mariner
Minos and Mariner are members of a large family of mariner transposable elements. The abundance of defective mariner-like elements in the insect genome made it difficult to isolate a functionally active element. Minos (Mos1) from Drosophila melanogaster is the only naturally occurring mariner element isolated from insects. Himar, another mariner-like element, was reconstructed based on the sequence of various copies of mariner elements isolated from the horn fly, Haematobia irritans (Lampe et al. 1996). Minos is an effective vector system tested in the Mediterranean fruit fly, and in Anopheles stephensi and Aedes aegypti mosquitoes (Coates et al. 1998; Catteruccia et al. 2000).
Identification of transformed insects
A marker gene whose product allows positive identification of transgenic insects must be used for differentiation from non-transgenic insects. In most cases, Green Fluorescent Protein (GFP) or another fluorescent protein is the marker of choice. A tissue-specific promoter such as 3XP3, which is expressed predominantly in the eye, or ubiquitous promoters such as polyubiquitin or actin have been used to drive the expression of marker genes. In principle, tissue-specific expression of markers is preferable to ubiquitous expression, to minimize possible negative effects of expression of a foreign protein in many insect tissues. The number of insertion events (number of copies of the element per genome) is usually assessed by Southern blot analysis of the transgenic insect’s genomic DNA, using transposable element DNA as a probe. A stable pattern of hybridization is proof of the stability of integration through generations. Insertion site sequencing provides definitive proof of transposon-mediated integration. This is achieved by sequencing of the integration junction fragment obtained by inverse polymerase chain reaction (PCR) (Tamura et al. 2000). The advantages of using GFP as a marker include the facts that it is dominant, and that there is no demonstrated effect on fitness or viability in laboratory experiments (Moreira et al. 2004). However, stringent tests must be performed under field conditions to ascertain these assumptions.
Genetic control of insects
The ability to genetically engineer insects of medical and agricultural importance opens the door for the development of novel approaches to control these organisms and the damage they cause. Current control measures heavily depend on insecticides that have adverse environmental effects and prolonged use of which eventually results in development of insecticide resistance. The two broad categories of novel genetic approach are (i) genetic suppression of the ability of vectors to transmit pathogens (vector competence) and (ii) genetic suppression of insect populations. The former approach is specific to the control of diseases transmitted by vectors such as mosquitoes, while the latter approach is applicable to the control of any insects in which genetic manipulation is feasible. Moreover, these genetic interventions are specific to the target insect species and do not affect any other species occupying the same biological niche.
Genetic suppression of vector competence
Vector borne diseases are transmitted by blood-sucking insects. Mosquitoes are by far the most important group of disease vectors. Mosquitoes acquire pathogens when they ingest blood from an infected individual. After a complex developmental cycle that includes traversal of the midgut epithelium, the pathogen invades the salivary gland, from where it is delivered to another human host when the mosquito takes the next blood meal. Different mosquito species differ dramatically in their ability to vector pathogens because of genetic differences and the ability of the mosquito’s innate immune system to clear the pathogens. A number of genes involved in mosquito immune defense against invading pathogens have been identified (Blandin et al. 2004; Osta et al. 2004; Abraham et al. 2005a). Over-expression of such genes in mosquitoes could be explored to reduce vector competence. For the implementation of genetic means to interfere with the insect’s vector competence, two major tasks need to be accomplished: (i) demonstrate that interference of transmission by genetic means is feasible; and (ii) devise ways to introduce the relevant effector genes (the genes that confer pathogen resistance to mosquitoes) into mosquitoes in the field.
The genetic approach has the important advantage over insecticide-based approaches that it leaves the biological niche occupied by insects incapable of transmitting the disease unharmed, thus providing an ecologically sustainable solution for reducing disease transmission. This is in sharp contrast with the use of insecticides that kill mosquitoes and leave an empty biological niche that is readily replenished as soon as insecticide treatment stops. Indoor insecticide campaigns (e.g. residual spraying, bed nets) effectively kill the mosquitoes that make it into houses but the outdoor breeding sites remain intact. This cycle of unimpaired outdoor breeding and indoor insecticide killing greatly accelerates the development of insecticide resistance.
Interference of transmission
Transgenic mosquitoes with a significantly reduced ability to vector the malaria parasite and the dengue virus have been created and successfully tested in the laboratory. Our laboratory has identified a peptide, named salivary gland and midgut peptide 1 (SM1), which interferes with invasion of the midgut and the salivary glands by the rodent malaria parasite Plasmodium berghei (Ghosh et al. 2000). A trans-genic mosquito expressing SM1 in the midgut significantly reduced parasite dissemination in these mosquitoes (Ito et al. 2002). Similarly, transgenic mosquitoes expressing a phospholipase A2 in their midgut were impaired in their ability to transmit the malaria parasite (Moreira et al. 2002b; Abraham et al. 2005b). Capurro et al. (2000) have shown that a single chain antibody against Plasmodium sporozoite surface protein is also effective in blocking parasite development in mosquitoes. Moreover, transgenic Anopheles gambiae mosquitoes overexpressing the antimicrobial peptide cecropin are compromised in their ability to support malaria parasite development (Kim et al. 2004). Similar efforts to limit the ability of Ae. aegypti to transmit the dengue virus have also been successful. A transgenic Ae. aegypti mosquito expressing a small interfering (si)RNA corresponding to a major DEN2 pre-membrane protein has been produced (Franz et al. 2006). This siRNA produced in transgenic Ae. aegypti interferes with virus development by inhibiting the formation of pre-membrane protein that is essential for virus replication. Thus, these mosquitoes have significantly reduced competence to vector the dengue virus.
Driving effector genes into field populations
The second critical step in interfering with an insect’s vector competence is the introgression of effector genes (genes that suppress pathogen development) into field populations using a genetic drive mechanism. The ultimate goal is to drive the effector genes into the vast majority of the mosquitoes in the field and in this way reduce or block their ability to transmit the pathogen. Several strategies are being considered for driving genes into populations, including the use of (i) transposable elements, (ii) the endosymbiont Wolbachia and (iii) meiotic drive. While arguments in favor of the use of each of these can be made, there are serious limitations that need to be overcome.
Transposable elements
In the case of transposable elements, there is a dramatic example of the successful spread of the P-element into the world population of D. melanogaster in only a few decades (Quesneville & Anxolabehere 1998). However, for use in the spread of effector genes in mosquitoes, several technical aspects need to be resolved. One is that transposition needs to be replicative (when moving, the element leaves an intact copy of itself behind) and to a distant location in the chromosome or a different chromosome (to allow for recombination). Unfortunately, replicative transposition is frequently associated with deletions and mutations, and the probability of generating such defects increases with the size of the “cargo” carried by the element. In the case of genetic control, a substantial cargo of at least four genes (approximately 10 kb) is required: two or more effector genes to avoid selection of resistant pathogens, a marker gene for identification of transgenic insects, and a driver gene (transposase). A transposable element that efficiently and replicatively transposes in mosquitoes without creating deletions has yet to be identified.
Wolbachia
Wolbachia is an intracellular bacterium that occurs in the germ cells of its host insect and can drive itself into insect populations by a phenomenon called cytoplasmic incompatibility (CI). Using Wolbachia to express genes of interest in mosquitoes is being explored. The molecular basis for CI is not understood. In some cases, Wolbachia also occurs in somatic cells. To develop a drive mechanism, it is essential that the driver gene be physically linked to the gene(s) to be driven, meaning that the effector genes need to be integrated into the Wolbachia genome itself. The major stumbling blocks are that Wolbachia transformation has not yet been achieved and, more importantly, at present it is technically not possible to deliver a protein produced in Wolbachia to the parasite (it needs to cross two cell membranes: that of the Wolbachia and that of its host cell).
Meiotic drive systems
These preferentially promote the spread of the gametes containing the drive gene into populations. Meiotic drive systems have been reported in several organisms across various taxa: plants, fungi, insects (flies, mosquitoes) and other animals (an arachnid, a lemming, mice and humans) (Pennisi 2003). In the mosquito meiotic drive system, gene products from driver male chromosomes destroy responder-bearing female chromosomes, theoretically leading to a population crash, otherwise quickly selecting for suppressor-linked responder alleles from sensitive target populations (Hickey & Craig 1966). However, Cha et al. (2006) have shown that the strong driver male containing an insensitive responder allele on the female chromosome can be introduced into a driver-sensitive target population for an efficient population replacement. Driver alleles would quickly sweep into the target population to replace sensitive alleles not with the driver suppressors from the target population, but with the insensitive responders that were introduced with driver alleles. During the allele replacement procedure, the driver would produce a genetic hitchhiking effect of the flanking region of insensitive responders, where anti-pathogen effector genes could be shuttled in. Compared to other drive mechanisms, such as transposable elements and Wolbachia, meiotic drive systems are stable and safe, with no possibility of horizontal transfer. Unfortunately, the molecular basis for meiotic drive and the identity of the driver and responder genes are not known, which prevents the development of protocols to stably combine effector genes with the drive system.
Recent investigation has brought to light a promising approach: the use of a maternal-effect dominant embryonic arrest (Medea) element (Chen et al. 2007). Experiments have been done in Drosophila using microRNA-based silencing of a maternally expressed gene essential for embryogenesis, coupled with early zygotic expression of a rescuing transgene. This new strategy has great promise for delivering genes into mosquito field populations.
An alternative approach to the genetic control of vector competence is to genetically modify bacteria living in the midgut of mosquitoes, rather than to modify the mosquitoes themselves (Riehle & Jacobs-Lorena 2005). This approach is commonly referred to as paratransgenesis. As do all higher organisms, mosquitoes carry a bacterial flora in their guts and the number of bacteria increases dramatically after a blood meal, probably due to the availability of nutrients. Because mosquitoes acquire the parasite with a blood meal, the use of bacteria to target the parasite is an attractive approach. Proof-of-concept experiments suggest that the approach is feasible (Riehle et al. 2007). Advantages of the paratransgenesis approach include: (i) growing large quantities of bacteria is “low-tech” and can easily be accomplished in disease-endemic countries; (ii) an unlimited number of effector molecules can be used by mixing bacteria that express different genes; (iii) if the parasite becomes resistant to an effector, a change in effectors can be easily accomplished; and (iv) the paratransgenesis approach is not sensitive to genetic reproductive barriers in the field (c.f. Perspectives below). A way to introduce the genetically modified bacteria into the mosquitoes in the field needs to be developed. The recent identification of bacteria that have the potential to spread among mosquito populations (Favia et al. 2007) represents a significant advancement for the implementation of this strategy.
Genetic suppression of insect populations
Sterile insect control has been used mainly for agricultural insect pests. It involves mass rearing, sterilization, and release of a large number of male insects into the field to compete with wild-type males for mating, thus drastically reducing the size of the pest population (female insects usually mate only once during their lifetime). Because of its species specificity, the sterile insect technique (SIT) is considered to be an ecologically safe program and has been successfully used to suppress or eradicate insects. For instance, the pink bollworm Pectinophora gossypiella in California (Walters et al. 2000), the tsetse fly Glossina spp. in Zanzibar (Vreysen 2001), and the Mediterranean fruit fly Ceratitis capitata (Rendon et al. 2004; Hendrichs et al. 1995) and New World screw worm Cochliomyia hominivorax in North and Central America (Wyss 2000).
Conventional SIT was generally carried out by releasing both sexes, even though sterilized females play no part in the control. Release of sterile males alone made a significant improvement in SIT by increasing the effectiveness of the released males, which disperse more widely in search of females, making the competition with wild-type males more effective. Separation of the undesired females has been achieved through genetic sexing. However, recent advances in molecular biology have promoted the transgenic approach for sex separation based on the female-specific expression of conditional lethal genes. This approach involves mass rearing of a strain homozygous for a repressible female specific dominant lethal under permissive conditions (e.g. Tet-off system in the presence of tetracycline). In the field, where the repressor is absent, female progeny from the released transgenic males die, resulting in decrease of population size (Gong et al. 2005).
Transcriptional activation system
For conditional lethality or sterility, a tightly controlled transcription system is essential so that the breeding population can survive and reproduce (the population used for releases cannot be maintained without females). Model biocontrol systems have been tested in D. melanogaster using dominant embryonic lethality of females (Horn & Wimmer 2003), or by inducing female-specific lethality by controlling alternate splicing (Fu et al. 2007). Two transcription activation systems widely used are the tetracycline-dependent transcription repression or activation systems, and the yeast GAL4/UAS transcriptional activation system.
Tetracycline-based conditional gene expression system
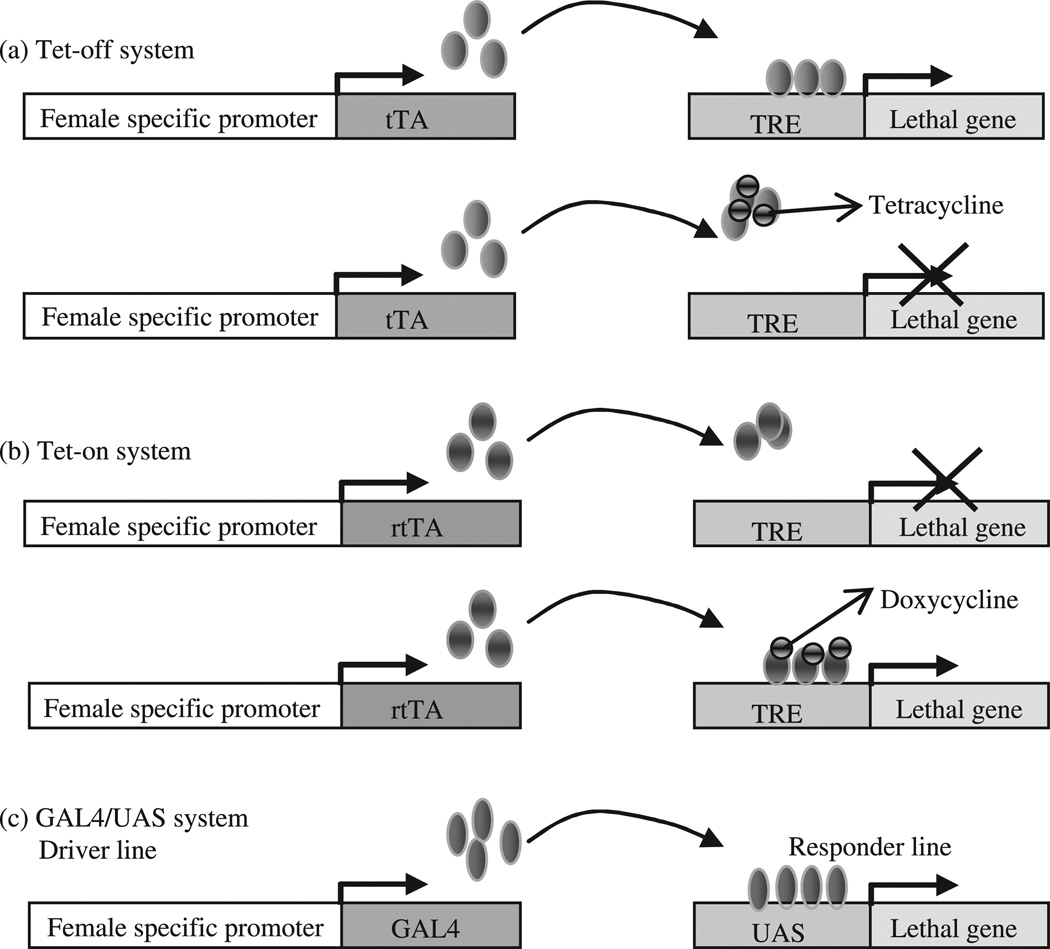
This conditional gene expression system is derived from the Escherichia coli Tn10 transposon and was developed into positive and negative gene expression regulatory systems, resulting in either the activation or inhibition of gene expression in the presence of tetracycline or its derivative doxycycline (Fig. 1; Gossen et al. 1995). The components of this system include transcriptional activators tTA or rtTA, and tetracycline responsive element (TRE). The expression of gene linked to TRE is positively or negatively regulated by the presence or absence of tetracycline or its derivative doxycycline, depending on the type of transcriptional activator present (Fig. 1a,b).
Figure 1.
A diagram illustrating transcriptional activation systems. (a) The Tet-off system, in which the expression of a gene linked to the tetracycline responsive element (TRE) is repressed by the binding of tetracycline to the transcription activator (tTA), driven by a female-specific promoter. In the absence of tetracycline, transgenic females having both the tTA and lethal genes will be killed. (b) The Tet-on system, in which the expression of the lethal gene linked to the TRE is induced by the binding of doxycycline to a mutated form of tTA, known as reverse transcriptional activator (rtTA). Therefore, in the presence of doxycycline, transgenic females having both the rtTA and lethal genes will be killed. (c) The GAL4/UAS gene expression system. In the driver line, a female-specific promoter drives the expression of the GAL4 transcription factor. In the responder line, UAS drives the expression of a lethal gene. In the progenies of a cross between the driver and responder lines, females will be killed.
The Tet-on and Tet-off systems have been demonstrated in Drosophila. A female-specific yolk protein 1 (YP1) regulatory sequence was used for the expression of tTA, and a cell death gene head involution defective (hid) linked to TRE was used to induce female-specific cell death (Grether et al. 1995). Because hid expression was repressed in the presence of tetracycline in the medium, both sexes survived, but in the absence of the antibiotic (which simulates the situation in the field), female-specific expression of hid killed all females. Thomas et al. (2000) also had demonstrated a Tet-off system using another female-specific yolk protein 3 promoter. Another example is the ecotopic expression of a proapoptotic gene that causes embryo-specific lethality when driven by tTA under regulation of a cellularization gene in Drosophila (Horn & Wimmer 2003). The lethality is specific to embryonic stages and can be suppressed by tetracycline provided maternally. Recently, the sex-specific alternate splicing of the C. capitata sex-determining gene Cctra was exploited to achieve the expression of functional tTA in females (Fu et al. 2007). Functional Cctra transcript is made only in females, while male transcripts carry additional exons with in-frame stop codons. These male-specific exons of the Cctra gene were inserted into tTA such that the functional protein is disrupted in male splice variants, thereby making tTA expression female specific.
In principle, the efficacy of this system can be further augmented by using homozygous males for the transgenes as well as using multiple integrations on different chromosomes. Release of homozygous males carrying the tetracycline-repressed dominant female lethal system is advantageous for population suppression because of the non-viability of the female offspring, thus extending the lethality to a proportion of F1 offspring. However, this has not yet been tested. The most important requirement for the success of this approach is the tight regulation of the expression by tTA trans activator. Although this has been achieved in the model organism, further study in medically and economically important insects is needed to confirm the tight regulation in the respective insect species.
Yeast GAL4/UAS binary gene expression system
GAL4 is a yeast transcription activator that binds to a specific enhancer sequence resulting in the expression of coding sequence linked to the enhancer (Brand & Perrimon 1993). This system has been optimized by mutation and reiterated sequences in the enhancer known as upstream activating sequence (UAS). The expression of a gene linked to UAS enhancer is regulated by GAL4, which may in turn be specified by a variety of tissue-specific or sex-specific promoter (Fig. 1c). Unlike the Tet systems, which are regulated by tetracycline, the GAL4/ UAS system is not regulated by any external treatment. A responder line expressing the lethal gene under the control of UAS and a second activator line expressing GAL4 by a female-specific promoter have to be maintained separately. Only male progeny from the interbreeding of responder and activator lines can be used for population suppression. Thus sex- or tissue-specific lethality can be achieved by having one transgenic line with the appropriate lethal gene under UAS control mating with another transgenic line with the GAL4 regulated by a sex- or tissue-specific promoter.
Perspectives
The development of insect transformation technology has opened novel avenues for insect and disease control. The identification of transposable elements as efficient vectors for introducing genes into the genome and the development of new dominant and universal transformation markers (fluorescent proteins) made possible the creation of trans-genic insects on a routine basis. Current work in many laboratories focusing on the elucidation of the molecular basis of insect–pathogen interactions will provide more and improved effector genes for the creation of refractory insects. The conditional gene expression systems provide tools to design better methods for genetic sexing and production of sterile males.
In the case of genetic suppression of insect populations, several caveats need to be considered. One is that most of the experiments have been done with the model organism Drosophila. While many of the genes used in these pilot experiments should have similar functional and regulatory properties in other insect species, this point remains to be established. Another critical consideration is that the effectiveness of SIT is crucially dependent on the size of the target population. For controlling insect pests of agricultural importance, SIT has great promise because in most cases, the aim is to eliminate a relatively small insect population. In the case of insects of medical importance, such as mosquitoes that naturally occur in large populations, to be successful, the SIT technique would require the release of an extremely large number of sterile males to compete with the existing wild-type males in the field. A recent study suggests that SIT using a late acting (pupal or early adult stage) lethal gene can significantly alleviate this concern in the dengue and yellow fever virus mosquito Ae. aegypti (Phuc et al. 2007). Moreover, the consideration about the intact biological niche made above in reference to insecticides (the breeding sites remain unaltered with SIT) also applies here.
In the case of genetic suppression of vector competence, additional considerations come into play. One is the genetic barrier, which is especially important in the case of malaria transmission. Anopheline mosquitoes occur in nature in reproductively isolated populations (cryptic species) that can occupy the same region (sympatric). Such cryptic species are an important barrier for the genetic introduction of novel genes. In this respect, paratransgenesis has the advantage that such barriers are not relevant. Moreover, because transgenes are usually introduced into the genome with transposable elements, one needs to ascertain that these are not mobilizable (destabilized) by elements naturally occurring in the target mosquito population.
For all applications of genetically modified insects, ethical considerations are crucially important. Before implementation of any of such strategy, much effort must be devoted to prove that the methods are absolutely safe. Moreover, implementation needs to be carried out with maximum transparency, working with the appropriate regulatory bodies and obtaining the consent of the affected human populations.
In conclusion, the advances in our understanding of insect genomics and molecular methods gives great hope for the development of new transgenic strains that will revolutionize our ability to control insect populations and their ability to vector pathogens. The most efficient use of this technology requires continued research into diverse genetic pathways, sex determination, and ecology of these insects. Given the intense public scrutiny of recombinant DNA use, all projects that involve release of transgenic insects into the environment should be treated with the utmost care. It is important to emphasize that even in the best possible scenario, a transgenic approach will never be able to, by itself, solve the difficult issue of disease control. There is strong consensus in the scientific community that the war on vector-transmitted infectious disease can only be won by a coordinated multi-pronged approach involving concomitant deployment of drugs, insecticides, vaccines and transgenic insects (Alphey et al. 2002; Jacobs-Lorena 2003).
References
- Abraham EG, Pinto SB, Ghosh A, et al. An immune-responsive serpin, SRPN6, mediates mosquito defense against malaria parasites. Proceedings of the National Academy of Sciences (USA) 2005a;102:16 327–16 332. doi: 10.1073/pnas.0508335102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abraham EG, Donnelly-Doman M, Fujioka H, Ghosh A, Moreira L, Jacobs-Lorena M. Driving midgut-specific expression and secretion of a foreign protein in transgenic mosquitoes with AgAper1 regulatory elements. Insect Molecular Biology. 2005b;14:271–279. doi: 10.1111/j.1365-2583.2004.00557.x. [DOI] [PubMed] [Google Scholar]
- Allen ML, O’Brochta DA, Atkinson PW, Levesque CS. Stable, germ-line transformation of Culex quinquefasciatus (Diptera: Culicidae) Journal of Medical Entomology. 2001;38:701–710. doi: 10.1603/0022-2585-38.5.701. [DOI] [PubMed] [Google Scholar]
- Alphey L, Beard CB, Billingsley P, et al. Malaria control with genetically manipulated insect vectors. Science. 2002;298:119–121. doi: 10.1126/science.1078278. [DOI] [PubMed] [Google Scholar]
- Atkinson PW, Pinkerton AC, O’Brochta DA. Genetic transformation systems in insects. Annual Review of Entomology. 2001;46:317–346. doi: 10.1146/annurev.ento.46.1.317. [DOI] [PubMed] [Google Scholar]
- Blandin S, Shiao SH, Moita LF, et al. Complement-like protein TEP1 is a determinant of vectorial capacity in the malaria vector Anopheles gambiae . Cell. 2004;116:661–670. doi: 10.1016/s0092-8674(04)00173-4. [DOI] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Capurro M de L, Coleman J, Beerntsen BT, et al. Virus-expressed, recombinant single-chain antibody blocks sporozoite infection of salivary glands in Plasmodium gallinaceum-infected Aedes aegypti . American Journal of Ttropical Medicine and Hygiene. 2000;62:427–433. doi: 10.4269/ajtmh.2000.62.427. [DOI] [PubMed] [Google Scholar]
- Catteruccia F, Nolan T, Loukeris TG, Blass C, Savakis C, Kafatos FC, Crisanti A. Stable germline transformation of the malaria mosquito Anopheles stephensi . Nature. 2000;405:959–962. doi: 10.1038/35016096. [DOI] [PubMed] [Google Scholar]
- Cha SJ, Mori A, Chadee DD, Severson DW. Cage trials using an endogenous meiotic drive gene in the mosquito Aedes aegypti, to promote population replacement. American Journal of Tropical Medicine and Hygiene. 2006;74:62–68. [PubMed] [Google Scholar]
- Chen CH, Huang H, Ward CM, Su JT, Schaeffer LV, Guo M, Hay BA. A synthetic maternal-effect selfish genetic element drives population replacement in Drosophila . Science. 2007;316:597–600. doi: 10.1126/science.1138595. [DOI] [PubMed] [Google Scholar]
- Coates CJ, Jasinskiene N, Miyashiro L, James AA. Mariner transposition and transformation of the yellow fever mosquito, Aedes aegypti . Proceedings of the National Academy of Sciences (USA) 1998;95:3748–3751. doi: 10.1073/pnas.95.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Favia G, Ricci I, Damiani C, et al. Bacteria of the genus Asaia stably associate with Anopheles stephensi, an Asian malarial mosquito vector. Proceedings of the National Academy of Sciences (USA) 2007;104:9047–9051. doi: 10.1073/pnas.0610451104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franz AW, Sanchez-Vargas I, Adelman ZN, Blair CD, Beaty BJ, James AA, Olson KE. Engineering RNA interference-based resistance to dengue virus type 2 in genetically modified Aedes aegypti . Proceedings of the National Academy of Sciences (USA) 2006;103:4198–4203. doi: 10.1073/pnas.0600479103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser MJ, Smith GE, Summers MD. Acquisition of host cell DNA sequences by baculoviruses: Relationship between host DNA insertions and FP mutants of Autographa californica and Galleria mellonella nuclear polyhedrosis viruses. Journal of Virology. 1983;47:287–300. doi: 10.1128/jvi.47.2.287-300.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu G, Condon KC, Epton MJ, et al. Female-specific insect lethality engineered using alternative splicing. Nature Biotechnology. 2007;25:353–357. doi: 10.1038/nbt1283. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Ribolla PE, Jacobs-Lorena M. Targeting Plasmodium ligands on mosquito salivary glands and midgut with a phage display peptide library. Proceedings of the National Academy of Sciences (USA) 2000;98:13 278–13 281. doi: 10.1073/pnas.241491198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong P, Epton MJ, Fu G, et al. A dominant lethal genetic system for autocidal control of the Mediterranean fruitfly. Nature Biotechnology. 2005;23:453–456. doi: 10.1038/nbt1071. [DOI] [PubMed] [Google Scholar]
- Gossen M, Freundlieb S, Bender G, Muller G, Hillen W, Bujard H. Transcriptional activation by tetracyclines in mammalian cells. Science. 1995;268:1766–1769. doi: 10.1126/science.7792603. [DOI] [PubMed] [Google Scholar]
- Grether ME, Abrams JM, Agapite J, White K, Steller H. The head involution defective gene of Drosophila melanogaster functions in programmed cell death. Genes and Development. 1995;9:1694–1708. doi: 10.1101/gad.9.14.1694. [DOI] [PubMed] [Google Scholar]
- Gubler DJ. Resurgent vector-borne diseases as a global health problem. Emerging Infectious Diseases. 1998;4:442–450. doi: 10.3201/eid0403.980326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handler AM. A current perspective on insect gene transformation. Insect Biochemistry and Molecular Biology. 2001;31:111–128. doi: 10.1016/s0965-1748(00)00159-4. [DOI] [PubMed] [Google Scholar]
- Hendrichs J, Franz G, Rendon P. Increased effectiveness and applicability of the sterile insect technique through male-only releases for control of Mediterranean fruit flies during fruiting seasons. Journal of Applied Entomology. 1995;119:371–377. [Google Scholar]
- Hickey WA, Craig GB. Distortion of sex ratio in populations of Aedes aegypti. Canadian Journal of Genetics and Cytology. 1966;8:260–278. doi: 10.1139/g66-033. [DOI] [PubMed] [Google Scholar]
- Horn C, Wimmer EA. A transgene-based, embryo-specific lethality system for insect pest management. Nature Biotechnology. 2003;21:64–70. doi: 10.1038/nbt769. [DOI] [PubMed] [Google Scholar]
- Ito J, Ghosh A, Moreira LA, Wimmer EA, Jacobs-Lorena M. Transgenic anopheline mosquitoes impaired in transmission of a malaria parasite. Nature. 2002;417:452–455. doi: 10.1038/417452a. [DOI] [PubMed] [Google Scholar]
- Jacobs-Lorena M. Interrupting malaria transmission by genetic manipulation of anopheline mosquitoes. Journal of Vector Borne Diseases. 2003;40:73–77. [PubMed] [Google Scholar]
- Jasinskiene N, Coates CJ, James AA. Structure of hermes integrations in the germline of the yellow fever mosquito, Aedes aegypti . Insect Molecular Biology. 2000;9:11–18. doi: 10.1046/j.1365-2583.2000.00153.x. [DOI] [PubMed] [Google Scholar]
- Kim W, Koo H, Richman AM, Seeley D, Vizioli J, Klocko AD, O’Brochta DA. Ectopic expression of a cecropin trans-gene in the human malaria vector mosquito Anopheles gambiae (Diptera: Culicidae): effects on susceptibility to Plasmodium . Journal of Medical Entomology. 2004;41:447–455. doi: 10.1603/0022-2585-41.3.447. [DOI] [PubMed] [Google Scholar]
- Lampe DJ, Churchill MEA, Robertson HM. A purified mariner transposase is sufficient to mediate transposition in vitro. EMBO Journal. 1996;15:5470–5479. [PMC free article] [PubMed] [Google Scholar]
- Lobo NF, Clayton JR, Fraser MJ, Kafatos FC, Collins FH. High efficiency germ-line transformation of mosquitoes. Nature Protocols. 2006;1:1312–1317. doi: 10.1038/nprot.2006.221. [DOI] [PubMed] [Google Scholar]
- Moreira LA, Ghosh AK, Abraham EG, Jacobs-Lorena M. Genetic transformation of mosquitoes: a quest for malaria control. International Journal of Parasitology. 2002a;32:1599–1605. doi: 10.1016/s0020-7519(02)00188-1. [DOI] [PubMed] [Google Scholar]
- Moreira LA, Ito J, Ghosh A, et al. Bee venom phospholipase inhibits malaria parasite development in transgenic mosquitoes. Journal of Biological Chemistry. 2002b;277:40 839–40 843. doi: 10.1074/jbc.M206647200. [DOI] [PubMed] [Google Scholar]
- Moreira LA, Wang J, Collins FH, Jacobs-Lorena M. Fitness of anopheline mosquitoes expressing transgenes that inhibit Plasmodium development. Genetics. 2004;166:1337–1341. doi: 10.1534/genetics.166.3.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris AC. Microinjection of mosquito embryos. In: Crampton JM, Beard CB, Louis C, editors. The Molecular Biology of Insect Disease Vectors. London: Chapman & Hall; 1997. pp. 423–429. [Google Scholar]
- Osta MA, Christophides GK, Kafatos FC. Effects of mosquito genes on Plasmodium development. Science. 2004;303:2030–2032. doi: 10.1126/science.1091789. [DOI] [PubMed] [Google Scholar]
- Pennisi E. Meiotic drive. Bickering genes shape evolution. Science. 2003;301:1837–1839. doi: 10.1126/science.301.5641.1837. [DOI] [PubMed] [Google Scholar]
- Phuc HK, Andreasen MH, Burton RS, et al. Late-acting dominant lethal genetic systems and mosquito control. BMC Biology. 2007;5:11. doi: 10.1186/1741-7007-5-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesneville H, Anxolabehere D. Dynamics of transposable elements in metapopulations: a model of P element invasion in Drosophila . Theoretical Population Biology. 1998;54:175–193. doi: 10.1006/tpbi.1997.1353. [DOI] [PubMed] [Google Scholar]
- Rendon P, McInnis D, Lance D, Stewart J. Medfly (Diptera: Tephritidae) genetic sexing: large-scale field comparison of males-only and bisexual sterile fly releases in Guatemala. Journal of Economic Entomology. 2004;97:1547–1553. doi: 10.1603/0022-0493-97.5.1547. [DOI] [PubMed] [Google Scholar]
- Riehle MA, Jacobs-Lorena M. Using bacteria to express and display anti-parasite molecules in mosquitoes: current and future strategies. Insect Biochemistry and Molecular Biology. 2005;35:699–707. doi: 10.1016/j.ibmb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Riehle MA, Moreira CK, Lampe D, Lauzon C, Jacobs-Lorena M. Using bacteria to express and display anti-Plasmodium molecules in the mosquito midgut. International Journal of Parasitology. 2007;37:595–603. doi: 10.1016/j.ijpara.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Schneider DS, James AA. Bridging the gaps in vector biology. Workshop on the Molecular and Population Biology of Mosquitoes and other Disease Vectors. EMBO Reports. 2006;7:259–262. doi: 10.1038/sj.embor.7400643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Thibert C, Royer C, et al. Germline transformation of the silkworm Bombyx mori L. using a piggyBac transposon-derived vector. Nature Biotechnology. 2000;18:81–84. doi: 10.1038/71978. [DOI] [PubMed] [Google Scholar]
- Thomas DD, Donnelly CA, Wood RJ, Alphey LS. Insect population control using a dominant, repressible, lethal genetic system. Science. 2000;287:2474–2476. doi: 10.1126/science.287.5462.2474. [DOI] [PubMed] [Google Scholar]
- Vreysen MJ. Principles of area-wide integrated tsetse fly control using the sterile insect technique. Médecine Tropicale. 2001;61:397–411. [PubMed] [Google Scholar]
- Walters ML, Staten RT, Roberson RC. Pink bollworm integrated management using sterile insects under field trial conditions, Imperial Valley, California. In: Tan KH, editor. Area-Wide Control of Fruit Flies and Other Insect Pests. Penang, Malaysia: Penerbit Universiti Sains Malaysia; 2000. pp. 201–206. [Google Scholar]
- Warren WD, Atkinson PW, O’Brochta DA. The Hermes transposable element from the house fly, Musca domestica, is a short inverted repeat-type element of the hobo, Ac, and Tam3 (hAT) element family. Genetical Research. 1994;64:87–97. doi: 10.1017/s0016672300032699. [DOI] [PubMed] [Google Scholar]
- Wimmer EA. Innovations: applications of insect transgenesis. Nature Reviews Genetics. 2003;4:225–232. doi: 10.1038/nrg1021. [DOI] [PubMed] [Google Scholar]
- Wyss JH. Screwworm eradication in the Americas – overview. In: Tan KH, editor. Area-Wide Control of Fruit Flies and Other Insect Pests. Penang, Malaysia: Penerbit Universiti Sains Malaysia; 2000. pp. 79–86. [Google Scholar]