Abstract
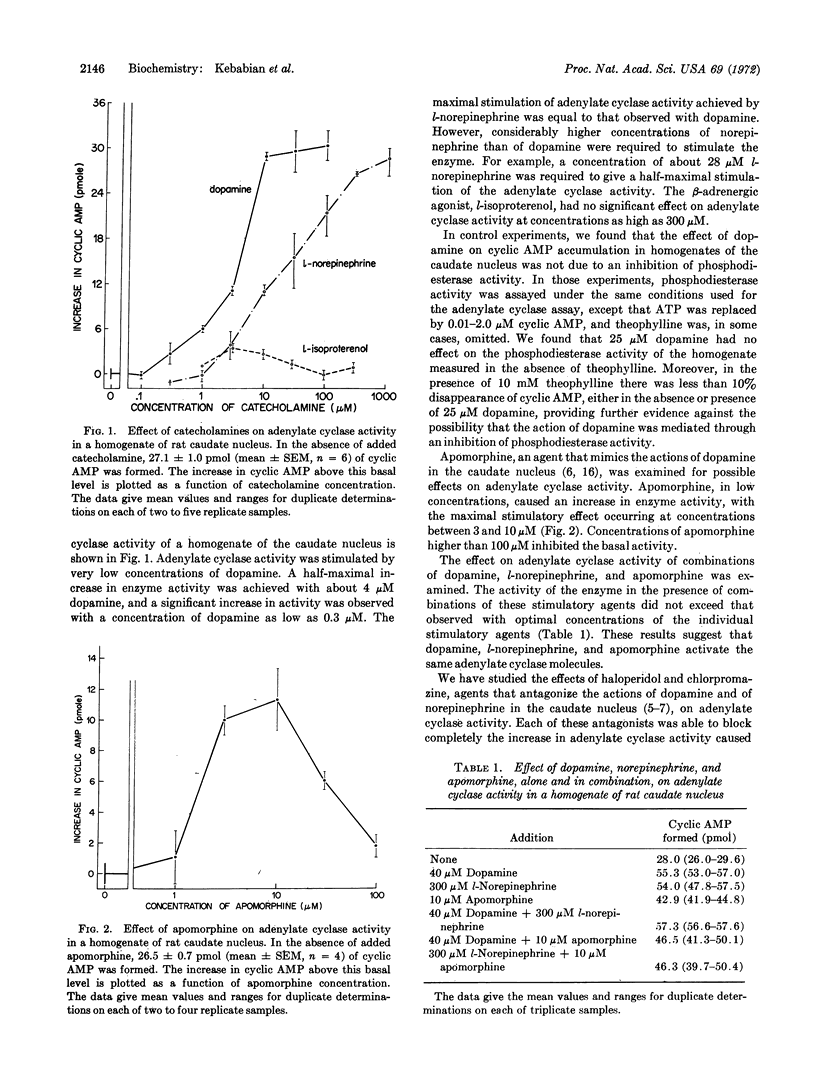
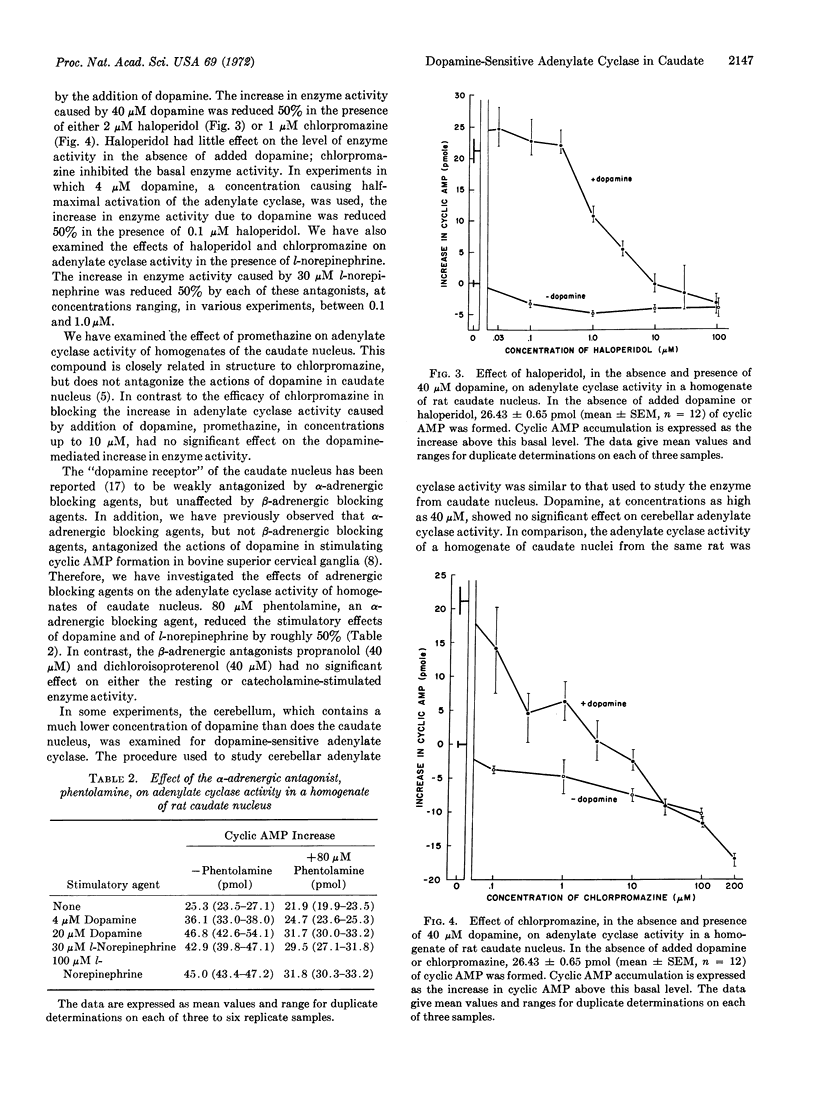
An adenylate cyclase that is activated specifically by low concentrations of dopamine has been demonstrated in homogenates of caudate nucleus of rat brain. A half-maximal increase in the activity of the enzyme occurred in the presence of 4 μM dopamine. Concentrations of dopamine as low as 0.3 μM stimulated the activity of the enzyme. The adenylate cyclase activity of the homogenates was also stimulated by low concentrations of apomorphine, a substance known to mimic the physiological and pharmacological effects of dopamine. The stimulatory effect of dopamine was blocked by low concentrations of either haloperidol or chlorpromazine, agents known to block the actions of dopamine in mammalian brain. The results suggest that dopamine-sensitive adenylate cyclase may be the receptor for dopamine in mammalian brain. The isolation of this enzyme from caudate nucleus should facilitate the search for new therapeutic agents useful in the treatment of extrapyramidal diseases.
Keywords: extrapyramidal disease, ganglia, neurotransmitter, dihydroxyphenylalanine, Parkinson's disease
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDEN N. E., DAHLSTROEM A., FUXE K., LARSSON K. FURTHER EVIDENCE FOR THE PRESENCE OF NIGRO-NEOSTRIATAL DOPAMINE NEURONS IN THE RAT. Am J Anat. 1965 Jan;116:329–333. doi: 10.1002/aja.1001160117. [DOI] [PubMed] [Google Scholar]
- Aghajanian G. K., Roth R. H. Gamma-hydroxybutyrate-induced increase in brain dopamine: localization by fluorescence microscopy. J Pharmacol Exp Ther. 1970 Oct;175(1):131–138. [PubMed] [Google Scholar]
- Andén N. E., Corrodi H., Fuxe K., Ungerstedt U. Importance of nervous impulse flow for the neuroleptic induced increase in amine turnover in central dopamine neurons. Eur J Pharmacol. 1971 Jul;15(2):193–199. doi: 10.1016/0014-2999(71)90173-7. [DOI] [PubMed] [Google Scholar]
- Andén N. E., Dahlström A., Fuxe K., Larsson K. Functional role of the nigro-neostriatal dopamine neurons. Acta Pharmacol Toxicol (Copenh) 1966;24(2):263–274. doi: 10.1111/j.1600-0773.1966.tb00389.x. [DOI] [PubMed] [Google Scholar]
- Andén N. E., Rubenson A., Fuxe K., Hökfelt T. Evidence for dopamine receptor stimulation by apomorphine. J Pharm Pharmacol. 1967 Sep;19(9):627–629. doi: 10.1111/j.2042-7158.1967.tb09604.x. [DOI] [PubMed] [Google Scholar]
- Bieger D., Larochelle L., Hornykiewicz O. A model for the quantitative study of central dopaminergic and serotoninergic activity. Eur J Pharmacol. 1972 Apr;18(1):128–136. doi: 10.1016/0014-2999(72)90140-9. [DOI] [PubMed] [Google Scholar]
- Bloom F. E., Costa E., Salmoiraghi G. C. Anesthesia and the responsiveness of individual neurons of the caudate nucleus of the cat to acetylcholine, norepinephrine and dopamine administered by microelectrophoresis. J Pharmacol Exp Ther. 1965 Nov;150(2):244–252. [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. H., Makman M. H. Stimulation by dopamine of adenylate cyclase in retinal homogenates and of adenosine-3':5'-cyclic monophosphate formation in intact retina. Proc Natl Acad Sci U S A. 1972 Mar;69(3):539–543. doi: 10.1073/pnas.69.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CARLSSON A., LINDQVIST M. EFFECT OF CHLORPROMAZINE OR HALOPERIDOL ON FORMATION OF 3METHOXYTYRAMINE AND NORMETANEPHRINE IN MOUSE BRAIN. Acta Pharmacol Toxicol (Copenh) 1963;20:140–144. doi: 10.1111/j.1600-0773.1963.tb01730.x. [DOI] [PubMed] [Google Scholar]
- Connor J. D. Caudate nucleus neurones: correlation of the effects of substantia nigra stimulaton with iontophoretic dopamine. J Physiol. 1970 Jul;208(3):691–703. doi: 10.1113/jphysiol.1970.sp009143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat W. C., Volle R. L. Interactions between the catecholamines and ganglionic stimulating agents in sympathetic ganglia. J Pharmacol Exp Ther. 1966 Nov;154(2):200–215. [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-hydroxytyramine) and brain function. Pharmacol Rev. 1966 Jun;18(2):925–964. [PubMed] [Google Scholar]
- Kebabian J. W., Greengard P. Dopamine-sensitive adenyl cyclase: possible role in synaptic transmission. Science. 1971 Dec 24;174(4016):1346–1349. doi: 10.1126/science.174.4016.1346. [DOI] [PubMed] [Google Scholar]
- LAVERTY R., SHARMAN D. F. THE ESTIMATION OF SMALL QUANTITIES OF 3,4-DIHYDROXYPHENYLETHYLAMINE IN TISSUES. Br J Pharmacol Chemother. 1965 Apr;24:538–548. doi: 10.1111/j.1476-5381.1965.tb01744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libet B. Generation of slow inhibitory and excitatory postsynaptic potentials. Fed Proc. 1970 Nov-Dec;29(6):1945–1956. [PubMed] [Google Scholar]
- Libet B., Tosaka T. Dopamine as a synaptic transmitter and modulator in sympathetic ganglia: a different mode of synaptic action. Proc Natl Acad Sci U S A. 1970 Oct;67(2):667–673. doi: 10.1073/pnas.67.2.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAfee D. A., Schorderet M., Greengard P. Adenosine 3',5'-monophosphate in nervous tissue: increase associated with synaptic transmission. Science. 1971 Mar 19;171(3976):1156–1158. doi: 10.1126/science.171.3976.1156. [DOI] [PubMed] [Google Scholar]
- Moore K. E., Von Voigtlander P. F. The release of H 3 -dopamine from cat brain following electrical stimulation of the substantia nigra and caudate nucleus. Neuropharmacology. 1971 Nov;10(6):733–741. doi: 10.1016/0028-3908(71)90088-8. [DOI] [PubMed] [Google Scholar]
- Ungerstedt U., Butcher L. L., Butcher S. G., Andén N. E., Fuxe K. Direct chemical stimulation of dopaminergic mechanisms in the neostriatum of the rat. Brain Res. 1969 Jul;14(2):461–471. doi: 10.1016/0006-8993(69)90122-x. [DOI] [PubMed] [Google Scholar]


