Abstract
OBJECTIVES
Latin America has a high prevalence of Helicobacter pylori infection and associated diseases, including gastric cancer. Antibiotic therapy can eradicate the bacterial infection and decrease associated morbidity and mortality. To tailor recommendations for optimal treatments, we summarized published literature and calculated region- and country-specific prevalences of antibiotic resistance.
METHODS
Searches of PubMed and regional databases for observational studies evaluating H. pylori antibiotic resistance yielded a total of 59 independent studies (56 in adults, 2 in children, and 1 in both groups) published up to October 2013 regarding H. pylori isolates collected between 1988 and 2011. Study-specific prevalences of primary resistance to commonly prescribed antibiotics were summarized using random-effects models. Between-study heterogeneity was assessed by meta-regression. As a sensitivity analysis, we extended our research to studies of patients with prior H. pylori-eradication therapy.
RESULTS
Summary prevalences of antimicrobial primary resistance among adults varied by antibiotic, including 12% for clarithromycin (n = 35 studies), 53% for metronidazole (n = 34), 4% for amoxicillin (n = 28), 6% for tetracycline (n = 20), 3% for furazolidone (n = 6), 15% for fluoroquinolones (n = 5), and 8% for dual clarithromycin and metronidazole (n = 10). Resistance prevalence varied significantly by country, but not by year of sample collection. Analyses including studies of patients with prior therapy yielded similar estimates. Pediatric reports were too few to be summarized by meta-analysis.
CONCLUSIONS
Resistance to first-line anti- H. pylori antibiotics is high in Latin American populations. In some countries, the empirical use of clarithromycin without susceptibility testing may not be appropriate. These findings stress the need for appropriate surveillance programs, improved antimicrobial regulations, and increased public awareness.
INTRODUCTION
Chronic Helicobacter pylori infection is causally related to serious benign and malignant upper gastrointestinal diseases, including peptic ulcer, gastric mucosa-associated lymphoid tissue lymphoma, and gastric cancer (1). Conversely, eradication of H. pylori is associated with ulcer healing (2), regression of mucosa-associated associated lymphoid tissue lymphoma (3), and decreased cancer risk (4). Successful treatment for H. pylori infection requires multidrug regimens, which are frequently based on clarithromycin as the central component. Eradication rates vary with level of antibiotic resistance (5,6), and, according to one guideline (7), the use of clarithromycin without susceptibility testing is not recommended in populations with more than 15–20% prevalence of resistant isolates.
Many Latin American countries have a high burden of H. pylori infection (8,9) and associated diseases, particularly gastric cancer (10). This geographic region also has multiple avenues of unfettered access to antibiotics, including self-medication, unnecessary prescriptions, and lax regulation of sales (11). In order to guide treatment choice and tailor eradication strategies for Latin American populations, we summarized the published literature on H. pylori antibiotic resistance in the region.
METHODS
Review methods and reporting were performed according to the PRISMA guidelines (12).
Search strategy and selection criteria
The literature databases PubMed (United States National Library of Medicine, Bethesda, MD), LILACS (Latin America and the Caribbean Literature on Health Sciences; http://lilacs.bvsalud.org/en), and SciELO (Scientific Electronic Library Online; http://www.scielo.org) were searched for observational studies evaluating H. pylori antibiotic resistance in the 20 countries comprising Latin America, as defined by the United Nations Educational Scientific and Cultural Organization (13), published in any language up to 31 October 2013.
To identify studies in PubMed, the following search strategy was used: Helicobacter pylori, and antimicrobial resistance or antibiotic resistance or “ Drug Resistance, Microbial ” [Mesh] or “ Microbial Sensitivity Tests ” [Mesh] and Latin America or Central America or South America or Argentina or Aruba or Bolivia or Brazil or Colombia or Costa Rica or Cuba or Chile, or Dominican Republic or Ecuador or El Salvador or Guatemala or Honduras or Mexico or Nicaragua or Panama or Paraguay or Peru or Uruguay or Venezuela. Analogous strategies were used to search the other two databases.
Two investigators (C.A.C. and T.H.-G.) independently reviewed titles and abstracts for selection of potentially relevant articles; any disagreement was resolved by consulting a third reviewer (A.G.). Full-text articles were retrieved for potential inclusion if data on resistance to at least one antibiotic were reported. Citations of retrieved articles were reviewed for studies that may have been missed or were absent from our database queries.
The following information was abstracted from each selected article: first author, year of publication, study location (country), year of sample collection, participant age (range or mean), prior antibiotic treatment for H. pylori, number of patients, histologic diagnoses, number of samples (gastric biopsies or H. pylori isolates), prevalence of antibiotic resistance, and method of resistance assessment (agar dilution, E-test, disk diffusion, or detection of point mutations by polymerase chain reaction). To ensure comparability across studies, we contacted the corresponding authors to enquire about missing data on prior antibiotic treatment for H. pylori. In all, 20 of the 21 authors contacted provided unpublished information. We also obtained additional unpublished data for 11 studies on year of sample collection and potentially overlapping sample sets.
In order to assess quality (risk of bias) of the included studies, we evaluated the following characteristics: (1) representativeness of the patients; (2) consecutive or random selection for inclusion; (3) adequacy of description of patient characteristics (e.g., demographics, year of sample collection, histologic diagnoses, and so on); (4) accounting of study flow (e.g., percentage of not consenting, percentage of failed cultures, percentage of inconclusive results, and so on); and (5) validity of testing methodology. Each domain was coded individually as “ + ” (i.e., low risk of bias), “ − ” (i.e., high risk of bias), or “?” (i.e., unclear).
Statistical analysis
All of our analyses were restricted to adults. We used random-effects models (14) to summarize double arcsine – transformed (15) prevalences of primary resistance to antibiotics, for which more than five articles were identified. Summary prevalences and corresponding 95% confidence intervals (CIs) were calculated by back-transformation. Study-specific results are presented for antibiotics reported in two to four articles, but antibiotics reported in a single study were not summarized. Given their similar antimicrobial activity, data on levofloxacin and ciprofloxacin resistance were combined. Between-study heterogeneity was assessed for statistical significance using the Q test and quantified with the I2 statistic as low (<25%), moderate (25–50%), or high (>50%) (16). If moderate or high heterogeneity was identified for a given antibiotic, meta-regression models were used to examine the extent to which country, year of sample collection (1988–1995, 1996–2000, 2001–2005, or 2006–2011), or test method may be explanatory. For one study that reported antibiotic resistance by anatomic subsite of sample collection (i.e., antrum or corpus), we used a random-effects model to estimate the overall prevalence. For 15 articles that did not state when samples were collected, year of collection was imputed as being 4 years prior to publication on the basis of the median difference between sample collection and publication year for the remaining articles. Sensitivity analyses were performed by including studies that evaluated secondary resistance (i.e., with prior antibiotic treatment for H. pylori) as well as those that did not specify prior antibiotic exposure.
For each of the evaluated antibiotics, publication bias was investigated by visual inspection of Begg’s funnel plots (17) and was formally tested using Egger’s regression asymmetry method (18). Meta-analyses were performed with Stata version 11 (Stata-Corp, College Station, TX, USA) using a combination of published macros (19). A P value of less than 0.05 was considered statistically significant for all tests, except the heterogeneity and Egger tests for which a P value less than 0.10 was considered significant. All statistical tests were two-sided.
RESULTS
Literature search and description of studies
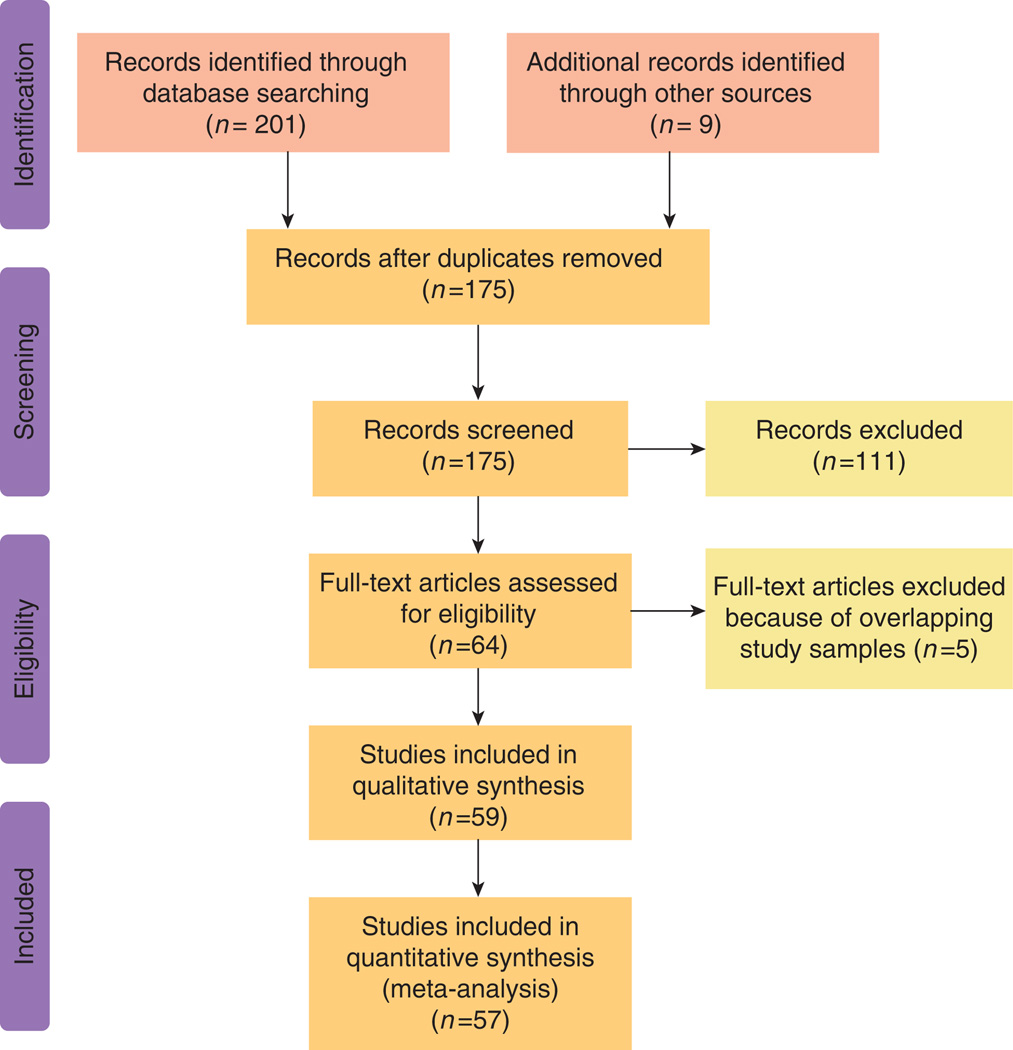
The literature searches identified a total of 201 articles: 106 from PubMed, 49 from LILACS, and 46 from SciELO (Figure 1). After excluding 146 irrelevant or duplicate publications, 55 full-text articles were retrieved for further evaluation; 9 additional publications were identified from citations of these articles. Five articles were excluded because the respective authors had other publications involving the same antibiotic in larger, but overlapping, samples. Thus, a total of 59 articles (38 written in English and 21 in Spanish) reporting on samples collected between 1988 and 2011 for assessing resistance to clarithromycin, metronidazole, amoxicillin, tetracycline, furazolidone, levofloxacin, and/or ciprofloxacin were included in this analysis (Supplementary Table online). Other antibiotics that were reported in single studies and therefore were not summarized included azithromycin, trovafloxacin, ampicillin, and doxycycline.
Figure 1.
Flow diagram of the literature search.
Fifty-six studies reported on adults, two only on children and one had both population groups. A total of 14 studies were conducted in Brazil, 11 in Colombia, 9 in Mexico, 5 in Chile, 5 in Argentina, 3 in Peru, 3 in Cuba, 3 in Costa Rica, 2 in Venezuela, 2 in Ecuador, 1 in Paraguay, and 1 in Uruguay. The total sample size ranged from 15 to 395 H. pylori isolates. In all, 6 studies included samples collected during the period 1988–1995, 20 during 1996–2000, 17 during 2001–2005, and 16 during 2006–2011. Forty-eight (81%) studies evaluated resistance by either agar dilution or E-test. Regarding the type of resistance, 50 (85%) studies evaluated only primary resistance, 8 (13%) included secondary resistance, and 1 (2%) did not specify this information.
Assessment of study quality
In general, the reviewed studies included a representative spectrum of patients and used valid methods to assess resistance (Table 1). There were some deficiencies with regard to the reporting of sampling strategy and patient characteristics. Nevertheless, based on the information for sample collection period, it seems likely that most studies used consecutive or random selection.
Table 1.
Methodological assessment of risk of bias
| Authors | Representativeness | Consecutive or random selection |
Adequate description of patient characteristics |
Accounting of study flow |
Validity of testing methodology |
|---|---|---|---|---|---|
| Argentina | |||||
| Vega et al. (1) | + | ? | Except collection time | + | + |
| Matteo et al.a (2) | ? | ? | Except age and diagnosis | + | + |
| Stege et al.b (3) | ? | ? | Except age | + | + |
| Matteo et al.a (4) | ? | ? | Except age and diagnosis | + | + |
| Antelo et al. (5) | + | + | Except collection time | + | + |
| Brazil | |||||
| Ogata et al. (6) | + | + | + | + | + |
| Suzuki et al.a (7) | + | + | + | + | + |
| Eisig et al. (8) | + | ? | Except collection time | + | + |
| Lins et al. (9) | + | + | + | + | + |
| Garcia et al. (10) | + | + | + | + | + |
| Eisig et al. (11) | + | ? | Except collection time | + | − |
| Godoy et al.a (12) | + | ? | Except collection time | + | + |
| Magalhaes Queiroz et al. (13) | + | + | Except collection time | + | + |
| Prazeres Magalhães et al. (14) | ? | ? | Except collection time and age | + | + |
| Ecclissato et al. (15) | ? | + | Except collection time | + | + |
| Mendonça et al. (16) | + | + | Except collection time | + | + |
| van Doorn et al. (17) | ? | ? | Missing collection time, age and diagnosis |
− | + |
| Salazar et al.a (18) | + | ? | Except age | + | + |
| Queiroz et al.b (19) | ? | ? | Except collection time | − | + |
| Chile | |||||
| Otth et al.a (20) | + | ? | + | + | + |
| Toledo et al. (21) | + | ? | Except age and diagnosis | + | + |
| Soto et al.a (22) | ? | ? | − | ? | + |
| Vallejos et al. (23) | + | ? | + | + | + |
| González et al.a (24) | + | ? | Except age | + | − |
| Colombia | |||||
| Bustamante-Rengifo et al. (25) | + | ? | + | + | + |
| Figueroa et al.a (26) | + | ? | + | + | + |
| Trespalacios et al.a (27) | + | ? | + | + | + |
| Trespalacios et al. (28) | + | ? | + | + | + |
| Henao et al. (29) | + | ? | + | + | + |
| Henao-Riveros et al. (30) | + | ? | + | + | − |
| Alvarez et al.a (31) | + | − | Except age | + | + |
| Alvarez et al.a (32). | + | − | Except age | − | + |
| Yepes et al (33) | + | + | + | + | − |
| Isaza et al. (34) | ? | ? | + | + | + |
| Gutierrez et al.a (35) | + | ? | + | + | + |
| Costa Rica | |||||
| Lang et al. (36) | + | ? | + | + | + |
| Rivas et al. (37) | + | ? | Missing collection time, age and diagnosis |
+ | + |
| Quintana-Guzmán et al. (38) | + | ? | Missing collection time, resistance type, age and diagnosis |
+ | − |
| Cuba | |||||
| Reyes-Zamora et al.a (39) | + | ? | Except age | + | + |
| Llanes et al.a (40) | + | + | + | + | + |
| Gutiérrez et al.a (41) | + | ? | Except age | + | + |
| Ecuador | |||||
| Zurita et al. (42) | + | ? | + | + | + |
| Debets-Ossenkopp et al.a (43) | + | ? | Except age | + | + |
| Mexico | |||||
| Ayala et al.a (44) | + | ? | Except diagnosis | + | + |
| Garza-Gonzalez et al. (45) | + | ? | + | + | + |
| Morales-Espinosa et al.a (46) | ? | ? | Except age | + | + |
| Chihu et al.a (47) | + | − | + | + | + |
| Garza-Gonzalez et al. (48) | + | ? | + | + | + |
| Dehesa et al. (49) | + | ? | Except collection time | + | + |
| Torres et al. (50) | + | ? | + | + | + |
| Dehesa et al. (51) | ? | ? | + | + | + |
| Lopez-Vidal et al. (52) | + | − | Except age | + | + |
| Paraguay | |||||
| Fariña et al. (53) | + | ? | + | + | + |
| Peru | |||||
| Mochizuki-Tamayo et al. (54) | + | + | + | + | + |
| Berg et al.a (55) | + | ? | + | + | − |
| Vasquez et al.a (56) | ? | ? | Except collection time and age | + | + |
| Uruguay | |||||
| Torres-Debat et al. (57) | + | − | Except diagnosis | + | + |
| Venezuela | |||||
| Ortiz et al.a (58) | + | ? | + | + | + |
| Urrestarazu et al.a (59) | + | ? | + | + | + |
+, low; −, high; ?, unclear.
Reference numbers correspond to online Supplementary Information.
Supplemented with unpublished data provided by personal communication.
Letter to the Editor.
Summary estimates of resistance in adults
Clarithromycin
Thirty-five studies examined primary resistance to clarithromycin. Study-specific prevalences ranged from 0% to 60% (Supplementary Figure S1). The overall summary prevalence was 12%, with high between-study heterogeneity (Table 2). Peru (50%, n = 1) reported the highest prevalence, where as Paraguay (2%, n = 1) reported the lowest. Pre valences did not differ significantly by period of collection (Table 2). Furthermore, prevalence estimates did not vary significantly by test method (P= 0.9): the estimates were 13% (n = 16) for agar dilution, 12% (n = 14) for E-test, 14% (n = 4) for disk diffusion, and 8% (n = 1) for PCR. The summary prevalence including studies evaluating secondary (n = 7) resistance was 12% (95% CI, 9–16%; n = 42).
Table 2.
Overall and period-specific summary prevalences of H. pylori antibiotic resistance among adults in Latin America
| Antibiotic | No. of studies | Summary prevalence of resistance (95% CI), % |
PQ for heterogeneity |
I2 for heterogeneity, % |
PEgger’s for publication bias |
|---|---|---|---|---|---|
| Clarithromycin | |||||
| Primary | 35 | 12 (9–16) | < 0.001 | 89.7 | 0.11 |
| Study period (P trend: 0.46a) | |||||
| 1988–1995 | 1 | 50 | — | — | |
| 1996–2000 | 15 | 10 (6–15) | < 0.001 | 86.0 | |
| 2001–2005 | 10 | 13 (5–23) | < 0.001 | 93.4 | |
| 2006–2011 | 9 | 13 (8–20) | < 0.001 | 88.3 | |
| All studies | 42 | 12 (9–16) | < 0.001 | 88.7 | 0.10 |
| Study period (P trend: 0.47a) | |||||
| 1988–1995 | 1 | 50 | — | — | |
| 1996–2000 | 19 | 11 (7–15) | < 0.001 | 85.1 | |
| 2001–2005 | 12 | 12 (5–21) | < 0.001 | 92.8 | |
| 2006–2011 | 10 | 14 (9–20) | < 0.001 | 86.8 | |
| Metronidazole | |||||
| Primary | 34 | 53 (46–60) | < 0.001 | 92.0 | 0.85 |
| Study period (P trend: 0.22) | |||||
| 1988–1995 | 5 | 65 (57–72) | 0.593 | 0 | |
| 1996–2000 | 14 | 54 (44–63) | < 0.001 | 90.9 | |
| 2001–2005 | 9 | 47 (30–64) | < 0.001 | 93.3 | |
| 2006–2011 | 6 | 50 (29–71) | < 0.001 | 95.7 | |
| All studies | 40 | 56 (49–63) | < 0.001 | 92.6 | 0.57 |
| Study period (P trend: 0.15) | |||||
| 1988–1995 | 6 | 71 (57–83) | < 0.001 | 78.4 | |
| 1996–2000 | 17 | 55 (47–63) | < 0.001 | 89.7 | |
| 2001–2005 | 11 | 54 (39–70) | < 0.001 | 94.1 | |
| 2006–2011 | 6 | 50 (29–71) | < 0.001 | 95.7 | |
| Amoxicillin | |||||
| Primary | 28 | 4 (2–8) | < 0.001 | 93.4 | 0.69 |
| Study period (P trend: 0.78) | |||||
| 1988–1995 | 2 | 1 (0–3) | 0.862 | 0 | |
| 1996–2000 | 11 | 7 (1–17) | < 0.001 | 95.6 | |
| 2001–2005 | 8 | 3 (1–8) | < 0.001 | 79.7 | |
| 2006–2011 | 7 | 4 (0–9) | < 0.001 | 92.6 | |
| All studies | 34 | 4 (2–8) | < 0.001 | 92.8 | 0.74 |
| Study period (P trend: 0.35) | |||||
| 1988–1995 | 3 | 10 (2–51) | < 0.001 | 96.6 | |
| 1996–2000 | 14 | 5 (1–12) | < 0.001 | 94.8 | |
| 2001–2005 | 10 | 3 (1–6) | < 0.001 | 75.2 | |
| 2006–2011 | 7 | 4 (0–9) | < 0.001 | 92.6 | |
| Tetracycline | |||||
| Primary | 20 | 6 (2–14) | < 0.001 | 96.4 | 0.13 |
| Study period (P trend: 0.61) | |||||
| 1988–1995 | 2 | 1 (0–5) | 0.855 | 0 | |
| 1996–2000 | 7 | 5 (1–11) | < 0.001 | 84.9 | |
| 2001–2005 | 6 | 13 (0–47) | < 0.001 | 98.8 | |
| 2006–2011 | 5 | 5 (0–15) | < 0.001 | 92.6 | |
| All studies | 24 | 7 (2–14) | < 0.001 | 96.6 | 0.10 |
| Study period (P trend: 0.62) | |||||
| 1988–1995 | 3 | 18 (7–83) | < 0.001 | 97.9 | |
| 1996–2000 | 8 | 4 (1–9) | < 0.001 | 83.7 | |
| 2001–2005 | 8 | 8 (0–30) | < 0.001 | 98.3 | |
| 2006–2011 | 5 | 5 (0–15) | < 0.001 | 92.6 | |
| Furazolidone | |||||
| Primary | 6 | 3 (0–9) | < 0.001 | 85.4 | 0.31 |
| Study period (P trend: NA) | |||||
| 1988–1995 | 1 | 0 | — | — | |
| 1996–2000 | 4 | 6 (1–14) | < 0.001 | 88.2 | |
| 2006–2011 | 1 | 0 | — | — | |
| Levofloxacin or ciprofloxacin | |||||
| Primary | 5 | 15 (6–28) | < 0.001 | 91.8 | 0.94 |
| Study period (P trend: NA) | |||||
| 2001–2005 | 1 | 4 | — | — | |
| 2006–2011 | 4 | 19 (7–34) | < 0.001 | 91.6 | |
| All studies | 15 (7–25) | < 0.001 | 88.6 | 0.93 | |
| Study period (P trend: 0.39) | |||||
| 1988–1995 | 1 | 7 | — | — | |
| 2001–2005 | 2 | 12 (0–35) | 0.002 | 89.2 | |
| 2006–2011 | 4 | 19 (7–34) | < 0.001 | 91.6 | |
| Clarithromycin and Metronidazole | |||||
| Primary | 10 | 8 (5–12) | < 0.001 | 74.6 | 0.34 |
| Study period (P trend: 0.35) | |||||
| 1996–2000 | 6 | 10 (5–16) | < 0.001 | 81.6 | |
| 2001–2005 | 3 | 5 (3–9) | 0.33 | 9.8 | |
| 2006–2011 | 1 | 5 | — | — | |
| All studies | 13 | 7 (4–10) | < 0.001 | 73.0 | 0.15 |
| Study period (P trend: 0.39) | |||||
| 1996–2000 | 8 | 8 (4–13) | < 0.001 | 78.7 | |
| 2001–2005 | 4 | 4 (2–7) | 0.360 | 6.6 | |
| 2006–2011 | 1 | 5 | — | — |
CI, confidence interval; NA, not applicable.
Excluding the period 1988–1995.
Metronidazole
Prevalences of primary resistance to metronidazole were reported in 34 studies. Study-specific prevalences ranged from 12.5% to 95% (Supplementary Figure S2). The summary prevalence was 53% with high heterogeneity among studies (Table 2). The highest resistance was reported in Colombia (83%, n = 4; Table 3), whereas the lowest was reported in Argentina (30%, n = 3). Prevalences did not differ significantly by period of collection (Table 2). Furthermore, prevalence estimates did not vary significantly by test method (P = 0.3): the estimates were 44% for agar dilution (n = 13), 57% (n = 16) for E-test, 62% (n = 3) for disk diffusion, and 59% (n =2) for PCR. The summary prevalence including studies evaluating secondary (n = 5) or unspecified (n = 1) resistance was 56% (95% CI, 49–63%; n = 40).
Table 3.
Country-specific summary prevalences of H. pylori antibiotic resistance among adults in Latin America
| Country | Clarithromycin | Metronidazole | Amoxicillin | Tetracycline | Furazolidone | Clarithromycin and metronidazole | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| No. of studies |
Resistance, % (95% CI) |
No. of studies |
Resistance, % (95% CI) |
No. of studies |
Resistance, % (95% CI) |
No. of studies |
Resistance, % (95% CI) |
No. of studies |
Resistance, % (95% CI) |
No. of studies |
Resistance, % (95% CI) |
|
| Primary | ||||||||||||
| Argentina | 3 | 14 (3–31) | 3 | 30 (20–42) | 3 | 0 (0–1) | 1 | NA | ||||
| Brazil | 7 | 11 (6–18) | 7 | 54 (47–61) | 5 | 15 (2–36) | 5 | 2 (0–9) | 6 | 3 (0–9) | 2 | 5 (1–11) |
| Chile | 3 | 9 (2–21) | 4 | 31 (16–49) | 3 | 2 (0–5) | 3 | 14 (0–45) | 2 | 7 (4–12) | ||
| Colombia | 7 | 18 (7–31) | 4 | 83 (73–90) | 5 | 7 (2–14) | 1 | NA | ||||
| Costa Rica | 2 | 6 (3–11) | 2 | 42 (34–50) | 2 | 3 (0–11) | 2 | 6 (2–38) | ||||
| Mexico | 7 | 13 (7–20) | 7 | 60 (47–72) | 6 | 4 (0–13) | 3 | 2 (0–9) | 4 | 13 (6–21) | ||
| Peru | 1 | NA | 2 | 66 (51–79) | 1 | NA | ||||||
| Venezuela | 2 | 8 (3–16) | 2 | 59 (41–77) | 2 | 0 (0–4) | 2 | 5 (1–13) | 1 | NA | ||
| All studies | ||||||||||||
| Argentina | 3 | 14 (3–31) | 3 | 30 (20–42) | 3 | 0 (0–1) | 1 | NA | ||||
| Brazil | 9 | 14 (8–21) | 7 | 54 (47–61) | 5 | 15 (2–36) | 5 | 2 (0–9) | 6 | 3 (0–9) | 2 | 5 (1–11) |
| Chile | 3 | 9 (2–21) | 4 | 31 (16–49) | 3 | 2 (0–5) | 3 | 14 (0–45) | 2 | 7 (4–12) | ||
| Colombia | 8 | 16 (7–28) | 5 | 83 (76–89) | 6 | 6 (2–12) | 2 | 33 (0–98) | 1 | NA | ||
| Costa Rica | 2 | 6 (3–11) | 3 | 63 (27–92) | 3 | 13 (0–46) | 3 | 26 (0–82) | ||||
| Cuba | 2 | 6 (1–16) | 2 | 68 (30–95) | 1 | NA | 2 | 5 (0–26) | ||||
| Ecuador | 2 | 9 (3–17) | 2 | 66 (32–92) | 2 | 0 (0–4) | 1 | NA | 1 | NA | ||
| Mexico | 8 | 12 (7–19) | 8 | 59 (48–70) | 7 | 3 (0–11) | 3 | 2 (0–9) | 5 | 11 (6–18) | ||
| Peru | 1 | NA | 2 | 66 (51–79) | 1 | NA | ||||||
| Venezuela | 2 | 8 (3–16) | 2 | 59 (41–77) | 2 | 0 (0–4) | 2 | 5 (1–13) | 1 | NA | ||
CI, confidence interval; NA, not applicable.
Summary prevalences not presented for single studies from Paraguay and from Uruguay.
Amoxicillin
Twenty-eight studies examined primary resistance to amoxicillin. Study-specific prevalences ranged from 0% to 39%. The summary prevalence was 4%, with high heterogeneity among the studies (Table 2). The highest resistance was reported in Brazil (15%, n = 5; Table 3). Prevalence did not differ significantly by period of collection (Table 2). Furthermore, prevalence estimates did not vary significantly by test method (P = 0.5): the estimates were 6% (n =14) for agar dilution, 3% (n =12) for E-test, and 3% (n =2) for disk diffusion. The summary prevalence including studies evaluating secondary (n = 5) or unspecified (n = 1) resistance was 4% (95% CI, 2–8%; n = 34).
Tetracycline
Primary resistance to tetracycline was evaluated in 20 studies. Study-specific prevalences ranged from 0% to 86%. The summary prevalence was 6% with high between-study heterogeneity (Table 2). The highest resistance was reported in Colombia (86%, n = 1; Table 3). Prevalence did not differ significantly by period of collection (Table 2). However, prevalence estimates varied significantly by test method (P= 0.0002): the estimates were 6% (n = 9) for agar dilution, 1% (n = 7) for E-test, 86% (n = 1) for disk diffusion, and 10% (n =3) for PCR. The summary prevalence including studies evaluating secondary (n = 3) or unspecified (n = 1) resistance was 7% (95% CI, 2–14%; n = 24).
Furazolidone
Primary resistance to furazolidone was studied only in Brazil (n = 6), with study-specific prevalences ranging from 0% to 14%. The summary prevalence was 3% with high heterogeneity across studies (Table 2). Prevalences did not differ significantly by period of collection (Table 2). All studies used agar dilution to assess resistance.
Fluoroquinolones
Five studies provided results about primary resistance to levofloxacin or ciprofloxacin (Supplementary Table) with study-specific prevalences ranging from 4% to 37%. The summary prevalence was 15% with high heterogeneity across studies (Table 2). Four studies used E-test to assess resistance (11%; 95% CI, 4–19%). The summary prevalence including studies evaluating secondary (n = 1) or unspecified (n = 1) resistance was 15% (95% CI, 7–25%; n = 7).
Clarithromycin and metronidazole
Dual primary resistance to clarithromycin and metronidazole was examined in 10 studies. Study-specific prevalences ranged from 0% to 18%. The summary prevalence was 8% with high heterogeneity among studies (Table 2). The highest resistance was reported in Mexico (13%, n = 4; Table 3), whereas the lowest was reported in Paraguay (2%, n = 1). Prevalences did not differ significantly by period of collection (Table 2). The summary prevalence including studies evaluating secondary resistance (n = 3) was 7% (95% CI, 4–10; n = 13).
Pediatric studies
As compared with adults, higher prevalences were observed in the three studies in children (Supplementary Table) for resistance to clarithromycin (ranging from 19% to 27%) and dual resistance to clarithromycin and metronidazole (18%), whereas lower prevalences were reported for metronidazole (ranging from 13% to 78%), tetracycline (0%), and furazolidone (0%). No pediatric data were available for resistance to fluoroquinolones.
Publication bias
For studies in adults, the P values for Egger’s test were equal or greater than 0.10 for all antibiotics (Table 2). Funnel plots confirmed symmetric distributions.
DISCUSSION
Antibiotic resistance patterns of H. pylori may predict the effi-cacy of current antibiotic regimens and may suggest new treatment strategies. Our study represents the first systematic effort to review and synthesize available data in Latin America. Our analysis indicates that H. pylori resistance to first-line antibiotics is high in some countries, which may contribute to high rates of treatment failure in this region.
Three-drug regimens including a proton pump inhibitor (PPI), clarithromycin, and either metronidazole or amoxicillin have been widely recommended as a first choice for H. pylori eradication with success rates of around 80% (7,20). However, this approach has decreased the efficacy of antibiotics in individuals with antibiotic-resistant strains (5,21,22). In the presence of clarithromycin or metronidazole resistance, the success rate of a PPI – clarithromycin–metronidazole regimen is significantly reduced by 35% and 18%, respectively. The decrease in success is 66% in case of clarithromycin resistance if the treatment contains PPI – clarithromycin – amoxicillin (5,23). Alternative first-line regimens include bismuth-containing quadruple, sequential, concomitant quadruple, and hybrid therapies (24).
According to the fourth edition of the European Maastricht Consensus (7), PPI clarithromycin – containing triple therapy without prior susceptibility testing should be abandoned in a given region when the local clarithromycin resistance rate is more than 15–20%. Levels within or above this range have already been reported in some European and Asian countries (25–28). Similarly, although the overall prevalence in Latin America (13%) was below this threshold, our meta-analysis suggests that empirical use of clarithromycin may not be appropriate in Peru, and perhaps in Colombia.
Levofloxacin is frequently substituted for clarithromycin in second-line treatment regimens for H. pylori infections. Given its broad spectrum of antibacterial activity, resistance to this agent may evolve rapidly. The overall prevalence of fluoroquinolone resistance in Latin America is higher than the overall levofloxacin resistance rates reported for Europe (14.1%) (25) and Asia (11.6%) (26). Urinary tract Escherichia coli isolates in Latin America also have a high prevalence of this resistance (29). Levofloxacin should not be used for first-line therapy of H. pylori infections. This antibiotic should only be considered for patients with clarithromycin-resistant isolates and for retreatment of patients who failed first-line clarithromycin-based therapies (7).
Regarding metronidazole, our meta-analysis found that resistance in Latin American populations was high and stable over the study period without remarkable trends within individual countries. Although concerns have been raised regarding lack of reproducibility of metronidazole testing for individual diagnosis (30,31), the trend of low, medium, or high prevalences provides useful information at a population level (32). In contrast to clarithromycin, metronidazole resistance is not of great clinical relevance, as it can be overcome by increasing the length of treatment or by adding bismuth to the regimen (33).
The relatively low overall prevalence of amoxicillin resistance in Latin America is similar to that of other regions (25). Thus, inclusion of this antibiotic in empiric eradication regimens is still appropriate worldwide.
In some areas, local variation in H. pylori antibiotic resistance is associated with the use of the same antibiotic in the general population (25,34). On the basis of retail sales data, total per capita consumption of antibiotics in Latin America was found to have increased by nearly 10% between 1997 and 2007 (35). The 10-year sales trends for macrolide, lincosamide, and streptogramin antibiotics showed large increases in Peru, Brazil, and Argentina but relatively little change, or even decreases, in Uruguay, Mexico, and Colombia. In 2007, the countries with highest per capita consumption of macrolides (including clarithromycin) and related antibiotics were Venezuela, Argentina, and Chile. Fluoroquinolone use, including levofloxacin, significantly increased throughout the region, with the highest consumption for 2007 recorded in the same three countries. The patterns in these retail data do not seem to correlate with the variation in prevalence of antimicrobial resistance, but further efforts should be directed to better monitor local and national antibiotic consumption.
Between-study heterogeneity was high in all meta-analyses and could not be explained by variation in country, period of sample collection, and test method. In previous studies, both patient (e.g., sex (36), age (25), and diagnosis (37)) and bacterial (e.g., cagA positivity (38,39)) characteristics have been associated with antibiotic resistance, and could therefore influence heterogeneity. Unfortunately, we did not have detailed data to assess such variation.
The majority of studies evaluated resistance by either agar dilution or E-test, which are both considered to be highly accurate and equivalently valid testing methodologies (40). Although in general we found no variation by testing method, studies using disk diffusion tended to provide higher resistance estimates. Nevertheless, these findings should be interpreted cautiously, as they were not based on studies directly comparing the techniques on the same H. pylori isolates.
The data we summarized mainly represent convenience samples of adults from 12 of the 20 Latin American countries. Most patients were recruited in specialized medical centers, with uncertain relevance to the appropriate target population for H. pylori eradication in Latin America. Also, there were only three reports regarding bacterial isolates from children. All the reviewed studies were at least moderate in quality with low risk of contributing bias to our systematic analysis. We found no evidence of publication bias.
Apart from antibiotic resistance, other determinants of eradication success include smoking, medication adherence, and host genetic variation. Smokers have a twofold increased risk of eradication failure (41). Poor adherence with the medication regimen is inversely associated with the probability of therapeutic success (42,43). Carriage of alleles encoding active cytochrome P450 2C19 isoenzymes is associated with increased PPI metabolism and diminished pharmacologic effect; these genotypes are common in Latin American populations (44,45). Success rates could be improved by strategies to reduce smoking and enhance adherence (e.g., counseling regarding medication side effects and risk of antibiotic resistance with treatment failure).
Surveillance systems for H. pylori antimicrobial resistance have proven useful for informing practitioners about empirical choices for treatment (25,36). Organized surveillance efforts in Latin American countries therefore warrant serious consideration. These systems could be set up using already established networks currently monitoring antibiotic resistance to other bacteria, such as the World Health Organization Collaborating Centre for Surveillance of Antimicrobial Resistance (WHONET), the Red Latinoamericana de Vigilancia de la Resistencia a los Antimicrobianos (ReLAVRA), the Alliance for the Prudent Use of Antibiotics (APUA), and the SENTRY antimicrobial surveillance program.
In conclusion, our meta-analysis demonstrated high H. pylori resistance to first-line antibiotics in Latin American countries. This finding stresses the need for appropriate surveillance programs, improved institutional and governmental antimicrobial regulations, and increased public awareness and knowledge. Implementation of safe and effective eradication regimens can help alleviate the burden of H. pylori -related diseases, and in particular, to reduce the high incidence of gastric cancer in this region.
Supplementary Material
Study Highlights.
WHAT IS CURRENT KNOWLEDGE
-
✓
Latin America has a high burden of Helicobacter pylori infection and associated diseases, particularly gastric cancer.
-
✓
Current H. pylori treatments have unacceptably high rates of failure.
-
✓
H. pylori resistance may decrease antibiotic efficacy, requiring alteration of eradication regimens.
WHAT IS NEW HERE
-
✓
Our meta-analysis found that primary resistance to first-line antibiotics is high in Latin America. In particular, empirical use of clarithromycin as the core antibiotic in H. pylori eradication regimens may already be an obsolete strategy for some countries.
-
✓
Better data on antibiotic resistance patterns are needed to improve region- and country-specific H. pylori-treatment strategies.
-
✓
This study represents the first systematic effort to review and synthesize available information on H. pylori antibiotic resistance in Latin America.
ACKNOWLEDGMENTS
We are grateful to the following colleagues who provided unpublished information: Drs Adalucy Alvarez Aldana, Universidad Tecnológica de Pereira, Colombia; Guadalupe Ayala Aguilar, Instituto Nacional de Salud Pública, México; Douglas E. Berg, University California San Diego, United States; Mariana Catalano, Universidad de Buenos Aires, Argentina; Yvette Debets-Ossenkopp, Vrije Universiteit, The Netherlands; Mercedes Figueroa Macca, Universidad del Valle, Colombia; Beatriz Gutierrez, Instituto de Oncología y Radiobiología, Cuba; Rafael Llanes, Instituto de Medicina Tropical Pedro Kourí, Cuba; Rosario Morales-Espinosa, Universidad Autónoma de México, México; Diana Ortiz, Universidad Central de Venezuela, Venezuela; Laura Otth Rademacher, Universidad Austral de Chile, Chile; José Pedrazzoli Jr, Universidade São Francisco, Brazil; Orlando Reyes, Centro Nacional de Investigaciones Científicas, Cuba; Patricia Rivera Moya, Hospital Nacional de Niños, Costa Rica; Claude Andre Solari, Instituto Oswaldo Cruz, Brazil; Márcia Aparecida Sperança, Universidade Federal do ABC, Brazil; Alba E. Vega, Universidad Nacional de San Luis, Argentina. We also thank Olga Mejia from the Revista Colombiana de Gastroenterología and Dr Juan Carlos Maldonado from the Revista de la Facultad de Ciencias Médicas, Universidad Central del Ecuador for their assistance in gathering articles for our systematic review.
Financial support: This work was supported by the Intramural Research Program of the United States National Institutes of Health, National Cancer Institute.
Footnotes
SUPPLEMENTARY MATERIAL is linked to the online version of the paper at http://www.nature.com/ajg
CONFLICT OF INTEREST
Guarantor of the article: M. Constanza Camargo, PhD.
Specific author contributions: Study concept and design: M.C.C., A.G. and C.S.R; acquisition of data: A.G., C.A.C., and T.H.; statistical analysis: M.C.C.; drafting of the manuscript: M.C.C. and C.S.R.; critical revision of the manuscript for important intellectual content: all authors.
Potential competing interests: The authors declare no conflict of interest. The findings and conclusions in this report are those of the authors and do not necessarily represent the official positions of the United States National Cancer Institute and/or the United States Centers for Disease Control and Prevention.
REFERENCES
- 1.International Agency for Research on Cancer. Monographs on the evaluation of carcinogenic risks to humans: Schistosomes, liver flukes and Helicobacter pylori. 1st edn. Lyon, France: IARC Press; 1994. [PMC free article] [PubMed] [Google Scholar]
- 2.Ford AC, Delaney BC, Forman D, et al. Eradication therapy for peptic ulcer disease in Helicobacter pylori positive patients. Cochrane Database Syst Rev. 2006;(2) doi: 10.1002/14651858.CD003840.pub4. CD003840. [DOI] [PubMed] [Google Scholar]
- 3.Zullo A, Hassan C, Cristofari F, et al. Effects of Helicobacter pylori eradication on early stage gastric mucosa-associated lymphoid tissue lymphoma. Clin Gastroenterol Hepatol. 2010;8:105–110. doi: 10.1016/j.cgh.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 4.Ma JL, Zhang L, Brown LM, et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J Natl Cancer Inst. 2012;104:488–492. doi: 10.1093/jnci/djs003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischbach L, Evans EL. Meta-analysis: the effect of antibiotic resistance status on the efficacy of triple and quadruple first-line therapies for Helicobacter pylori. Aliment Pharmacol Ther. 2007;26:343–357. doi: 10.1111/j.1365-2036.2007.03386.x. [DOI] [PubMed] [Google Scholar]
- 6.McMahon BJ, Hennessy TW, Bensler JM, et al. The relationship among previous antimicrobial use, antimicrobial resistance, and treatment outcomes for Helicobacter pylori infections. Ann Intern Med. 2003;139:463–469. doi: 10.7326/0003-4819-139-6-200309160-00008. [DOI] [PubMed] [Google Scholar]
- 7.Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection—the Maastricht IV/Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 8.Coelho LG, Leon-Barua R, Quigley EM. Latin-American Consensus Conference on Helicobacter pylori infection. Latin-American National Gastroenterological Societies affiliated with the Inter-American Association of Gastroenterology (AIGE) Am J Gastroenterol. 2000;95:2688–2691. doi: 10.1111/j.1572-0241.2000.03174.x. [DOI] [PubMed] [Google Scholar]
- 9.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 10.Ferlay J, Shin HR, Bray F, et al. International Agency for Research on Cancer (ed) Lyon: France: 2010. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10. (ed). [Google Scholar]
- 11.Wolf MJ. Use and misuse of antibiotics in Latin-America. Clin Infect Dis. 1993;17:S346–S351. doi: 10.1093/clinids/17.supplement_2.s346. [DOI] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.UNESCO The State of Education in Latin America and the Caribbean Guaranteeing Quality Education for All 2008
- 14.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 15.Freeman MF, Tukey JW. Transformations related to the angular and the square root. Annals Math Stat. 1950;21:607–611. [Google Scholar]
- 16.Higgins JP, Tompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sterne JAC. Meta-Analysis in Stata: An Updated Collection from the Stata Journal. College Station, TX, USA: Stata Press; 2009. [Google Scholar]
- 20.Chey WD, Wong BC. American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 2007;102:1808–1825. doi: 10.1111/j.1572-0241.2007.01393.x. [DOI] [PubMed] [Google Scholar]
- 21.You WC, Brown LM, Zhang L, et al. Randomized double-blind factorial trial of three treatments to reduce the prevalence of precancerous gastric lesions. J Natl Cancer Inst. 2006;98:974–983. doi: 10.1093/jnci/djj264. [DOI] [PubMed] [Google Scholar]
- 22.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–1153. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 23.Megraud F. Basis for the management of drug-resistant Helicobacter pylori infection. Drugs. 2004;64:1893–1904. doi: 10.2165/00003495-200464170-00003. [DOI] [PubMed] [Google Scholar]
- 24.Kuo CH, Kuo FC, Hu HM, et al. The optimal first-line therapy of Helicobacter pylori Infection in Year 2012. Gastroenterol Res Pract. 2012;2012:168361. doi: 10.1155/2012/168361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Megraud F, Coenen S, Versporten A, et al. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut. 2013;62:34–42. doi: 10.1136/gutjnl-2012-302254. [DOI] [PubMed] [Google Scholar]
- 26.De Francesco V, Giorgio F, Hassan C, et al. Worldwide H. pylori antibiotic resistance: a systematic review. J Gastrointestin Liver Dis. 2010;19:409–414. [PubMed] [Google Scholar]
- 27.Gao W, Cheng H, Hu F, et al. The evolution of Helicobacter pylori antibiotics resistance over 10 years in Beijing, China. Helicobacter. 2010;15:460–466. doi: 10.1111/j.1523-5378.2010.00788.x. [DOI] [PubMed] [Google Scholar]
- 28.Kobayashi I, Murakami K, Kato M, et al. Changing antimicrobial susceptibility epidemiology of Helicobacter pylori strains in Japan between 2002 and 2005. J Clin Microbiol. 2007;45:4006–4010. doi: 10.1128/JCM.00740-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andrade SS, Sader HS, Jones RN, et al. Increased resistance to first-line agents among bacterial pathogens isolated from urinary tract infections in Latin America: time for local guidelines? Mem Inst Oswaldo Cruz. 2006;101:741–748. doi: 10.1590/s0074-02762006000700006. [DOI] [PubMed] [Google Scholar]
- 30.Hirschl AM, Hirschl MM, Rotter M. Comparison of three methods for the determination of the sensitivity of Helicobacter pylori to metronidazole. J Antimicrob Chemother. 1993;32:45–49. doi: 10.1093/jac/32.1.45. [DOI] [PubMed] [Google Scholar]
- 31.Glupczynski Y, Broutet N, Cantagrel A, et al. Comparison of the E test and agar dilution method for antimicrobial suceptibility testing of Helicobacter pylori. Eur J Clin Microbiol Infect Dis. 2002;21:549–552. doi: 10.1007/s10096-002-0757-6. [DOI] [PubMed] [Google Scholar]
- 32.Megraud F, Lehours P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin Microbiol Rev. 2007;20:280–322. doi: 10.1128/CMR.00033-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malfertheiner P, Bazzoli F, Delchier JC, et al. Helicobacter pylori eradication with a capsule containing bismuth subcitrate potassium, metronidazole, and tetracycline given with omeprazole versus clarithromycin-based triple therapy: a randomised, open-label, non-inferiority, phase 3 trial. Lancet. 2011;377:905–913. doi: 10.1016/S0140-6736(11)60020-2. [DOI] [PubMed] [Google Scholar]
- 34.Boyanova L, Mitov I. Geographic map and evolution of primary Helicobacter pylori resistance to antibacterial agents. Expert Rev Anti Infect Ther. 2010;8:59–70. doi: 10.1586/eri.09.113. [DOI] [PubMed] [Google Scholar]
- 35.Wirtz VJ, Dreser A, Gonzales R. Trends in antibiotic utilization in eight Latin American countries, 1997–2007. Rev Panam Salud Publica. 2010;27:219–225. doi: 10.1590/s1020-49892010000300009. [DOI] [PubMed] [Google Scholar]
- 36.Tveit AH, Bruce MG, Bruden DL, et al. Alaska sentinel surveillance study of Helicobacter pylori isolates from Alaska Native persons from 2000 to 2008. J Clin Microbiol. 2011;49:3638–3643. doi: 10.1128/JCM.01067-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Khan A, Farooqui A, Manzoor H, et al. Antibiotic resistance and cagA gene correlation: a looming crisis of Helicobacter pylori. World J Gastroenterol. 2012;18:2245–2252. doi: 10.3748/wjg.v18.i18.2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Correa P, van Doorn LJ, Bravo JC, et al. Unsuccessful treatment results in survival of less virulent genotypes of Helicobacter pylori in Colombian patients. Am J Gastroenterol. 2000;95:564–566. doi: 10.1111/j.1572-0241.2000.t01-1-01813.x. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki T, Matsuo K, Sawaki A, et al. Systematic review and meta-analysis: importance of CagA status for successful eradication of Helicobacter pylori infection. Aliment Pharmacol Ther. 2006;24:273–280. doi: 10.1111/j.1365-2036.2006.02994.x. [DOI] [PubMed] [Google Scholar]
- 40.Piccolomini R, DiBonaventura G, Catamo G, et al. Comparative evaluation of the E test, agar dilution, and broth microdilution for testing susceptibilities of Helicobacter pylori strains to 20 antimicrobial agents. J Clin Microbiol. 1997;35:1842–1846. doi: 10.1128/jcm.35.7.1842-1846.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suzuki T, Matsuo K, Ito H, et al. Smoking increases the treatment failure for Helicobacter pylori eradication. Am J Med. 2006;119:217–224. doi: 10.1016/j.amjmed.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 42.Al-Eidan FA, McElnay JC, Scott MG, et al. Management of Helicobacter pylori eradication--the influence of structured counselling and follow-up. Br J Clin Pharmacol. 2002;53:163–171. doi: 10.1046/j.0306-5251.2001.01531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zullo A, De Francesco V, Hassan C. Predicting Helicobacter pylori eradication: how to teach an old dog new tricks! J Clin Gastroenterol. 2012;46:259–261. doi: 10.1097/MCG.0b013e318247177e. [DOI] [PubMed] [Google Scholar]
- 44.Isaza C, Henao J, Martinez JH, et al. Phenotype-genotype analysis of CYP2C19 in Colombian mestizo individuals. BMC Clin Pharmacol. 2007;7:6–10. doi: 10.1186/1472-6904-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bravo-Villalta HV, Yamamoto K, Nakamura K, et al. Genetic polymorphism of CYP2C9 and CYP2C19 in a Bolivian population: an investigative and comparative study. Eur J Clin Pharmacol. 2005;61:179–184. doi: 10.1007/s00228-004-0890-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.