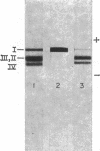
Abstract
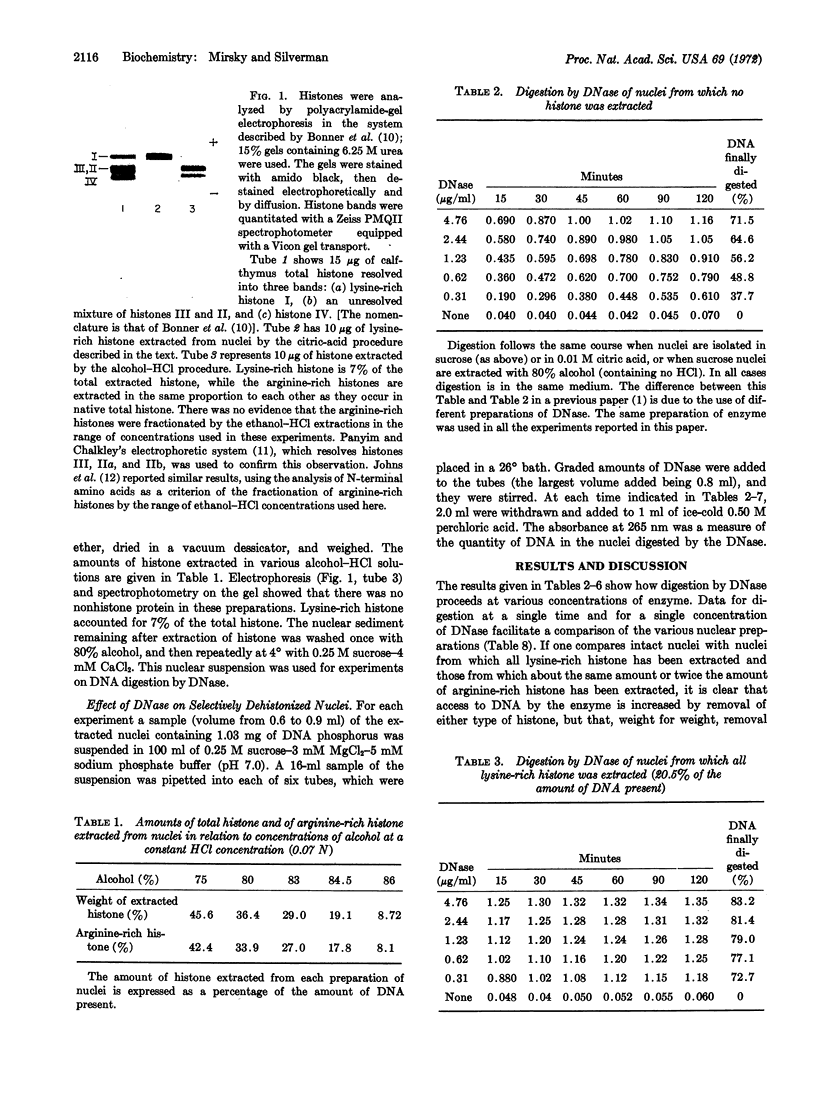
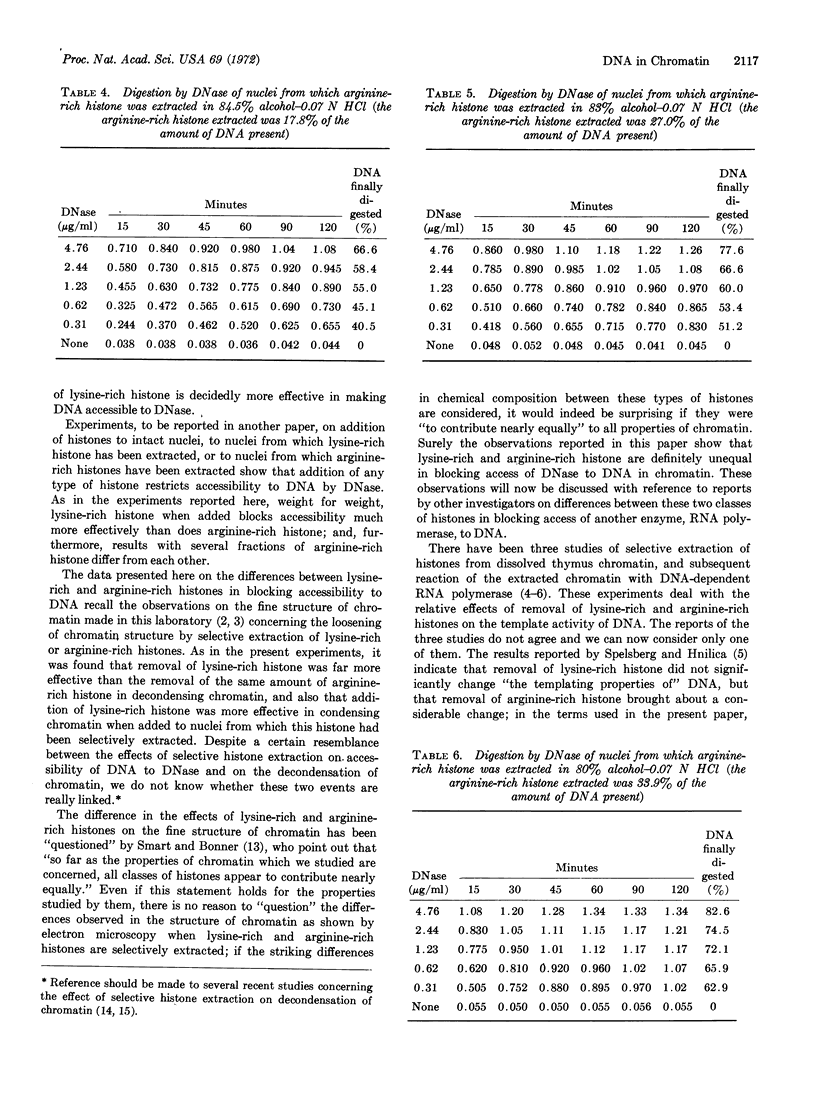
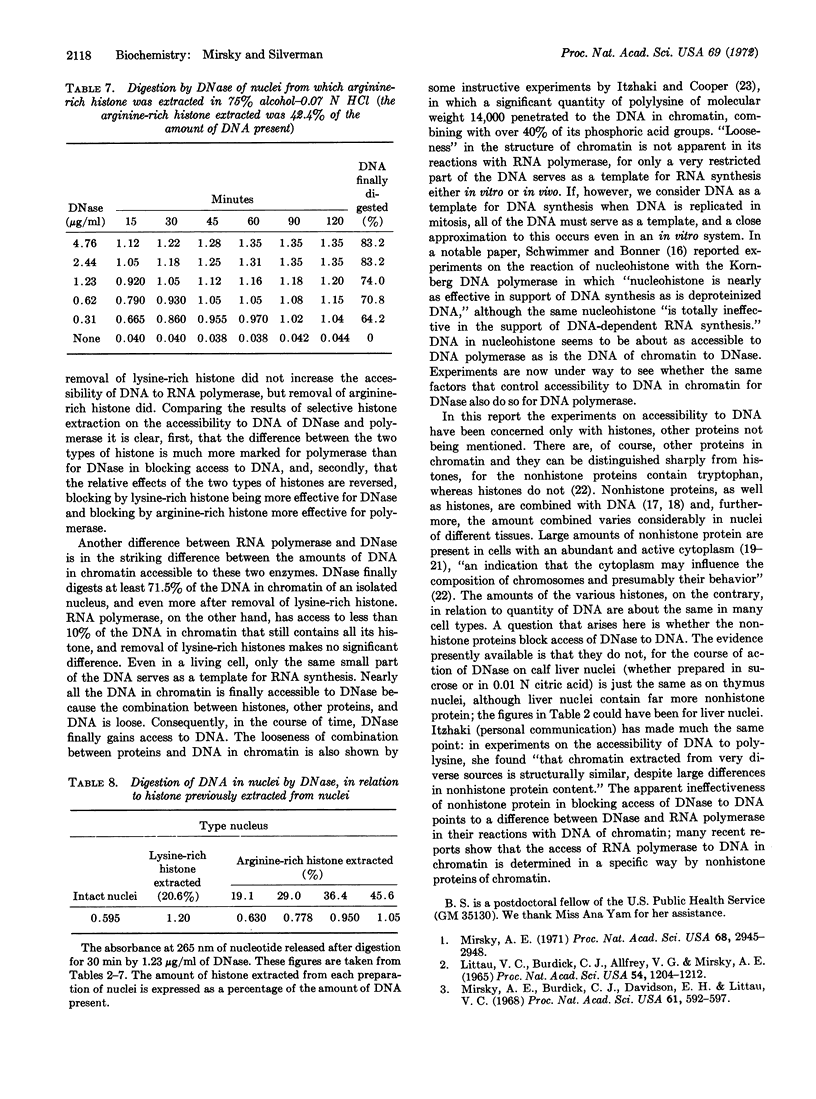
The effect of histones on accessibility of DNA to DNase in chromatin of thymus nuclei has been studied by selective extraction of either lysine-rich or arginine-rich histones. It was found that all histones block accessibility but that, weight for weight, lysine-rich histones block much more effectively than do arginine-rich histones. We point to the contrast between accessibility of DNA to DNase and of DNA to RNA polymerase, and to what may be the similarity between accessibility to DNase and DNA polymerase.
Keywords: DNase, RNA polymerase, DNA polymerase
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLFREY V. G., MIRSKY A. E., OSAWA S. Protein synthesis in isolated cell nuclei. J Gen Physiol. 1957 Jan 20;40(3):451–490. doi: 10.1085/jgp.40.3.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berlowitz L., Kitchin R., Pallotta D. Histones and RNA synthesis: selective binding of histones by a synthetic polyanion in calf thymus nuclei. Biochim Biophys Acta. 1972 Mar 14;262(2):160–168. doi: 10.1016/0005-2787(72)90229-8. [DOI] [PubMed] [Google Scholar]
- Brasch K., Seligy V. L., Setterfield G. Effects of low salt concentration on structural organization and template activity of chromatin in chicken erythrocyte nuclei. Exp Cell Res. 1971 Mar;65(1):61–72. doi: 10.1016/s0014-4827(71)80050-2. [DOI] [PubMed] [Google Scholar]
- DALY M. M., MIRSKY A. E. Histones with high lysine content. J Gen Physiol. 1955 Jan 20;38(3):405–413. doi: 10.1085/jgp.38.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DINGMAN C. W., SPORN M. B. STUDIES ON CHROMATIN. I. ISOLATION AND CHARACTERIZATION OF NUCLEAR COMPLEXES OF DEOXYRIBONUCLEIC ACID, RIBONUCLEIC ACID, AND PROTEIN FROM EMBRYONIC AND ADULT TISSUES OF THE CHICKEN. J Biol Chem. 1964 Oct;239:3483–3492. [PubMed] [Google Scholar]
- Georgiev G. P., Ananieva L. N., Kozlov J. V. Stepwise removal of protein from a deoxyribonucleoprotein complex and de-repression of the genome. J Mol Biol. 1966 Dec 28;22(2):365–371. doi: 10.1016/0022-2836(66)90140-9. [DOI] [PubMed] [Google Scholar]
- JOHNS E. W., PHILLIPS D. M., SIMSON P., BUTLER J. A. Improved fractionations of arginine-rich histones from calf thymus. Biochem J. 1960 Dec;77:631–636. doi: 10.1042/bj0770631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KUNITZ M. Crystalline desoxyribonuclease; isolation and general properties; spectrophotometric method for the measurement of desoxyribonuclease activity. J Gen Physiol. 1950 Mar;33(4):349–362. doi: 10.1085/jgp.33.4.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littau V. C., Burdick C. J., Allfrey V. G., Mirsky S. A. The role of histones in the maintenance of chromatin structure. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1204–1212. doi: 10.1073/pnas.54.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIRSKY A. E., RIS H. The composition and structure of isolated chromosomes. J Gen Physiol. 1951 May;34(5):475–492. doi: 10.1085/jgp.34.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MIRSKY A. E. The chemical composition of chromosomes. Harvey Lect. 1950 1951;Series 46:98–115. [PubMed] [Google Scholar]
- Mirsky A. E., Burdick C. J., Davidson E. H., Littau V. C. The role of lysine-rich histone in the maintenance of chromatin structure in metaphase chromosomes. Proc Natl Acad Sci U S A. 1968 Oct;61(2):592–597. doi: 10.1073/pnas.61.2.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirsky A. E. The structure of chromatin. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2945–2948. doi: 10.1073/pnas.68.12.2945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panyim S., Chalkley R. High resolution acrylamide gel electrophoresis of histones. Arch Biochem Biophys. 1969 Mar;130(1):337–346. doi: 10.1016/0003-9861(69)90042-3. [DOI] [PubMed] [Google Scholar]
- Schwimmer S., Bonner J. Nucleohistone as template for the replication of DNA. Biochim Biophys Acta. 1965 Sep 6;108(1):67–72. doi: 10.1016/0005-2787(65)90108-5. [DOI] [PubMed] [Google Scholar]
- Smart J. E., Bonner J. Studies on the role of histones in relation to the template activity and precipitability of chromatin at physiological ionic strengths. J Mol Biol. 1971 Jun 28;58(3):675–684. doi: 10.1016/0022-2836(71)90032-5. [DOI] [PubMed] [Google Scholar]
- Smart J. E., Bonner J. Studies on the role of histones in the structure of chromatin. J Mol Biol. 1971 Jun 28;58(3):661–674. doi: 10.1016/0022-2836(71)90031-3. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C., Hnilica L. S. Proteins of chromatin in template restriction. I. RNA synthesis in vitro. Biochim Biophys Acta. 1971 Jan 1;228(1):202–211. doi: 10.1016/0005-2787(71)90560-0. [DOI] [PubMed] [Google Scholar]
- Spelsberg T. C., Hnilica L. S. Proteins of chromatin in template restriction. II. Specificity of RNA synthesis. Biochim Biophys Acta. 1971 Jan 1;228(1):212–222. doi: 10.1016/0005-2787(71)90561-2. [DOI] [PubMed] [Google Scholar]
- Wang T. Y. The isolation, properties, and possible functions of chromatin acidic proteins. J Biol Chem. 1967 Mar 25;242(6):1220–1226. [PubMed] [Google Scholar]