Abstract
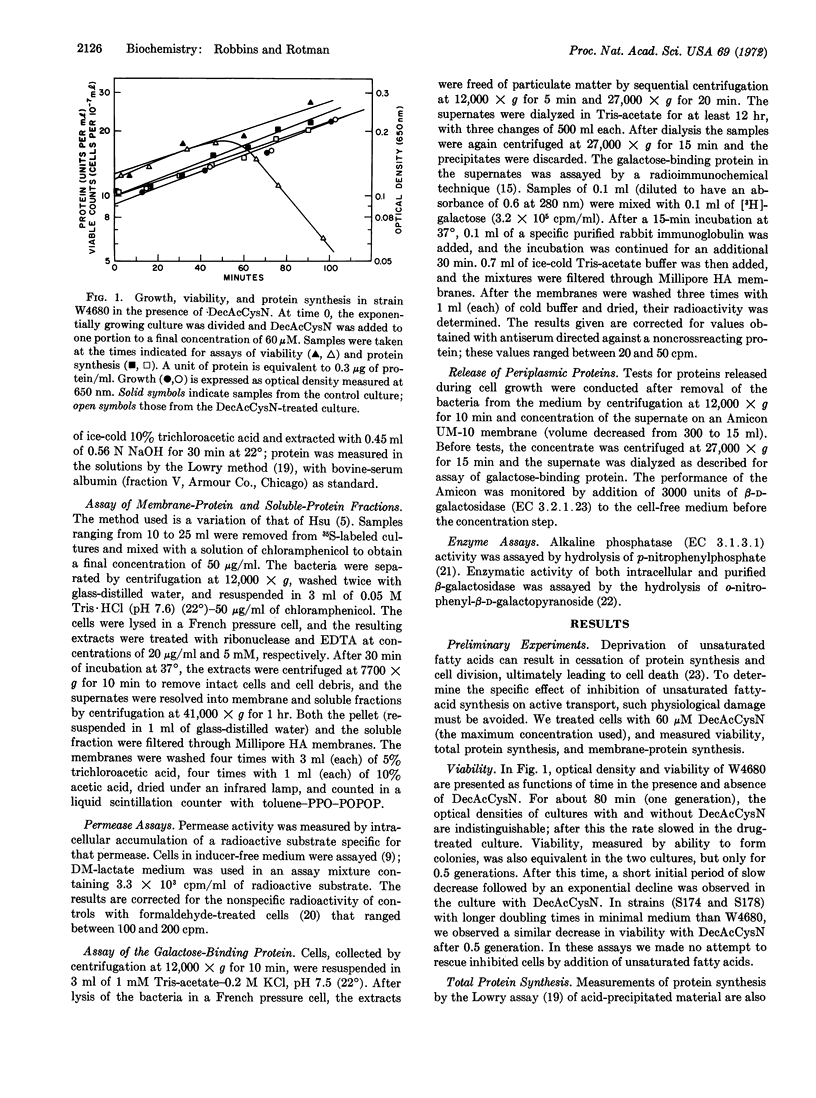
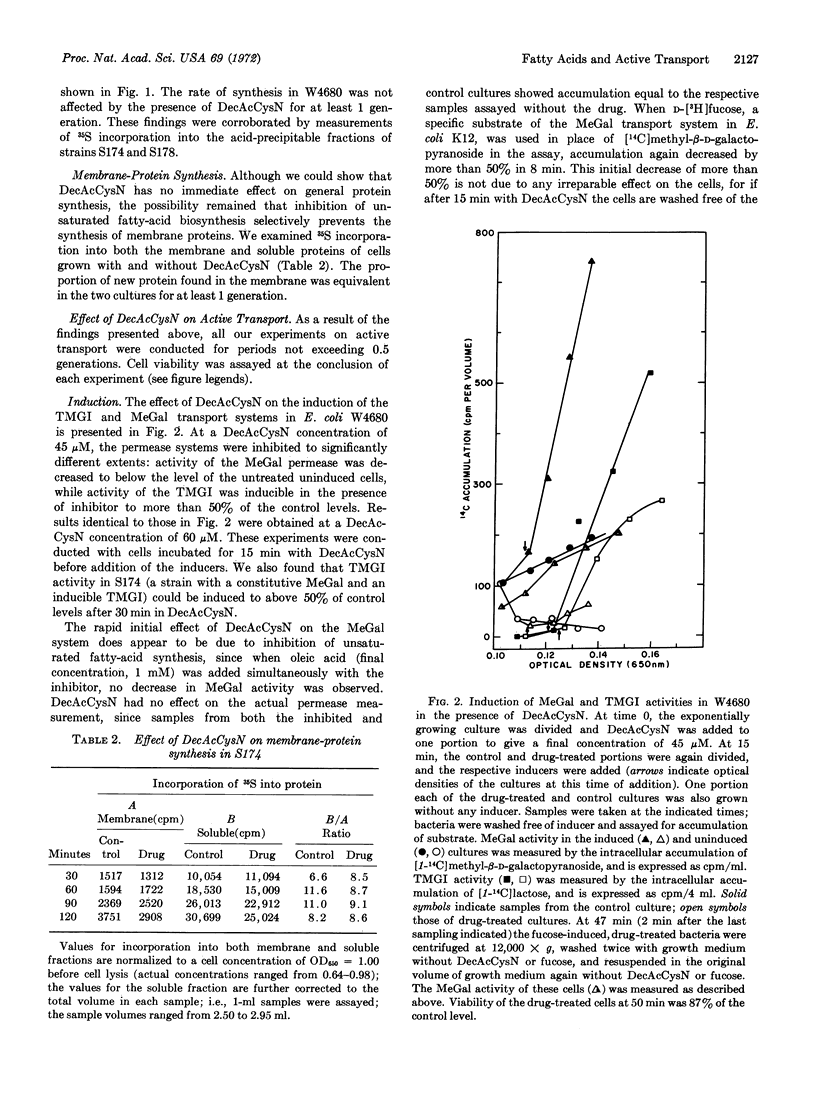
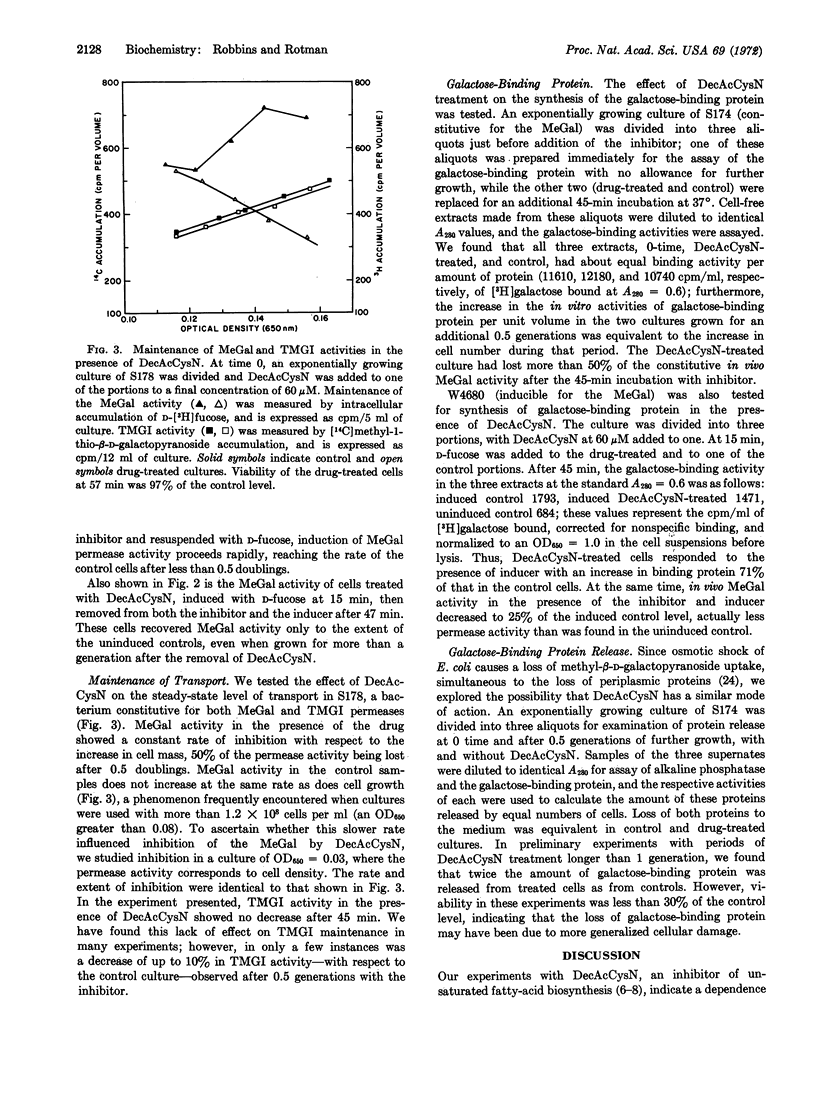
The activity of the methylgalactoside transport system of E. coli is impaired upon treatment with 3-decynoyl-N-acetylcysteamine, an inhibitor of unsaturated fatty-acid synthesis. Treated cells are unable to be induced for permease activity, while transport sites synthesized before treatment show a regular loss of activity. The inhibition of methylgalactoside transport occurs at a step after translation of the galactose-binding protein, a component of the permease, and appears to be highly specific, since drug-treated cells show normal viability, protein synthesis, and membrane integrity when transport activity is greatly reduced. A second transport system, the galactoside permease, shows significantly less sensitivity to the inhibitor. That the activity of this permease is maintained in the presence of this inhibitor suggests that the inhibitor does not impair energy coupling.
Keywords: thiomethylgalactoside, 3-decynoyl-N-acetylcysteamine, viability, protein synthesis, membrane structure
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anraku Y. Transport of sugars and amino acids in bacteria. I. Purification and specificity of the galactose- and leucine-binding proteins. J Biol Chem. 1968 Jun 10;243(11):3116–3122. [PubMed] [Google Scholar]
- Boos W., Lengeler J., Hermann K. O., Unsöld H. J. The regulation of the beta-methylgalactoside transport system and of the galactose binding protein of Escherichia coli K12. Eur J Biochem. 1971 Apr 30;19(4):457–470. doi: 10.1111/j.1432-1033.1971.tb01336.x. [DOI] [PubMed] [Google Scholar]
- Boos W., Sarvas M. O. Close linkage between a galactose binding protein and the beta-methylgalactoside permease in Escherichia coli. Eur J Biochem. 1970 Apr;13(3):526–533. doi: 10.1111/j.1432-1033.1970.tb00956.x. [DOI] [PubMed] [Google Scholar]
- Brock D. J., Kass L. R., Bloch K. Beta-hydroxydecanoyl thioester dehydrase. II. Mode of action. J Biol Chem. 1967 Oct 10;242(19):4432–4440. [PubMed] [Google Scholar]
- Fox C. F. A lipid requirement for induction of lactose transport in Escherichia coli. Proc Natl Acad Sci U S A. 1969 Jul;63(3):850–855. doi: 10.1073/pnas.63.3.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Carter J. R., Kennedy E. P. GENETIC CONTROL OF THE MEMBRANE PROTEIN COMPONENT OF THE LACTOSE TRANSPORT SYSTEM OF Escherichia coli. Proc Natl Acad Sci U S A. 1967 Mar;57(3):698–705. doi: 10.1073/pnas.57.3.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., LEVINTHAL C. A fine-structure genetic and chemical study of the enzyme alkaline phosphatase of E. coli. I. Purification and characterization of alkaline phosphatase. Biochim Biophys Acta. 1960 Mar 11;38:470–483. doi: 10.1016/0006-3002(60)91282-8. [DOI] [PubMed] [Google Scholar]
- Ganesan A. K., Rotman B. Transport systems for galactose and galactosides in Escherichia coli. I. Genetic determination and regulation of the methyl-galactoside permease. J Mol Biol. 1966 Mar;16(1):42–50. doi: 10.1016/s0022-2836(66)80261-9. [DOI] [PubMed] [Google Scholar]
- Hazelbauer G. L., Adler J. Role of the galactose binding protein in chemotaxis of Escherichia coli toward galactose. Nat New Biol. 1971 Mar 24;230(12):101–104. doi: 10.1038/newbio230101a0. [DOI] [PubMed] [Google Scholar]
- Helmkamp G. M., Jr, Bloch K. Beta-hydroxydecanoyl thioester dehydrase. Studies on molecular structure and active site. J Biol Chem. 1969 Nov 10;244(21):6014–6022. [PubMed] [Google Scholar]
- Helmkamp G. M., Jr, Brock D. J., Bloch K. Beta-hydroxydecanoly thioester dehydrase. Specificity of substrates and acetylenic inhibitors. J Biol Chem. 1968 Jun 25;243(12):3229–3231. [PubMed] [Google Scholar]
- Henning U., Dennert G., Rehn K., Deppe G. Effects of oleate starvation in a fatty acid auxotroph of Escherichia coli K-12. J Bacteriol. 1969 May;98(2):784–796. doi: 10.1128/jb.98.2.784-796.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- Hsu C. C., Fox C. F. Induction of the lactose transport system in a lipid-synthesis-defective mutant of Escherichia coli. J Bacteriol. 1970 Aug;103(2):410–416. doi: 10.1128/jb.103.2.410-416.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalckar H. M. The periplasmic galactose binding protein of Escherichia coli. Science. 1971 Nov 5;174(4009):557–565. doi: 10.1126/science.174.4009.557. [DOI] [PubMed] [Google Scholar]
- Kass L. R. The antibacterial activity of 3-decynoyl-n-acetylcysteamine. Inhibition in vivo of beta-hydroxydecanoyl thioester dehydrase. J Biol Chem. 1968 Jun 25;243(12):3223–3228. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Overath P., Hill F. F., Lamnek-Hirsch I. Biogenesis of E. coli membrane: evidence for randomization of lipid phase. Nat New Biol. 1971 Dec 29;234(52):264–267. doi: 10.1038/newbio234264a0. [DOI] [PubMed] [Google Scholar]
- Overath P., Schairer H. U., Stoffel W. Correlation of in vivo and in vitro phase transitions of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1970 Oct;67(2):606–612. doi: 10.1073/pnas.67.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROTMAN B., GUZMAN R. Transport of galactose from the inside to the outside of Escherichia coli. Pathol Biol. 1961 Apr;9:806–810. [PubMed] [Google Scholar]
- ROTMAN B. Separate permeases for the accumulation of methyl-beta-D-galactoside and methyl-beta-D-thiogalactoside in Escherichia coli. Biochim Biophys Acta. 1959 Apr;32:599–601. doi: 10.1016/0006-3002(59)90659-6. [DOI] [PubMed] [Google Scholar]
- Rotman B., Ganesan A. K., Guzman R. Transport systems for galactose and galactosides in Escherichia coli. II. Substrate and inducer specificities. J Mol Biol. 1968 Sep 14;36(2):247–260. doi: 10.1016/0022-2836(68)90379-3. [DOI] [PubMed] [Google Scholar]
- Silbert D. F., Ruch F., Vagelos P. R. Fatty acid replacements in a fatty acid auxotroph of Escherichia coli. J Bacteriol. 1968 May;95(5):1658–1665. doi: 10.1128/jb.95.5.1658-1665.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L. Current linkage map of Escherichia coli. Bacteriol Rev. 1970 Jun;34(2):155–175. doi: 10.1128/br.34.2.155-175.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G., Fox C. F. Biogenesis of microbial transport systems: evidnce for coupled incorporation of newly synthesized lipids and proteins into membrane. J Mol Biol. 1971 Jan 14;55(1):49–60. doi: 10.1016/0022-2836(71)90280-4. [DOI] [PubMed] [Google Scholar]



