Abstract
Although one-third of children and adolescents are overweight or obese, developmental changes in food craving and the ability to regulate craving remain poorly understood. We addressed this knowledge gap by examining behavioral and neural responses to images of appetizing unhealthy foods in individuals aged 6-23 years. On “Close” trials (assessing unregulated craving), participants focused on a pictured food's appetitive features. On “Far” trials (assessing effortful regulation), participants focused on a food's visual features and imagined it was farther away. Across conditions, age predicted less craving, less striatal recruitment, greater prefrontal activity and stronger frontostriatal coupling. When effortfully regulating, all participants reported less craving and exhibited greater lateral prefrontal and less vmPFC recruitment. Body mass predicted less regulation-related prefrontal activity, particularly among children. These results suggest that children experience stronger craving than adults but can also effectively regulate craving. Moreover, the mechanisms underlying regulation may differ for heavy and lean children.
Today, one-third of youth are overweight or obese (Ogden, Carroll, Kit, & Flegal, 2012). This is troubling, given that childhood obesity predicts adult obesity and long-term health problems – leading some to characterize childhood and adolescence as sensitive periods for the acquisition of obesity (Dietz, 1994). As such, this developmental window is an ideal target for early intervention aimed at preventing weight gain. While prior work indicates that children's reward sensitivity (Verbeken, Braet, Lammertyn, Goossens, & Moens, 2012) and self-regulatory ability (Batterink, Yokum, & Stice, 2010; Francis & Susman, 2009; Schlam, Wilson, Shoda, Mischel, & Ayduk, 2013) are associated with concurrent and future body mass indices (BMI), little is known about how food craving, its regulation, or its association with BMI relates to age. Using neuroimaging and behavioral methods, the present study addresses three questions about food craving in children, adolescents, and adults.
The first question is whether food craving differs as a function of age. Numerous studies have shown that impulse control improves from childhood to adulthood, but may be compromised in certain contexts in adolescence (Somerville & Casey, 2010). Relative to adults, adolescents perform better on cognitive and decision making tasks when incentives are at stake (Geier, Terwilliger, Teslovich, Velanova, & Luna, 2010; Teslovich et al., 2013), and impulsively approach positive social stimuli (Somerville, Hare, & Casey, 2011). This tendency for motivational factors to modulate cognitive control has been linked to exaggerated responses in reward regions like the ventral striatum (VS) (Galvan et al., 2006; Van Leijenhorst et al., 2010).
To date, two neuroimaging studies have compared how adolescents and adults respond to food. The first gave participants juice and found that adolescents showed heightened VS responses and reported enjoying the juice more than adults (Galvan & McGlennen, 2013). The second study showed participants food images and found no age effects in the VS and did not collect online self-reports (Killgore & Yurgelun-Todd, 2005). These mixed findings make it unclear whether adolescents are more responsive to food than adults and, given that neither study tested participants under the age of 9, how they differ from children.
The second question is whether age predicts general changes in food craving or specifically, the capacity to regulate craving using cognitive strategies like reappraisal, which involves thinking about a stimulus differently so as to alter its affective impact. In adults, reappraising food reduces craving (Giuliani, Mann, Tomiyama, & Berkman, 2014; Kober et al., 2010; Siep et al., 2012; Wang et al., 2009), recruits dorsolateral, ventrolateral and dorsomedial prefrontal regions (dlPFC; vlPFC; dmPFC) implicated in cognitive control (Giuliani et al., 2014; Kober et al., 2010; Scharmuller, Ubel, Ebner, & Schienle, 2012), and attenuates activation of reward-related circuitry (Kober et al., 2010; Wang et al., 2009), including the VS and ventromedial prefrontal cortex (vmPFC). One prior neuroimaging study found that adolescents recruited vlPFC, but did not reduce craving, vmPFC or VS activity when reappraising food (Yokum & Stice, 2013). These mixed results make it unclear whether youth can reappraise food and, together with the fact that no adults were scanned, leave open the question of whether age predicts differences in the ability to regulate craving.
Our third question is the extent to which BMI is associated with behavioral and neural responses to food in youth. Obese and overweight adults report more food craving in everyday life than lean individuals (Delahanty, Meigs, Hayden, Williamson, & Nathan, 2002), and show heightened VS activity (Giuliani et al., 2014) and reduced lateral prefrontal recruitment both when responding naturally (Brooks, Cedernaes, & Schioth, 2013), and when regulating food craving (Giuliani et al., 2014). It is unknown, however, how BMI relates to food craving and its regulation in childhood and adolescence.
To address these three questions, participants aged 6-23 years were scanned while viewing appetizing unhealthy foods and alternately focusing more or less on the food's appetitive features so as to regulate craving. To test whether age predicts general changes in responsivity to food, developmental differences were characterized across experimental conditions with regards to self-reported craving and frontostriatal activity. To test whether the ability to cognitively regulate craving increases with age, we compared age effects for regulation and baseline trials. Finally, age-adjusted BMI was examined to determine its influence on food craving and neural responses.
METHODS
Participants
105 healthy individuals 6 to 23 years of age participated in the experiment (71 female; mean age = 14.27 years, S.D. = 4.85; Figure S1). Participants were prescreened prior to participation to ensure that they could read and write in English, had normal or corrected vision, had never been diagnosed with a developmental or psychiatric disorder, had no conditions contraindicated for scanning, and had never been prescribed psychotropic medication. All participants were of normal intelligence as indexed the Wechsler Abbreviated Scale of Intelligence (mean = 114.57, S.D. = 15.93) and no relationship was observed between age and IQ scores (r=−.08, p>.05). A subset of participants (N=45; 25 female; mean age = 17.71 years, S.D. = 3.59 years) completed the SCOFF questionnaire, a screen for eating disorders (Morgan, Reid, & Lacey, 2000). SCOFF scores did not correlate with age or with craving on Close or Far trials (p's>.10)suggesting that age-related changes in craving were not attributable to restrained or disordered eating.
In addition to the 105 participants whose data are presented in this manuscript, 10 participants (6 female; mean age = 8.36 years, S.D. = 1.92) were scanned but excluded from analyses due to excessive head motion. The initial target sample size was set at 100 participants but 115 participants were scanned because it was anticipated that some data might need to be excluded due to head motion. The Columbia University Institutional Review Board approved this study and participants provided informed consent and assent.
Participants were not given explicit instructions regarding fasting pre-scanning. A subset of participants (n=57) were asked about the last time they ate before scanning and this was unrelated to craving for the Close and Far conditions, as well as the difference in craving between the Close and Far conditions (p's>.20). Feasibility constraints prevented participants from being scanned at the same time of day. 15.2% of participants were scanned in the morning (8-30-11:59AM), 55.2% were scanned in the afternoon (12-4:59PM), and 29.5% were scanned in the evening (5-7:30PM). Time of day did not predict craving in the Close or Far condition, nor did it interact with condition or age to predict craving (p's>.27).
After scanning, participants were weighed and their height was measured. Prior work has suggested that BMI percentile, which calculates a child's BMI relative to same-aged peers, is a more accurate assessment of body composition than is BMI for children and adolescents (Mei et al., 2002). The Center for Disease Control's BMI-for-age growth chart was used to calculate BMI percentile. BMI percentile data is not published for individuals over the age of 20 and thus 17 participants ranging in age from 20-23 were not included in analyses examining BMI. BMI was not recorded for two participants. Thus, in total 86 individuals (53 female; Mean age = 13.71 years, S.D. = 4.04) were included in BMI analyses. Neither age nor gender was associated with BMI percentile (p's>.32).
Experimental task
Participants completed a regulation of craving task consisting of 40 experimental trials, all of which involved the presentation of a picture of unhealthy but appetizing food (Figure 1). Food stimuli were downloaded from public online sources. Care was taken to present participants with a variety of types of food stimuli (e.g., equivalent representation of salty and sweet foods). Piloting in a prior sample of children, teenagers, and adults confirmed that all food stimuli were highly desirable.
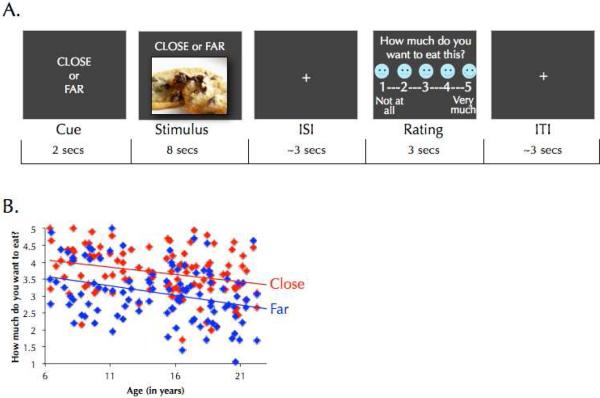
Figure 1.
(A) Trial structure and (B) behavioral results for the regulation of craving task. Participants reported less craving when reappraising (on Far versus Close trials), p<.001, and older individuals reported less craving than younger individuals, p<.001.
On each experimental trial, participants implemented the strategy indicated by a cue word (‘Close’ or ‘Far’, shown for 2 seconds prior to the food image) while viewing the food stimulus for 8 seconds. After a jittered fixation period (~3 seconds), participants rated how much they wanted to eat the food they had just seen on a five-point scale (1=not at all, 5=very much) via button press. The trial concluded with a second jittered fixation period (~3 seconds).
On half of all trials, participants were cued to use the Close strategy and on the other half they were cued to use the Far strategy. Assignment of pictures to strategy was counterbalanced between participants. Prior to the task, participants were trained on both strategies in accordance with procedures that have been well-validated in developmental populations (Silvers et al., 2012). On Close trials, participants were told to imagine the food was in front of them and were told to focus on the taste and smell of the food. On Far trials, participants were told to imagine the food was farther away and to focus more on the visual aspects of the food stimuli (e.g., the shape, and color) than its appetitive features. Participants were not told that Close trials were intended to assess baseline appetitive responsiveness whereas Far trials were used to assess regulation.
fMRI acquisition
Whole-brain fMRI data were acquired on a 3T Siemens Magnetom Trio scanner. Structural images were acquired using a high-resolution, T1-weighted MPRAGE sequence (TR=2170 ms, TE=4.33 ms, 120 1.5 mm slices). Functional images were acquired with a T2*-sensitive EPI BOLD sequence. Thirty-four axial slices were collected with a TR of 2000 ms (TE of 34 ms, flip angle of 90°, field of view of 22.4 cm and 3.5 × 3.5 × 4 mm voxels). Stimuli were presented using E-Prime and were projected onto a flat screen mounted in the scanner bore. Subjects viewed the screen using a mirror mounted on a 16-channel head coil. Participants made their responses using a five-finger-button response pad.
Behavioral data analysis
Effects of strategy (Close, Far) mean-centered age and BMI percentile were analyzed using a repeated measures GLM, as implemented in SPSS 19.0.
fMRI analysis
Preprocessing was conducted using the statistical parametric mapping software (SPM8, Wellcome Department of Cognitive Neurology, London, UK). Preprocessing steps consisted of slice time correction, realignment, and coregistration of the functional and structural data. Images were segmented and normalized (warped) to the standard MNI template brain. Normalized functional images were interpolated to 3 × 3 × 3 mm voxels and spatially smoothed with a 6-mm Gaussian filter. A gray-matter mask based on the MNI-standardized Colin-brain was used to constrain the functional data.
Prior to scanning, children under the age of 10 were acclimated to the scanning environment with the use of a mock scanner so as to enhance participants’ comfort and to give them an opportunity to practice staying still. After scanning, motion parameters were estimated during preprocessing and volumes that contained motion greater than 1.5 mm (translation) or 2 degrees (rotation) relative to the proceeding volume were censored from further analyses. If 10% or more volumes from a given run were excluded, the entire run was discarded. Participants were excluded if more than two out of four runs were discarded. Activation values from clusters identified in age-related analyses were tested offline while controlling for the number of volumes removed to examine whether age effects still held. These adjusted age effects are reported in Tables S1, S2, S3, S5, and S8.
First-level GLM analyses were implemented in NeuroElf (http://neuroelf.net). Cue, stimulus-viewing and response portions of each trial were modeled as boxcar regressors convolved with a canonical hemodynamic response function. Separate regressors were made for the stimulus-viewing period of Close and Far trials. Robust regression was used in first level analyses. Motion parameters, a high-pass temporal filter and estimates of global signal in white matter, gray matter and CSF were included as regressors of no interest. Age was not correlated with temporal signal to noise ratios (tSNR) calculated for each participant's neuroimaging data – the mean value of each voxel's time series intensity divided by its standard deviation (Welvaert & Rosseel, 2013) – (r=−.03, p=.78) suggesting that age did not predict significant differences in data quality.
Following GLM computation for each participant, second-level random effects analyses were performed on the group data. The first analysis examined the effects of strategy (Close, Far) and mean-centered age, and the second examined the effects of strategy, mean-centered age and mean-centered BMI percentile. Both linear and quadratic effects of age were examined and were found to identify nearly identical clusters in the brain. To determine model fit, a conjunction analysis was performed to isolate clusters identified by both the linear and quadratic models. Parameter estimates were then extracted from these clusters and examined offline using the extra sum-of-squares F-test. Significant main effects and interactions were interrogated with follow-up t test and correlational analyses to ascertain the directionality of effects. Significant clusters were identified using joint voxel and extent thresholds that preserved alpha < .05, as determined by AlphaSim (1000 iterations; smoothness estimated at 10.6 mm by AlphaSim), implemented in NeuroElf (uncorrected p<.005 and 79 voxels).
To examine age-related changes associated with VS connectivity, a PPI analysis was also conducted. The seed region used was the right VS cluster identified by the main effect of age term in the activation-based analyses described above (MNI coordinates: 18, 12, −6). In this analysis, regressors were created for each task condition, the seed region time series, and interaction terms for the time series and the two experimental conditions (i.e., the Close x PPI interaction term and the Far x PPI interaction term). Given that this VS seed region was identified by the main effect of age term in the activation-based analyses (i.e., the effect of age collapsing across Close and Far trials), the primary question of interest for the PPI analysis involved examining the effect of age across the two experimental conditions compared to fixation (Close x PPI + Far x PPI > fixation). Follow-up regression analyses were conducted on clusters identified in this analysis to determine whether age predicted comparable changes in connectivity for the Close and Far conditions. No clusters identified (Table S2) showed differential connectivity on Close versus Far trials (p>.14). Whole-brain tests were additionally performed to identify clusters that showed age-related variation in baseline VS connectivity and overall connectivity during the task (Baseline connectivity + [Close x PPI + Far x PPI > fixation]) and are reported in the Supplemental Materials as well as in Table S3. Significant clusters were identified using a joint voxel and extent threshold that preserved alpha < .05, as determined by AlphaSim (1000 iterations; smoothness estimated at 9.3 mm by AlphaSim), implemented in NeuroElf (uncorrected p<.005 and 56 voxels).
RESULTS
Behavioral results
To characterize developmental differences associated with food craving, the main effect of age on craving was computed (Figure 1). Results revealed that older individuals reported less craving than younger individuals, F(1, 103)=12.09, p<.001; η 2p=.13. Adding a quadratic age term (age2) did not improve model fit, as determined by the extra sum-of-squares F-test, and thus the simpler linear model was used (F(1,102)=.12, p=.73). To examine whether reappraisal reduced craving across age, the main effect of strategy was tested and as expected, participants reported less craving on Far than Close trials, F(1, 103)=68.29, p<.001; η 2p=.40. Age effects were not unique to the reappraisal condition as no interaction was observed between age and strategy, F(1, 103)=.86, p=.36; η 2p=.008. Participants of different ages used the craving scale similarly, as evidence by the fact that age did not predict differences in within-subject standard deviations for craving ratings (Mean S.D.=1.22; r=−.08, p=.40). Age-adjusted BMI did not predict craving, nor did the interaction term between BMI and strategy (p's>.29). Even when the top and bottom 25th percentiles for BMI were examined, no differences in craving for either condition were identified (p's>.71).
Imaging results
Main effect of age
Age predicted increased recruitment of right lateral prefrontal and bilateral posterior parietal cortices (Figure 2; Table S1) and decreased recruitment of subcortical structures implicated in reward and emotional processing such as the VS (Liu, Hairston, Schrier, & Fan, 2011) and amygdala (Buhle et al., 2013). Similar regions of interest (ROIs) were identified by linear and quadratic models of age, but the more complex quadratic model did not improve model fit relative to the simpler linear model in any regions (p>.09).
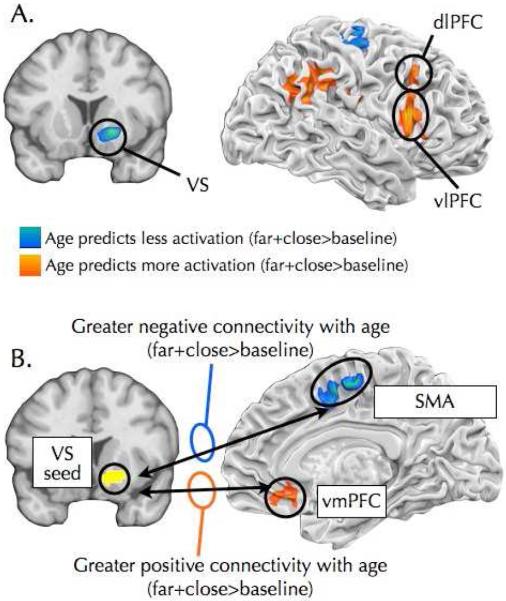
Figure 2.
(A) Brain regions showing differential as a main effect of age (Far + Close > baseline). Warm colors indicate brain regions showing greater activation in older than younger individuals, and included right lateral prefrontal and parietal cortices. Cool colors indicate brain regions showing attenuated activation in older individuals, and included the ventral striatum (MNI coordinates: 18, 12, −6). (B) Brain regions showing differential functional connectivity with the ventral striatum (18, 12, −6) as a function of age. Warm colors indicate increasingly positive connectivity as a function of age and included vmPFC. Cool colors indicate increasingly negative connectivity as a function of age and included SMA and the central sulcus.
To further investigate age effects observed in the VS, a psychophysiological interaction (PPI) analysis was conducted using the VS as a seed region (MNI coordinates: 18, 12, −6). Connectivity between vmPFC and the VS was positive at baseline (see Supplemental Materials for additional details) and age predicted even more positive vmPFC-VS connectivity during Close and Far trials relative to fixation (Figure 2; Table S2). Age effects on vmPFC-VS functional connectivity did not differ significantly between Close and Far trials (p>.14). In younger participants, vmPFC-VS connectivity was weaker (i.e., less positive) during the task relative to baseline, while for older participants connectivity was stronger (i.e., more positive) during the task than at baseline. Task-related vmPFC-VS connectivity became stronger than baseline connectivity starting at 15.64 years for Close trials and at 15.54 years for Far trials (Close = −.22 + .014*age; Far = −.34 +. 022*age). Task-related increases in vmPFC-VS coupling predicted less craving (β=−.65, t(103)=2.13, p<.05), but not after controlling for age (β=− .24, t(103)=.76, p=.45). Age continued to predict less craving after controlling for vmPFC-VS connectivity (β =−.05, t(103)=4.32, p<.005).
In addition to vmPFC, age predicted task-related VS connectivity with the supplementary motor area (SMA) and the central sulcus (CS). As with vmPFC, age-related effects on functional connectivity with SMA and CS did not differ between Close and Far trials (p's>.42). At baseline, the VS was negatively coupled with the SMA and CS (see Supplemental Materials for details). In younger participants, SMA-VS and CS-VS connectivity was weaker (i.e., less negative) during the task than at baseline, while in older participants connectivity was stronger during the task than at baseline (i.e., more negative). SMA-VS and CS-VS connectivity became stronger during the task relative to baseline during early to mid adolescence (SMAClose = .19 − .014*age; SMAFar = .20 − .014*age; CSClose = .19 - .014*age; CSFar = .22 − .017*age; Table S2). Stronger (i.e., more negative) SMA-VS (β=1.48, t(103)=3.05, p<.005) and CS-VS (β=.87, t(103)=2.39, p<.05) coupling during task compared to baseline predicted less craving across Close and Far trials, but not after controlling for age (p's>.15).
Main effect of strategy and interaction between age and strategy
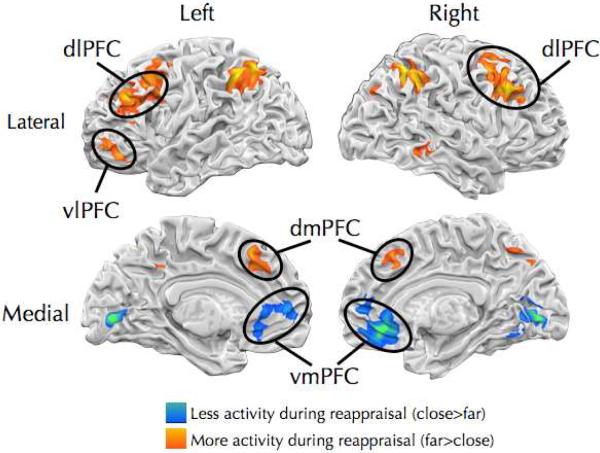
Regulation of craving (i.e., Far > Close contrast) was associated with robust recruitment of vlPFC, dlPFC, and dmPFC and posterior parietal cortex (Figure 3; Table S4). Regulation of craving attenuated activation of vmPFC as well as the cuneus and fusiform gyrus. Age and strategy interacted to predict recruitment of the putamen (Table S5). Linear and quadratic models of age identified similar ROIs, but the more complex quadratic model did not improve model fit relative to the simpler linear model in any regions (p>.2). A whole-brain PPI analysis examining whether the VS showed differential connectivity for Far and Close trials (i.e., Far > Close x PPI) was conducted but no brain regions identified by this analysis survived FWE correction. No brain regions identified by the Far > Close x PPI interaction differed significantly as a function of age.
Figure 3.
Brain regions showing differential activation during regulation. Warm colors indicate brain regions showing greater activation during regulation and included left vlPFC, bilateral dlPFC, dmPFC and posterior parietal cortex. Cool colors indicate brain regions showing attenuated activation during regulation and included vmPFC.
Effects of BMI
Across both Close and Far trials, leaner individuals recruited mPFC more strongly than heavier individuals (Table S6). When regulating craving (i.e. on Far relative to Close trials), leaner individuals activated left vlPFC more than heavier individuals (Figure 4; Table S7). In a partially overlapping portion of vlPFC an interaction between BMI, strategy and age was observed such that BMI predicted less vlPFC activity during regulation in younger, but not older individuals (Figure 4; Table S8; MNI coordinates of overlap: −42, 30, 10, 20 voxels). No regions were identified by the interaction between age and BMI.
Figure 4.
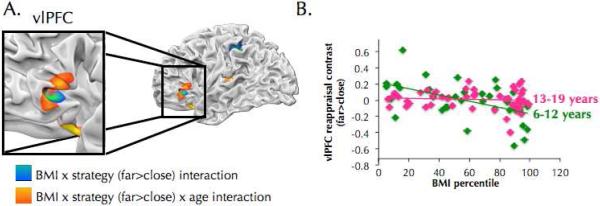
Brain regions where BMI predicts brain activity as a function of strategy and/or age. (A) BMI x strategy (Far > Close) interaction is shown in cool colors. Heavier individuals recruited left vlPFC and parietal cortex to a lesser degree than leaner individuals during regulation of craving (Far > Close). Results of BMI x strategy x age interaction are shown in warm colors. BMI predicted less reappraisal-related recruitment at younger ages than at older ages. The results of these two analyses intersect in left vlPFC (MNI coordinates: −42, 30, 0; 20 voxels). (B) For illustrative purposes, the data from the BMI x strategy x age interaction in left vlPFC are plotted here (MNI coordinates: −54, 33, −12; shown in 4A). Contrast values for Far > Close are plotted on the y axis with BMI percentile on the x axis. Age was analyzed continuously but for ease of interpretation, age is shown split into two groups to highlight that BMI predicted less vlPFC recruitment during reappraisal in younger individuals, but not in older individuals.
DISCUSSION
This study reveals three key findings about how food craving, and its regulation, varies with age and BMI. First, across experimental conditions, age predicted less craving, attenuated VS responses, and enhanced dlPFC recruitment. Second, both behavioral and neuroimaging data showed that children and adolescents can use reappraisal to regulate food craving. Third, BMI predicted diminished vlPFC recruitment during reappraisal, particularly for children. These data have significant implications for basic and translational work on childhood obesity.
Implications for developmental models of cognition and motivation
Over the past 20 years, the prevalence of overweight and obese youth has increased in the United States and remains high (Ogden et al., 2012). Despite widespread concern over these trends’ implications, no prior work has examined how food craving changes across development. The present results provide insight into how developing cognitive and motivational systems impact food craving. We found that age predicts decreased craving, decreased activation of subcortical structures involved in reward and emotional processing, enhanced frontostriatal connectivity, and enhanced lateral prefrontal recruitment both when simply viewing food images and when actively regulating craving.
These age-related effects suggest that children crave food to a greater extent than adolescents or adults. In contrast, developmental studies using monetary or social incentives, rather than food, have found that VS responses peak in adolescence (Galvan et al., 2006; Somerville et al., 2011; Van Leijenhorst et al., 2010). Together, these findings suggest that age is associated with a shift in what individuals find rewarding – from basic needs (food) in childhood to more socially relevant ones in adolescence (E. E. Nelson, Leibenluft, McClure, & Pine, 2005).
With age, comes an increased tendency to evaluate appetitive stimuli in more sophisticated ways. Consistent with this, our PPI analysis showed that age-related decreases in VS recruitment were paralleled by enhanced vmPFC-striatal connectivity, with stronger connectivity predicting less craving. vmPFC is critical for integrating past experiences, present context, and goals to update value representations about stimuli (Roy, Shohamy, & Wager, 2012). While vmPFC tracks subjective craving, VS tracks actual food consumption (Lawrence, Hinton, Parkinson, & Lawrence, 2012) – suggesting that the two play complementary roles in appetitive processing. As such, developmental changes in vmPFC-striatal connectivity may reflect a growing ability to represent value and reward in ways that extend beyond simple hedonics.
Age-related reductions in craving may also be a downstream effect of increased lateral prefrontal and parietal recruitment. Activation of these regions, which are broadly implicated in cognitive control and emotional regulation, followed two patterns. First, right dlPFC and posterior parietal cortex, structures that support core working memory processes (Rottschy et al., 2012), sustained attention (Langner & Eickhoff, 2013), and reappraisal (Buhle et al., 2013), were recruited during regulation of craving (i.e., Far > Close) and also showed general age-related increases (i.e., across both the Far and Close conditions). Second, right vlPFC recruitment, which supports response inhibition (Wager et al., 2005) and inhibition of appetitive impulses (Casey et al., 2011; Somerville et al., 2011), did not differ as a function of strategy (i.e., Far > Close contrast was non-significant) but showed age-related increases in recruitment across conditions. This suggests that older individuals may spontaneously engage cognitive control in response to food, perhaps because of chronic regulatory goals.
By contrast, an age-independent pattern of prefrontal recruitment was observed when participants reappraised to regulate craving. Participants of all ages reported less craving when they focused on a food's visual, rather than appetitive, features and imagined it being farther away. Reduced craving was paralleled by increased recruitment of prefrontal and parietal regions implicated in the cognitive regulation of emotion (Buhle et al., 2013), and by decreased vmPFC recruitment, which supports valuation of stimuli (Roy et al., 2012). At the same time, age predicted reduced craving during reappraisal and unregulated responding. These findings are consistent with a growing body of evidence that children can use cognitive strategies to self-regulate (McRae et al., 2012; W. Mischel & Baker, 1975; Silvers et al., 2012), but also that one's capacity and tendency to use cognitive strategies improves with age and experience (McRae et al., 2012; H. N. Mischel & Mischel, 1983; Silvers et al., 2012).
Implications for translational work on obesity in childhood and adolescence
From a translational perspective, the present study has implications for basic obesity research as well as for interventions seeking to prevent childhood obesity.
With regards to basic research, it is intriguing to consider why food craving decreases with age given epidemiological work showing that obesity rates rise with age, particularly after age 20 (M. C. Nelson, Story, Larson, Neumark-Sztainer, & Lytle, 2008). This could be explained in one of three ways. First, adults may crave less and eat less food than children and adolescents, but gain weight because of biological or lifestyle factors (e.g., metabolism, sedentariness). Second, craving and consumption may be tightly coupled in childhood but become less so with age, such that adults eat more but experience less craving than younger individuals – a possibility supported by data showing that young adults eat less healthily than adolescents (M. C. Nelson et al., 2008). Third, craving and food consumption may increase in adulthood after decreasing during adolescence – a possibility that was untestable in the present study because of participant age range but could be tested in future work.
Also striking was our finding that heavier individuals recruited left vlPFC less strongly than leaner individuals during regulation. This result dovetails nicely with a recent study showing that BMI predicts less lateral PFC recruitment during reappraisal in adults (Giuliani et al., 2014). The fact that vlPFC was also identified in the main effect of strategy analysis suggests that while most individuals recruited vlPFC during reappraisal, this depended in part on BMI. Given vlPFC's involvement in emotion regulation more generally (Buhle et al., 2013), these findings could be interpreted in one of two ways. The first is that heavier individuals recruit vlPFC to a lesser degree because they are less effective at regulating craving. However, this seems unlikely given that BMI did not predict craving. The second possibility is that although all participants were instructed to reappraise using the same strategy, heavy and lean individuals implemented reappraisal differently. In prior work, left vlPFC has been more strongly implicated in reinterpretation variants of reappraisal – i.e., generating a narrative that provides an alternative meaning for a stimulus – than distancing variants like the one used in the present study (Ochsner, Silvers, & Buhle, 2012). That BMI-related differences in vlPFC recruitment were most pronounced in younger individuals suggests that perhaps leaner children incorporate elements of reinterpretation, even when distancing, to a greater extent than heavier children.
With respect to interventions, it is noteworthy that after little training, participants as young as 6 years learned to regulate craving. It is particularly striking that the reappraisal strategy was so effective given that participants were not explicitly told it was intended to reduce craving. At present, the majority of childhood obesity interventions aim to restrict access to unhealthy foods or increase access to exercise (Sobol-Goldberg, Rabinowitz, & Gross, 2013). While changing children's environments is clearly important, so too is teaching them to regulate cravings that lead to unhealthy choices in the first place. Teaching children how to regulate cravings when they inevitably do encounter temptations deserves to be tested as a tool for combating obesity.
Limitations
The present study has two limitations that deserve mention. First, age effects were identified using a cross-sectional design. Future work may examine within-subject changes in food craving longitudinally to test whether similar results are obtained. Second, participants did not rate their current hunger prior to scanning. Given that hunger may influence food craving, this too ought to be incorporated into future study designs.
Supplementary Material
Acknowledgements
We thank Danielle Dellarco, Chelsea Helion, Alexa Hubbard, Natasha Mehta, Gloria Pedersen, and Theresa Teslovich Woo for their help in participant recruitment and testing.
Funding
This work was supported by the National Institutes of Health (R01 NICHD 0691780, F31 NIMH 94056).
Footnotes
Author Contributions
J.A. Silvers and K.N. Ochsner developed the study concept. J.A. Silvers, C. Insel, A. Powers, B.J. Casey, W. Mischel and K.N. Ochsner designed the study. J.A. Silvers, C. Insel, A. Powers, and P. Franz collected the data. J.A. Silvers, C. Insel, P. Franz, and J. Weber performed data analyses under the supervision of K.N. Ochsner. J.A. Silvers drafted the first manuscript with extensive input from K.N. Ochsner. B.J. Casey, W. Mischel, and K.N. Ochsner provided critical revisions. All authors approved the final version of the manuscript for submission.
References
- Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. Neuroimage. 2010;52(4):1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SJ, Cedernaes J, Schioth HB. Increased prefrontal and parahippocampal activation with reduced dorsolateral prefrontal and insular cortex activation to food images in obesity: a meta-analysis of fMRI studies. PLoS One. 2013;8(4):e60393. doi: 10.1371/journal.pone.0060393. doi: 10.1371/journal.pone.0060393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhle JT, Silvers JA, Wager TD, Lopez R, Onyemekwu C, Kober H, Ochsner KN. Cognitive Reappraisal of Emotion: A Meta-Analysis of Human Neuroimaging Studies. Cereb Cortex. 2013 doi: 10.1093/cercor/bht154. doi: 10.1093/cercor/bht154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Somerville LH, Gotlib IH, Ayduk O, Franklin NT, Askren MK, Shoda Y. Behavioral and neural correlates of delay of gratification 40 years later. Proc Natl Acad Sci U S A. 2011;108(36):14998–15003. doi: 10.1073/pnas.1108561108. doi: 10.1073/pnas.1108561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahanty LM, Meigs JB, Hayden D, Williamson DA, Nathan DM. Psychological and behavioral correlates of baseline BMI in the diabetes prevention program (DPP). Diabetes Care. 2002;25(11):1992–1998. doi: 10.2337/diacare.25.11.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz WH. Critical periods in childhood for the development of obesity. Am J Clin Nutr. 1994;59(5):955–959. doi: 10.1093/ajcn/59.5.955. [DOI] [PubMed] [Google Scholar]
- Francis LA, Susman EJ. Self-regulation and rapid weight gain in children from age 3 to 12 years. Arch Pediatr Adolesc Med. 2009;163(4):297–302. doi: 10.1001/archpediatrics.2008.579. doi: 10.1001/archpediatrics.2008.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. J Neurosci. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. doi: 26/25/6885 [pii] 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A, McGlennen KM. Enhanced striatal sensitivity to aversive reinforcement in adolescents versus adults. J Cogn Neurosci. 2013;25(2):284–296. doi: 10.1162/jocn_a_00326. doi: 10.1162/jocn_a_00326. [DOI] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cereb Cortex. 2010;20(7):1613–1629. doi: 10.1093/cercor/bhp225. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuliani NR, Mann T, Tomiyama AJ, Berkman ET. Neural Systems Underlying the Reappraisal of Personally Craved Foods. J Cogn Neurosci. 2014 doi: 10.1162/jocn_a_00563. doi: 10.1162/jocn_a_00563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore WD, Yurgelun-Todd DA. Developmental changes in the functional brain responses of adolescents to images of high and low-calorie foods. Dev Psychobiol. 2005;47(4):377–397. doi: 10.1002/dev.20099. doi: 10.1002/dev.20099. [DOI] [PubMed] [Google Scholar]
- Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proc Natl Acad Sci U S A. 2010;107(33):14811–14816. doi: 10.1073/pnas.1007779107. doi: 1007779107[pii]10.1073/pnas.1007779107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Eickhoff SB. Sustaining attention to simple tasks: A meta-analytic review of the neural mechanisms of vigilant attention. Psychological Bulletin. 2013;139(4):870–900. doi: 10.1037/a0030694. doi: 10.1037/a0030694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence NS, Hinton EC, Parkinson JA, Lawrence AD. Nucleus accumbens response to food cues predicts subsequent snack consumption in women and increased body mass index in those with reduced self-control. Neuroimage. 2012;63(1):415–422. doi: 10.1016/j.neuroimage.2012.06.070. doi: 10.1016/j.neuroimage.2012.06.070. [DOI] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: A meta-analysis of functional neuroimaging studies. Neuroscience & Biobehavioral Reviews. 2011;35(5):1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRae K, Gross JJ, Weber J, Robertson ER, Sokol-Hessner P, Ray RD, Ochsner KN. The development of emotion regulation: an fMRI study of cognitive reappraisal in children, adolescents and young adults. Soc Cogn Affect Neurosci. 2012;7(1):11–22. doi: 10.1093/scan/nsr093. doi: 10.1093/scan/nsr093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei Z, Grummer-Strawn LM, Pietrobelli A, Goulding A, Goran MI, Dietz WH. Validity of body mass index compared with other body-composition screening indexes for the assessment of body fatness in children and adolescents. Am J Clin Nutr. 2002;75(6):978–985. doi: 10.1093/ajcn/75.6.978. [DOI] [PubMed] [Google Scholar]
- Mischel HN, Mischel W. The development of children's knowledge of self-control strategies. Child Dev. 1983;54(3):603–619. [Google Scholar]
- Mischel W, Baker N. Cognitive appraisals and transformations in delay behavior. Journal of Personality and Social Psychology. 1975;31(2):254–261. [Google Scholar]
- Morgan JF, Reid F, Lacey JH. The SCOFF questionnaire: a new screening tool for eating disorders. West J Med. 2000;172(3):164–165. doi: 10.1136/ewjm.172.3.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: a neuroscience perspective on the process and its relation to psychopathology. Psychol Med. 2005;35(2):163–174. doi: 10.1017/s0033291704003915. [DOI] [PubMed] [Google Scholar]
- Nelson MC, Story M, Larson NI, Neumark-Sztainer D, Lytle LA. Emerging adulthood and college-aged youth: an overlooked age for weight-related behavior change. Obesity (Silver Spring) 2008;16(10):2205–2211. doi: 10.1038/oby.2008.365. doi: 10.1038/oby.2008.365. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251(1):E1–E24. doi: 10.1111/j.1749-6632.2012.06751.x. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999-2010. JAMA. 2012;307(5):483–490. doi: 10.1001/jama.2012.40. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Eickhoff SB. Modelling neural correlates of working memory: a coordinate-based meta-analysis. Neuroimage. 2012;60(1):830–846. doi: 10.1016/j.neuroimage.2011.11.050. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy M, Shohamy D, Wager TD. Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends Cogn Sci. 2012;16(3):147–156. doi: 10.1016/j.tics.2012.01.005. doi: S1364-6613(12)00027-7[pii]10.1016/j.tics.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharmuller W, Ubel S, Ebner F, Schienle A. Appetite regulation during food cue exposure: a comparison of normal-weight and obese women. Neurosci Lett. 2012;518(2):106–110. doi: 10.1016/j.neulet.2012.04.063. doi: 10.1016/j.neulet.2012.04.063. [DOI] [PubMed] [Google Scholar]
- Schlam TR, Wilson NL, Shoda Y, Mischel W, Ayduk O. Preschoolers' delay of gratification predicts their body mass 30 years later. J Pediatr. 2013;162(1):90–93. doi: 10.1016/j.jpeds.2012.06.049. doi: 10.1016/j.jpeds.2012.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siep N, Roefs A, Roebroeck A, Havermans R, Bonte M, Jansen A. Fighting food temptations: the modulating effects of short-term cognitive reappraisal, suppression and up-regulation on mesocorticolimbic activity related to appetitive motivation. Neuroimage. 2012;60(1):213–220. doi: 10.1016/j.neuroimage.2011.12.067. doi: 10.1016/j.neuroimage.2011.12.067. [DOI] [PubMed] [Google Scholar]
- Silvers JA, McRae K, Gabrieli JD, Gross JJ, Remy KA, Ochsner KN. Age-Related Differences in Emotional Reactivity, Regulation, and Rejection Sensitivity in Adolescence. Emotion. 2012 doi: 10.1037/a0028297. doi: 10.1037/a0028297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobol-Goldberg S, Rabinowitz J, Gross R. School-based obesity prevention programs: A meta-analysis of randomized controlled trials. Obesity (Silver Spring) 2013 doi: 10.1002/oby.20515. doi: 10.1002/oby.20515. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Casey BJ. Developmental neurobiology of cognitive control and motivational systems. Curr Opin Neurobiol. 2010;20(2):236–241. doi: 10.1016/j.conb.2010.01.006. doi: 10.1016/j.conb.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. J Cogn Neurosci. 2011;23(9):2123–2134. doi: 10.1162/jocn.2010.21572. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teslovich T, Mulder M, Franklin NT, Ruberry EJ, Millner A, Somerville LH, Casey BJ. Adolescents let sufficient evidence accumulate before making a decision when large incentives are at stake. Dev Sci. 2013 doi: 10.1111/desc.12092. doi: 10.1111/desc.12092. [DOI] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SA, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cereb Cortex. 2010;20(1):61–69. doi: 10.1093/cercor/bhp078. doi: bhp078 [pii]10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Verbeken S, Braet C, Lammertyn J, Goossens L, Moens E. How is reward sensitivity related to bodyweight in children? Appetite. 2012;58(2):478–483. doi: 10.1016/j.appet.2011.11.018. doi: 10.1016/j.appet.2011.11.018. [DOI] [PubMed] [Google Scholar]
- Wager TD, Sylvester CY, Lacey SC, Nee DE, Franklin M, Jonides J. Common and unique components of response inhibition revealed by fMRI. Neuroimage. 2005;27(2):323–340. doi: 10.1016/j.neuroimage.2005.01.054. doi: 10.1016/j.neuroimage.2005.01.054. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Telang F, Jayne M, Ma Y, Pradhan K, Fowler JS. Evidence of gender differences in the ability to inhibit brain activation elicited by food stimulation. Proc Natl Acad Sci U S A. 2009;106(4):1249–1254. doi: 10.1073/pnas.0807423106. doi: 10.1073/pnas.0807423106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welvaert M, Rosseel Y. On the definition of signal-to-noise ratio and contrast-to-noise ratio for FMRI data. PLoS One. 2013;8(11):e77089. doi: 10.1371/journal.pone.0077089. doi: 10.1371/journal.pone.0077089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokum S, Stice E. Cognitive regulation of food craving-effects of three cognitive reappraisal strategies on neural response to palatable foods. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2013.39. doi: 10.1038/ijo.2013.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.