Abstract
Background
People using long-term indwelling urinary catheters experience multiple recurrent catheter problems. Self-management approaches are needed to avoid catheter-related problems.
Objectives
The aim was to determine effectiveness of a self-management intervention in prevention of adverse outcomes (catheter-related urinary tract infection, blockage, and accidental dislodgement). Healthcare treatment associated with the adverse outcomes and catheter-related quality of life was also studied.
Method
A randomized clinical trial was conducted. The intervention involved learning catheter-related self-monitoring and self-management skills during home visits by a study nurse (twice during the first month and at four months—with a phone call at two months). The control group received usual care. Data were collected during an initial face-to-face home interview followed by bimonthly phone interviews. A total of 202 adult long-term urinary catheter users participated. Participants were randomized to treatment or control groups following collection of baseline data. Generalized estimating equations (GEE) were used for the analysis of treatment effect.
Results
In the intervention group, there was a significant decrease in reported blockage in the first six months (p = .02), but the effect did not persist. There were no significant effects for catheter-related urinary tract infection or dislodgment. Comparison of baseline rates of adverse outcomes with subsequent periods suggested that both groups improved over 12 months.
Discussion
A simple–to–use catheter problems calendar and the bimonthly interviews might have functioned as a modest self-monitoring intervention for persons in the control group. A simplified intervention using a self-monitoring calendar is suggested—with optimal and consistent fluid intake likely to add value.
Keywords: longitudinal research, quality of life, randomized clinical trial, self-management, urinary catheterization
Indwelling urethral or suprapubic catheters are used by individuals with chronic urinary retention who are unable to perform intermittent-catheterization because of poor hand dexterity, no caregiver assistance, difficulty in using the bathroom, or in select cases of incontinence. While many catheter users have spinal cord injury (SCI) or multiple sclerosis (MS), the population also includes those with other neurological disorders, diabetes, or disease/injury to the bladder region. Unfortunately, indwelling urinary catheters are seldom trouble free (Cottenden et al., 2013; Wilde, McDonald et al., 2013). Data collected in a longitudinal study of 43 long-term catheter users indicated that recurrent problems affect the great majority. Prevalence rates during eight months of catheter use were 70% for catheter-associated urinary tract infection (CAUTI) with a rate of 8.4/1000 catheter days; 74% for blockage; and 33% for catheter expulsion or dislodgement (Wilde, et al., 2010). For a two-month period prior to the beginning of this randomized clinical trial (RCT), catheter problems were reported as: 31% for CAUTI, 24% blockage, and 12% dislodgment (Wilde, McDonald et al., 2013).
These complications are distressing to patients/families (Wilde & Cameron, 2003; Wilde, 2003) and contribute to increased healthcare expenses, such as additional clinic, home care, or emergency department visits or hospitalization (Wilde, McDonald et al., 2013). Interventions to address prevention of CAUTI in long-term catheter users such as the use of silver coated or antimicrobial coatings on the catheter (Parker et al., 2009) installations to the drainage bag (Thompson et al., 1984), or special cleaning of the urinary meatus (Burke, Jacobson, Garibaldi, Conti, & Alling, 1983) have not been successful. Although research in self-management of chronic conditions in diabetes (Coyle, Francis, & Chapman, 2013), stroke (Lennon, McKenna, & Jones, 2013), and asthma (van Gaalen et al., 2013) has grown, no self-management clinical trials have been found in catheter users (Cottenden et al., 2013; Niël-Weise, van den Broek, Peterhans, da Silva, & Silva, 2012).
The research clinical trial (RCT) for the current report was developed inductively through six previous studies conducted primarily by the first author (MHW). These studies included research with 30 individuals who kept a urinary diary and were interviewed twice to determine what their self-care practices were (Wilde & Dougherty, 2006). A concept analysis was next conducted of self-monitoring, delineating key attributes and how it fit within self-management literature (Wilde& Garvin, 2007). Then a pilot study teaching self-monitoring of urine flow in long-term catheter users was conducted. The results of the pilot study indicated that optimal fluid intake and preventing dislodgment were the most useful self-management components reported by study participants. Importantly, CAUTI decreased in the six months during the single group pilot (Wilde & Brasch, 2008a). Taken together, the theoretical model for the current study proposed that the intervention would affect catheter self-management indirectly through self-efficacy, and directly; and that higher levels of awareness, monitoring, and behaviors related to catheter care would improve outcomes (Figure 1; Wilde, Zhang et al., 2013).
FIGURE 1.
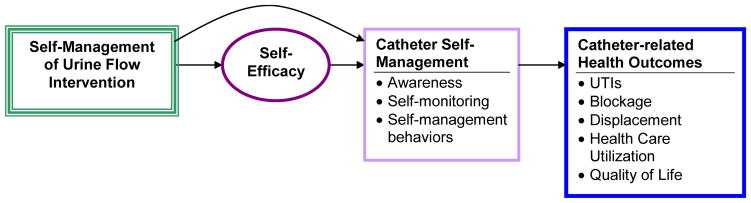
Theoretical model for self-management of urine flow intervention (Wilde, Zhang et al., 2013). Used with permission.
The aim of the study was to determine the effect of the self-management intervention on health outcomes compared to the usual care control. It was hypothesized that the self-management group would: (a) have fewer episodes of UTI (and severity), blockage, and dislodgement of the catheter; (b) have decreased unplanned catheter-related healthcare utilization, including hospitalizations, emergency department visits, and fewer nurse home/or clinic visits; and (c) report higher catheter-related quality of life.
Methods
Design
The study was a randomized, single-blinded experimental design with repeated measures every two months over a 12-month period. The design called for 101 individuals to receive the self-management intervention and 101 to receive usual care (catheter-related care provided by home care nurses, clinics, or private providers).
Inclusion/Exclusion Criteria
Eligible participants were adults age 18 and over. Inclusion criteria were: (a) expect to use an indwelling urethral or suprapubic catheter for at least one year, and will be in the study region for at least four months; (b) can complete study measurements alone or with the help of a caregiver; (c) speak English; and (d) have access to a telephone for data collection. Because we wanted to target only individuals who might benefit from this study and, thus, better determine effectiveness, our participants also must have had a catheter problem of CAUTI within the last year, or blockage or dislodgement within the last six months, or be new to a catheter within the last year. Individuals were excluded for terminal illness or cognitive impairments. Children under 18 were not included because they might not have the capacity for self-care, which includes directing others.
Setting and Recruitment
Participants consisted of community-dwelling individuals recruited in two distinct regions by two study sites: (a) a university in a large northeastern U.S. state, and (b) a home care agency which conducts research in a large metropolitan area in the same state. For the university site, participants were recruited through nurses or physicians in home care agencies, medical center clinics, hospitals, and private medical offices (e.g., urology). Screening for eligibility and interest in participation was conducted by phone by the first author (MHW) or the project coordinator after contact information was received from providers who had permission to do so from potential participants. Some catheter users contacted the researchers themselves. In the home care agency, their database was used to identify potential participants with a catheter for telephone call screenings and recruitment by trained study interviewers. Using data from the agency’s U.S. Outcome and Assessment Information Set (OASIS) for home care, catheter users were screened and excluded if they had a poor prognosis/life expectancy, cognitive impairment, confused, severe speech impairment, behavior problems, diagnosis indicating dementia, or had been previously interviewed (in another study or declined). Data were collected from June 2009-June 2012 in the homes of community-dwelling individuals and through telephone contacts over 12 months of participation.
Sample Size and Power Calculations
Power calculations were performed to determine a sample size for an adequately powered study to detect clinically meaningful effects across multiple outcomes. For each of the primary outcomes, a priori range of clinically meaningful effect sizes was determined based on previous research. All calculations employed a significance level of .05 and 80% power. Power analyses were performed with SAS 9.1 using Monte Carlo simulation resampling techniques for general estimating equation (GEE) analysis (Gastañaga, McLaren, & Delfino, 2006; Yuan & Hayashi, 2003). The analysis indicated that a sample of 220 (160 completers) would provide sufficient power to detect medium effect sizes (15% to 30% differences between groups) for the primary health status outcomes. However, health care utilization measures, such as hospitalizations and ED visits, require larger samples due to their relatively rare frequency of occurrence.
Ethics/Human Subjects
The study was approved at each site by their respective bodies for protection of human subjects. A coordinated approach assured that the same processes were used, including the same consent form with stamps and contact information from both sites. A Data Safety Monitoring Board was formed and convened annually to assess interim results and potential adverse events.
Randomization and Blinding
Randomization was conducted by the main study statistician who directed the processes with the study coordinators at each site—each of whom subsequently enrolled participants at their own site and allocated them to treatment or usual care after completion of the baseline home visit (HV) interview. Participants were stratified by site to balance the large number of study subjects in the large metropolitan area (75% of participants), as compared with the university site (25% of participants) which was a combination of urban, suburban, and rural areas. Block randomization with random block sizes of 4, 6, or 8 was carried out independently at the two sites to balance the two treatment groups. For each site, a sequence of random assignments was generated by the study statistician and sealed in sequentially numbered envelopes by the study coordinator. The participants were sequentially assigned the treatment after completing the consent and initial HV interview; then, the study nurse called those allocated to the intervention and made arrangements for the first nurse contact in the home. Study investigators, data gathering teams, and statisticians were blinded to allocation status until the final analyses were completed.
The Urinary Catheter Self-Management Intervention
The intervention designed to improve self-management in people with long-term, indwelling urinary catheters was based on self-efficacy theory (Bandura, 1997). Sources of urinary catheter self-efficacy were targeted in the nurse home visit interventions—specifically mastery experiences—vicarious observation, verbal persuasion, and knowledge about physiological status.
Each of the two study sites followed identical intervention protocols, which consisted of three home visits and one telephone call by a trained registered nurse to deliver the intervention. Two home visits took place in the first month and a third (booster) visit occurred at four months. During the first home visit, participants were taught to conduct self-monitoring using a three-day urinary diary to record observations and measurements of fluid intake and output (I & O), urine characteristics, and sensations of flow. This was to teach awareness of urine flow, basic self-monitoring skills, and to increase their level of mastery, thus, contributing to increased catheter related self-efficacy. During the second home visit, about a week later, self-management skills were taught first by reviewing the information from the urinary diary, calculating the intake and output averages and comparing these to an optimal volume (30ml/kg body weight), and identifying the individual’s catheter-related problems. Anything notable about I & O, the color/character of urine, or of urinary sensations, was discussed and implications for self-management pointed out.
An educational booklet which had been piloted and viewed as very helpful (Wilde, Zhang, et al., 2013) was then provided and discussed, which focused on basic catheter self-management skills related to: (a) maintaining optimal and consistent fluid intake; and (b) preventing catheter dislodgement—which were the key components of the intervention. In the presence of certain bacteria which cause urea in the urine to split, sodium, magnesium, and calcium will precipitate from the urine—often at about a pH of 6.8, causing sediment and encrustation. However, researchers found that urine pH could increase to as high as 9 or 10, and the catheter might not block if fluid intake is increased to dilute the concentration of minerals (Khan, Housami, Melotti, Timoney, & Stickler, 2010). This is our foundation for the fluid intake requirements, which we set at 30ml/kg body weight (Gray & Krissovich, 2003).
Other modules of the booklet were reviewed briefly or in-depth, depending on interest or need. These were: recognizing early symptoms of CAUTI; living with the catheter; promoting optimal catheter change intervals; decreasing caffeine; decreasing leakage; emptying and cleaning the drainage bag; making adjustments for sex; and recognizing early symptoms of autonomic dysreflexia (for people with spinal cord injury/disease). Goals, if any, were written in the educational booklet. A motivational bookmark with quotes and pictures was reviewed to help encourage participants to be attentive to urine flow.
Two weeks later, the study nurses called to answer questions and, if needed, helped the participants revise goals or plans. At four months, a third home visit served as a booster of the intervention to further refine or modify goals/plans as the catheter user desired. Family or caregivers were encouraged to be present, but the intervention was delivered to the catheter user.
Intervention compliance and fidelity
Two study nurses (one at each site), who were trained together at the beginning of the project, delivered all the intervention components. Multiple strategies were used to establish and sustain the fidelity and integrity of the intervention. These involved: (a) standardization of the intervention and training, including use of a detailed training manual that incorporated Bandura’s self-efficacy concepts (Bandura, 1997); (b) randomly selected fidelity assessments of 10% of the interventions—half by audiotape and half in-person home observations; (c) at least monthly conference calls; (d) training study nurses together; (e) tracking study nurse activities and responsiveness of the participants; (f) assessment of participant skills at the end of the study; and (g) inclusion of fidelity assessment in the analysis plan. The results of the audiotape and in person fidelity assessment indicated that competence and adherence to the intervention parameters were highly scored with most means between 4–5 (5 was the highest possible score). Also, because there was little variability in the proportion of the intervention participants who received the intervention contacts (i.e., 98% for HV1, 95% for HV2, 93% TC at two weeks, and 91% HV3 at four months), we decided it was not necessary to adjust for fidelity in the main outcomes analysis. All participants, regardless of group allocation, continued to receive usual nursing and medical care.
Usual Care
Participants randomized to the control group received usual care.
Measures
Measures were developed for this study based on previous research of the first-author’s team (Wilde & Brasch, 2008a; Wilde & Dougherty, 2006). Instrumentation was modeled on the Stanford Chronic Disease Self-Management programs (http://patienteducation.stanford.edu/). Participants in both groups were administered identical data collection instruments. At baseline, self-report was used for data collection related to the two-month timeframe prior to the study (for evaluating equivalence of the groups), and every two months for 12 months thereafter through telephone call interviews. To improve accuracy of recall, all participants were asked to maintain an ongoing catheter calendar over the 12 months of the study, using letter symbols for problems: CAUTI (U), blockage (B), and dislodgement (D). Treatments were identified as antibiotic (A), extra nurse home visit (HV), extra office visit (O), hospitalization (H), and emergency department visit (ED). For missed interviews, data were collected for the primary outcomes at the next scheduled interview—if the study participant had kept track of problems in their catheter calendar. This occurred only nine times.
Outcomes
Primary outcomes consisted of catheter-related complications of CAUTI, blockage, and dislodgement. CAUTI was defined as a urinary tract infection that was treated with an antibiotic prescribed by the person’s healthcare provider. Blockage was defined as an occurrence in which the urine would not flow through the catheter due to an obstruction of the catheter tube. Blockage was distinguished from kinks or twists that are external to the catheter tube. Dislodgement occurred when the catheter fell out or became displaced accidentally due to traction (i.e., pulling on it). Catheter-related quality of life was also a primary outcome using our previously developed measure with a five-point Likert scale from 1 = strongly agree to 5 = strongly disagree; higher scores reflect better quality of life (Wilde, Brasch, Getliffe, McMahon et al., 2010).
Excess healthcare expenditures (treatments) related to catheter problems were also primary outcomes; these included extra nurse or clinic visits, hospitalizations, rehabilitation, and emergency department visits. Additional information was asked about each episode of CAUTI regarding the perceived severity of the infection (on a scale of 1–10, with 1 = very mild and 10 = most severe imaginable), and the number of days hospitalized or in rehabilitation specifically related to the CAUTI.
Data Analysis
Standard data cleaning procedures were applied to screen for errors and potential univariate and multivariate outliers. These procedures led to the removal of blockage data (percentages/month, counts, treatments) for one participant due to inconsistent and contradictory responses. All other data were used; several outliers for blockage were adjusted by windsorizing to 9 as the maximum number of events in a two-month period for testing group differences.
Intention-to-treat analysis was used. Data were analyzed with SAS 9.3 (SAS Institute Inc., Cary, NC, USA). Generalized linear models were utilized with an identity link function for continuous outcomes (CAUTI severity and QOL) and a logit link function for binary outcomes. Randomization achieved comparability on demographic characteristics of participants and aspects of catheter use (Tables 1 and 2) in treatment and usual care groups. The groups were similar on key outcome variables during the two months prior to the study, except for catheter blockage (p < .05) and days hospitalized (p < .01) (Table 2). Thus, we fitted each of the models with and without controlling for baseline information on the outcome variable. First, data for the first six months were modeled; then, data from the entire 12 month period of the study were modeled.
TABLE 1.
Baseline Characteristics by Group
| Characteristic | Intervention
|
Control
|
||
|---|---|---|---|---|
| M | (SD) | M | (SD) | |
| Age (years) | 60.6 | (16.6) | 62.2 | (18.2) |
| Catheter (months) | 73.1 | (87.4) | 71.9 | (83.8) |
| Catheter size (Fr) | 18.4 | (3.3) | 18.7 | (3.2) |
|
|
|
|||
| n | (%) | n | (%) | |
|
|
||||
| Gender (male) | 53 | (52.4) | 50 | (50.0) |
| Catheter type | ||||
| Urethral | 57 | (56.4) | 55 | (54.5) |
| Suprapubic | 43 | (42.6) | 46 | (45.5) |
| Both | 1 | (0.0) | 0 | (0.0) |
| Catheter balloon size | ||||
| 5–10 ml | 64 | (63.4) | 60 | (59.4) |
| 30 ml | 11 | (10.9) | 18 | (17.8) |
| Other | 1 | (1.0) | 1 | (1.0) |
| Unknown/no answer | 25 | (24.8) | 22 | (21.8) |
Note. There were 101 participants in each group. None of the characteristics was significantly associated with treatment group assignment. Median duration of catheter use in the intervention group was 42 months; control group median was 37 months. Additional detailed information about the sample is available in Wilde, McDonald et al. (2013).
TABLE 2.
Baseline Comparison of Key Outcomes by Group (For the Two Months Prior to the Study)
| Outcome | Intervention
|
Control
|
p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| M | (SD) | %a | Rateb | 95% CI | M | (SD) | %a | Rateb | 95% CI | ||
| Catheter-related health status | |||||||||||
| Urinary tract infection (yes) | .42 | (0.71) | 33 | 6.9 | [5.0, 9.4] | .33 | (0.53) | 30 | 5.50 | [3.8, 7.7] | ns |
| Catheter blockage (yes) | .56 | (1.55) | 21 | 9.3 | [7.0, 12.1] | .69 | (1.67) | 26 | 11.50 | [9.0, 14.6] | .05 |
| Dislodgement (yes) | .17 | (0.68) | 8 | 2.8 | [1.6, 4.5] | .26 | (0.68) | 17 | 4.33 | [2.8, 6.4] | ns |
| Healthcare related to CAUTI | |||||||||||
| Hospitalization (number) | 0.11 | (0.37) | 9 | 1.8 | [0.9, 3.3] | 0.1 | (0.32) | 8 | 1.50 | [0.69, 2.85] | ns |
| Hospitalization (days) | 0.61 | (2.33) | 9 | 10.2 | [7.8, 13.1] | 1.0 | (4.26) | 8 | 17.17 | [14.01, 20.82] | .01 |
Note. CAUTI = catheter-associated urinary tract infection; CI = confidence interval; ns = not significant at p < .05; SD = standard deviation.
Percent experiencing the event during the two months prior to the study.
Rate per 1000 catheter days.
First six months
We let “group” be the indicator of treatment assignment (0 = usual care; 1 = treatment), and yit be the outcome for the ith subject at month t. Three models were developed for each outcome over the first six months of the study: a group differences model, a model for interaction of group and time; and a model controlling for baseline and time. The group differences model is
| (1) |
where f is the appropriate link function, the intercept β0 is the y-intercept in the usual care group when f is an identity function and mean log odds for the usual care group when f is the logit link, β1 is the treatment effect, and the “*” symbol denotes multiplication. The interaction model, controlling for baseline and time is
| (2) |
When the interactions in Equation 2 were not significant, we further modeled the data controlling for baseline and time, with
| (3) |
In the models given in Equations 1 and 3, inference about β1 provides information about the treatment effect, either as difference in outcome (CAUTI severity, quality of life) or difference in log odds of the outcome (binary outcomes) as a function of group (Equation 1) or group, baseline, and time in months (Equation 3).
Complete 12 month outcomes
We applied the same group difference model in Equation 1 to the 12-month data (i.e., t = 2, 4, 6, 8, 10 and 12). For the models controlling for baseline and time (Equations 2 and 3), an indicator variable “second” was added to allow modeling differences between the first and second six months of the study (scored 0 when t = 2, 4, or 6 months and 1 when t = 8, 10, and 12 months). An interaction term between “second” and “group” was added to the models described in Equations 2 and 3 to detect possible treatment effects between the first and last six months of the study, shown as
| (4) |
and
| (5) |
To deal with the dependency among the repeated measures, generalized estimating equations (GEE) with a first order autoregressive structure for the working correlation were utilized. We chose to use GEE because of the complexity and difficulty in modeling the correlations among the repeated measures—especially for discrete outcomes. As a semiparametric approach, GEE has the advantage that the inference is robust to the misspecification of the working correlation matrix—in the sense that estimates are consistent even when the working correlation departures from the true correlations among repeated measures (Diggle, Heagerty, Liang, & Zeger, 2002). Nominal p-values of .05 for two-tailed tests were used.
Results
Sample Description
Baseline characteristics of participants are displayed in Table 1. Ages ranged from 19–96 years (Mdn = 61). The range in duration of catheter use was 1 to 470 months (39 years). Self-reported diagnoses involved SCI (40%), MS (23%), diabetes (12%), stroke (2%), prostate (10%), spina bifida (1%), neurogenic bladder not otherwise reported (8%), Parkinson’s disease (2%), and other (3%). Indications for indwelling catheter use were: immobility or difficulty moving around (58%), incontinence (57%), neurogenic bladder (54%), obstructed urine (32%), healing wounds (11%), and other reasons (11%). More than one indication could be listed. Additional detailed information about the participants at baseline is available in Wilde, McDonald et al. (2013).
Table 2 lists catheter-related health status, healthcare related to CAUTI, healthcare related to blockage, and quality of life at baseline by treatment group. Following randomization, groups appeared equivalent except that, during the two months prior to the study, the percentage of participants who had catheter dislodgement and the number of days hospitalized for a catheter-related CAUTI were higher in the control group.
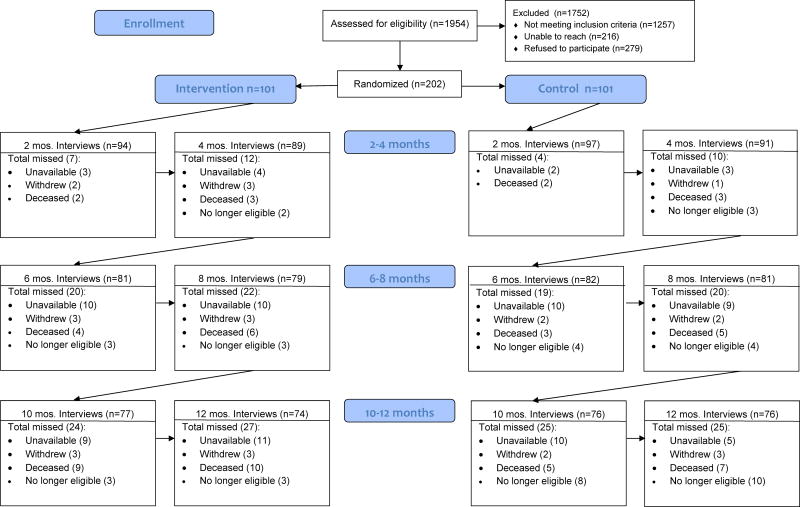
Attrition was similar in both groups (Figure 2). There were more deaths in the experimental group than the control group (10 vs. 7). More participants whose catheters were removed were in the control group, mostly late in the study (3 vs. 10). Three persons withdrew from each arm of the study.
FIGURE 2.
CONSORT flow diagram
Treatment Effects
Table 3 lists treatment effects for primary outcomes for models depicted in Equation 3 (short term effects during the first six months of the study, controlling for baseline value and time) and Equation 5 (longer-term effects, controlling for baseline value, time, and first vs. second half of study). Complete statistical results are available from the authors (MHW).
TABLE 3.
Treatment Effects for Primary Outcomes
| Outcome | First six monthsa
|
Full 12 monthsb
|
|||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
(SE) | p | 95% CI |
|
(SE) | p | 95% CI | ||
| Catheter-related health status | |||||||||
| CAUTI (yes) | −0.17 | (0.266) | ns | [−0.69, 0.35] | 0.16 | (0.222) | ns | [−0.27, 0.60] | |
| CAUTI severityc | 2.76 | (0.590) | < .001 | [1.60, 3.92] | 1.60 | (0.467) | < .01 | [0.68, 2.51] | |
| Blockage (yes) | −0.74 | (0.343) | < .05 | [−1.42, −0.07] | −0.23 | (0.291) | ns | [−0.81, 0.34] | |
| Dislodgment (yes) | 0.29 | (0.328) | ns | [−0.36, 0.93] | 0.01 | (0.287) | ns | [−0.55, 0.57] | |
| Healthcare for CAUTI | |||||||||
| Hospitalized (yes) | 0.81 | (0.527) | ns | [−0.23, 1.84] | 1.33 | (0.428) | < .01 | [0.49, 2.17] | |
| Emergency visit (yes) | 0.88 | (0.373) | < .05 | [0.15, 1.61] | 1.06 | (0.337) | < .001 | [0.40, 1.72] | |
| Nurse home visit (yes) | −0.06 | (0.402) | ns | [−0.85, 0.73] | 0.00 | (0.317) | ns | [−0.62, 0.62] | |
| Clinic visit (yes) | −0.37 | (0.408) | ns | [−1.17, 0.43] | −0.06 | (0.333) | ns | [−0.71, 0.59] | |
| Healthcare for blockage | |||||||||
| Emergency visit (yes) | −0.62 | (0.978) | ns | [−2.53, 1.30] | 1.10 | (0.575) | ns | [−0.02, 2.23] | |
| Nurse home visit (yes) | −1.35 | (0.642) | < .05 | [−2.61, −0.09] | −1.02 | (0.470) | < .05 | [−1.95, −0.10] | |
| Clinic visit (yes) | −0.62 | (0.629) | ns | [−1.86, 0.61] | −0.42 | (0.560) | ns | [−1.52, 0.68] | |
| Quality of lifed | −1.66 | (1.581) | ns | [−4.76, 1.44] | −2.03 | (1.389) | ns | [−4.75, 0.69] | |
Note. CAUTI = catheter-associated urinary tract infection; CI = confidence interval; ns = not significant at p < .05; SE = standard error. A positive sign for B1 favors the control (usual care group); a negative sign favors the intervention group. Complete statistical results are available from the authors (MHW).
Treatment effects were obtained controlling for baseline outcomes and time (Equation 3).
Treatment effects were obtained controlling for baseline outcomes, time from 2 to 12 months, and first vs. second half of the study (Equation 5).
Scored from 1 = very mild to 10 = most severe imaginable.
Scored from 1 = strongly agree to 5 = strongly disagree; higher scores reflect better self-rated quality of life.
First six months
Without controlling for any covariates, the overall short-term effect of the intervention relative to the control condition was assessed over the first six months (Equation 1). During the first six months, patients in the experimental group were less likely to report catheter blockage (p < .05) and fewer blockage-related nurse visits (p < .05), but they reported experiencing more severe CAUTI (p < .001) and significantly more CAUTI-related emergency room visits (p < .05), in terms of the percentage reporting such events. As shown in Table 3, we obtained similar conclusions controlling for the outcome variables at baseline (such as CAUTI) as well as time (Equation 3).
Complete 12 month outcomes
Without controlling for any covariates, the overall long-term effect of the intervention compared to the control condition was assessed over the whole 12-month period (Equation 1, with t = 2, 4, 6, 8, 10, 12). However, the overall difference in blockage between the two groups was not significant over this longer period. The experimental group continued to report significantly higher CAUTI severity scores (p < .01) and CAUTI-related emergency room visits (percentage reporting these events [p < .001] and frequencies of events [p < .01], as well as more hospitalizations for CAUTI (percentage [p < .01], frequencies [p < .01], and days hospitalized [p < .05]; Table 3; Table 4 [days hospitalized]).
TABLE 4.
Rates of Blockage, CAUTI, Dislodgement and Hospitalization in Treatment and Control Groups Over Time
| Outcome | Experimental
|
Control
|
p | Within groupsa (p)
|
|||
|---|---|---|---|---|---|---|---|
| Rateb | 95% CI | Rateb | 95% CI | E | C | ||
| Blockage | |||||||
| Baseline | 9.26 | [6.98, 12.05] | 11.50 | [8.95, 14.55] | ns | ||
| First 6 months | 4.28 | [3.32, 5.43] | 7.41 | [6.14, 8.86] | < .01 | < .0001 | .004 |
| Second 6 months | 5.31 | [4.15, 6.67] | 4.45 | [3.41, 5.71] | ns | < .0001 | < .0001 |
| Full 12 months | 4.76 | [4.00, 5.62] | 6.04 | [5.20, 6.99] | .03 | < .0001 | < .0001 |
| CAUTI | |||||||
| Baseline | 6.93 | [5.00, 9.37] | 5.50 | [3.79, 7.72] | ns | ||
| First 6 months | 4.37 | [3.40, 5.53] | 4.83 | [3.82, 6.03] | ns | .02 | ns |
| Second 6 months | 5.48 | [4.31, 6.87] | 3.29 | [2.41, 4.39] | .01 | ns | .02 |
| Full 12 months | 4.89 | [4.12, 5.75] | 4.12 | [3.42, 4.91] | ns | .05 | ns |
| Dislodgement | |||||||
| Baseline | 2.80 | [1.63, 4.49] | 4.33 | [2.83, 6.35] | ns | ||
| First 6 months | 2.60 | [1.86, 3.52] | 2.74 | [1.99, 3.67] | ns | ns | ns |
| Second 6 months | 1.45 | [0.89, 2.24] | 2.44 | [1.69, 3.41] | ns | .05 | .03 |
| Full 12 months | 2.06 | [1.58, 2.65] | 2.60 | [2.06, 3.24] | ns | ns | .02 |
| Hospitalizations (number) | |||||||
| Baseline | 1.82 | [0.91, 3.25] | 1.50 | [0.69, 2.85 | ns | ||
| First 6 months | 1.01 | [0.58, 1.65] | 0.43 | [0.17, 0.89] | ns | ns | .01 |
| Second 6 months | 1.68 | [1.07, 2.52] | 0.22 | [0.04, 0.63] | <.001 | ns | .004 |
| Full 12 months | 1.32 | [0.94, 1.81] | 0.33 | [0.16, 0.61] | <.001 | ns | .001 |
| Hospitalization (days) | |||||||
| Baseline | 10.23 | [7.84, 13.12] | 17.17 | [14.01, 20.82] | .01 | ||
| First 6 months | 7.73 | [6.4, 9.23] | 4.03 | [3.11, 5.13] | .01 | ns | < .0001 |
| Second 6 months | 8.26 | [6.81, 9.93] | 1.01 | [0.55, 1.69] | .001 | ns | < .0001 |
| Full 12 months | 7.98 | [6.99, 9.06] | 2.63 | [2.08, 3.28] | .001 | ns | < .0001 |
Note. C = control; E = experimental.
Change from baseline.
Rate is per 1000 catheter days.
Additional statistical tests of treatment effect controlling for baseline outcomes, time, and study period (“second”) were conducted (Equation 4, not shown in a table.). The experimental group tended to report more CAUTI compared to the control group in the second half ( ; 95% CI [0.03, 1.30], p = .04), and no difference between the two groups during the first half year. The experimental group reported higher CAUTI severity than the control group in the first half year ( ; 95% CI [−4.62, −0.97], p < .0001), but no difference between the two groups during the second half. This indicates that the experimental group’s CAUTI severity scores decreased relative to the control group from the first to the second half of the year. Blockages were fewer in the experimental group (by percentage) compared with the control group in the first half of the year ( , 95% CI [0.38, 1.97], p < .05), but not in the second half of the year.
For other outcomes, the interaction was not significant, and was therefore removed. Treatment effect was estimated controlling for baseline outcomes and time. The experimental group had higher hospitalization for CAUTI (p < .01) and emergency room visits (p < .001; Table 3). Catheter-related quality of life did not differ significantly by group (Table 3).
Rates Comparisons
Table 4 provides detailed information about rates of blockage, CAUTI, dislodgement, and hospitalizations within groups over the course of the study and between groups. See Table 4 for a figure displaying the percentage of persons who reported experiencing catheter adverse outcomes of blockage, CAUTI, or dislodgement by group over time.
Catheter-related health status
Comparison of between- and within-groups rates per 1000 catheter days from baseline provided further details about changes in the primary outcomes over the first six months, second six months, and full study of 12 months. Blockage rates were significantly lower in the experimental group during the first six months (p < .01) and for the full study (p = .03), but there were no differences during the second half of the study (p = .31). Blockage improved significantly within each group at each assessment time from baseline.
For CAUTI, there were significant rate differences favoring the control group during the second half of the study (p = .01), but no differences for the first half (p = .55) or the full study (p = .16). Compared with baseline rate estimates, the experimental group had significant decreases in CAUTI rates during the first half of the study (p = .02), and for the overall full study time period of 12 months (p = .05). The control group had a significant decrease in CAUTI during the second half of the study (p = .02).
For dislodgement, rates decreased steadily in both groups by six months and 12 months, and there were no significant group differences. Dislodgement rates were lower in the experimental group at baseline, and larger decreases in dislodgement took place over time in the control group. During the second half of the study, the experimental group rate was slightly better than the control, but not significantly so (p = .06).
Hospitalization rates
Hospitalizations were significantly higher in the experimental group for most time points (Table 4). While the hospitalization rates favored the control group, rates decreased in both groups compared with their respective rates at baseline. Slight increases in rates were found during the second six months in the experimental group, compared with the first six months, but control group rates continued to decrease during the second six months. With further analysis at the individual level, we found that one person (in the experimental group) was hospitalized six times during the study, and five of these occurred in the second six months of the study. All others hospitalized in either group were hospitalized either once or twice during the study.
Discussion
Key Findings
Four Cochrane reviews concluded that there was an astounding lack of evidence to guide practice in long-term catheter use (Cottenden et al., 2013). Almost all intervention research in the past has focused on applications such as silver or antibiotic coatings (Johnson, Kuskowski, & Wilt, 2006) to the catheter, special cleaning of the urinary meatus (Burke, Jacobson, Garibaldi, Conti, & Alling, 1983), or drainage bag additives (Washington, 2001). None have proven effective in long-term catheter users. We believe this study is a unique contribution because it is the first known study of its kind using a randomized clinical trial with an inductively derived, theory-based behavioral intervention (Wilde, 2002; Wilde & Brasch, 2008b; Wilde and Dougherty, 2006; Wilde & Garvin, 2007) to assess whether teaching catheter users self-management skills could decrease short-term, catheter-related problems, and whether improvements could be sustained over 12 months.
The GEE analyses indicated that there was a significant group difference in the first six months only for the blockage outcome—favoring the experimental group. Several interactions suggested that effects of the intervention were stronger for the first six months than the long-term effects over 12 months. Comparisons of rates for the first six months, second six months, and full study of 12 months—as well as the changes in rates from baseline—provided additional information suggesting that both groups improved over time. The line graphs (see Supplemental Digital Content) also indicate a general downward trend for both groups over the 12-month study.
The decreases in rates for CAUTI and blockage are believed to be clinically meaningful in both groups. In a recent report from the Agency on Healthcare Research and Quality (2013, p. 26), decreases in CAUTI in hospitalized persons went down in two months from 2.56 to 2.39/1000 catheter days—a relative reduction of 6.3%. The rate decreased even further at 14 months to 2.14/1000 catheter days—a 16.1% relative reduction in 14 months. These changes reflected that “progress has been made” toward national goals. CAUTI rates in community-dwelling persons can be as high as 8.4/1000 catheter days (Wilde, Brasch, Getliffe, Brown et al., 2010). In our study in the experimental group, the baseline CAUTI rate of 6.93/1000 catheter days decreased to 4.89 (a 29% relative reduction) and in the control group from 5.5/1000 catheter days to 4.12 (a 25% relative reduction; see Table 4).
Blockage prevalence is often cited as about 50% (Getliffe, 2003), but our previous research reflects a wide range of 74% over eight months in 43 persons (Wilde, Brasch, Getliffe, Brown et al., 2010) to 24% in our cross-sectional analysis—before random assignment at baseline in the current study (Wilde, McDonald et al., 2013). Importantly, there is no agreement of whether blockage is a one-time occurrence or a persistent pattern, and this might explain the wide range in blockage prevalence. Because our study tested group differences in this randomized trial, the blockage rates reported should be viewed with caution because blockage outliers were adjusted statistically to a maximum of 9 in a two-month time period. Prior to our study, there were no known reports of dislodgement rates. Therefore, we recommend that in future research, rates per 1000 catheter days should be calculated for blockage and dislodgement, as well as CAUTI.
Although blockage decreased significantly in the first six months in the experimental group—as compared with the control group—it is not known whether this was truly of benefit since the control group also improved and started with more blockages. In addition, since this effect did not last over the full 12 months, and the three nurse home visits took place in months one and four, there might be a benefit in expanding the intervention dose over time.
One major issue remains: whether the self-management intervention contributed to more hospitalizations, whether the experimental group was sicker and more prone to severe CAUTIs requiring hospitalization, or whether this group simply noticed signs of CAUTI and acted on them more quickly. A priori, we had identified hospitalization as an indicator of CAUTI severity. Nevertheless, we had hypothesized that the intervention participants would contact care providers earlier and, thus, avoid some of the hospitalizations. However, if the experimental group were to have been more prone to serious CAUTIs—as severity scores suggested—then seeking early care—even if it included hospitalization—might have kept them from more serious consequences, such as long hospitalizations, sepsis, or death. There is no way to know this.
In a recent analysis addressing reasons for rehospitalization, chronic disease and vulnerability in patients’ conditions seem to play a big role. Also, morbidity and mortality appeared to be inversely related, meaning sicker patients were treated more often at a hospital and extending their lives. Thus, hospitalization is not necessarily an indicator of poor quality in care (American Hospital Association, 2011). As with the other primary outcomes, we found a pattern of both groups improving over time in relation to hospitalization. That is, rates in hospitalization (times hospitalized and days hospitalized) decreased in both groups, and there were larger decreases in the first six months of the study (see Supplemental Digital Content).
The experimental and control groups both appeared to have improved during the study. Simple self-monitoring through use of the catheter calendar for bimonthly data collection by study participants in both groups could have contributed to fewer catheter problems overall. Essentially, it is possible that study participants in both groups became more aware of catheter problems due to the calendar, and were reminded of their catheter through the phone call interviews every two months. This could have contributed to changes in self-management behaviors, such as increasing fluids for early CAUTI symptoms. Teaching people with indwelling urinary catheters to keep track of key catheter problems (self-monitoring) in a simple notation calendar could be easy and practical to implement in practice. Because blockage decreased significantly in the first six months of the study, in the intervention group, there could be added value in teaching about optimal and consistent fluid intake.
Research implications include replication with additional nurse contacts over time, simplifying the intervention to focus on optimal fluid intake and preventing dislodgement, and using a simple catheter calendar as a self-monitoring intervention. Testing in a multisite RCT using data collection forms embedded in the home care patient records could eliminate self-reported data for outcomes. Evidence-based policies will lag until more randomized trials—or other forms of scientifically sound research—are conducted in this understudied and vulnerable population that use indwelling catheters for long-term bladder management.
Limitations
Self-reported data were used because standardized health records were not available. The catheter calendar was used to minimize self-report error. In addition, recall during the pilot study was excellent using bimonthly telephone interviews (Wilde & Brasch, 2008a). Agreement between self-reported catheter problems (CAUTI, blockage, dislodgement) and chart data was high in another study (97%; Wilde, Brasch, Getliffe, Brown et al., 2010).
Use of a medical diagnosis with antibiotic treatment for defining CAUTI could include some inaccurate diagnoses because some providers could have treated asymptomatic bacteriuria. Because bacteriuria is universal in this population after 30 days, colony counts would be useless. It was not possible to review multiple sources of data in multiple agencies to determine symptoms which might have been used for treatment decision making. Moreover, symptoms vary among individuals and over time. Thus, the decision for treating symptomatic CAUTI is complex, and it requires clinical judgment of the provider.
Attrition was similar in both groups. It was addressed regularly by the team during monthly meetings. Each of the 17 deaths that occurred during the study was evaluated. At the large home care agency site, charts were audited to determine whether there was any person whose death might have been related to the study. In one or two instances, details about comorbidities were discussed with the urologist on the team. The data safety monitoring board (DSMB) met annually with the lead investigators, statistician, urologist, and an outside researcher to review information about each death. The DSMB conclusion was that no deaths were related to the study, and that comorbidities contributed to each event.
Conclusions
Adults with a variety of health conditions are challenged with managing urinary catheter self-care to avoid complications and enhance quality of life. In a one-year RCT setting, participants receiving a self-monitoring, self-management intervention had less catheter blockage during the first six months. No other differences between treatment and control groups were noted. Participants in experimental and control groups improved over 12 months, compared with baseline. The simple-to-use catheter problems calendar and bimonthly interviews used for data collection may have served as a modest intervention in both groups.
Supplementary Material
A figure displaying the percentage in each group reporting catheter problems at each timepoint during the trial.
Acknowledgments
The authors acknowledge funding by National Institute of Nursing Research, National Institutes of Health (U.S.) #R01 NR01553.
The authors also acknowledge Penny Feldman, PhD, Sr. Vice President at the Center for Home Care Policy and Research, Visiting Nurse Service of New York; Harriet Kitzman, RN, PhD, Senior Associate Dean for Research, University of Rochester School of Nursing; and Robert Mayer, MD, Professor, University of Rochester/Strong Memorial Hospital.
Footnotes
Clinical Trial Registration number from clinicaltrials.gov is NCT00883220.
Conflicts of Interest Statements: Mary H. Wilde has been a consultant with NovaBay Pharmaceutics Inc. since June 2013. The remaining authors have no conflicts of interest to report.
Contributor Information
Mary H. Wilde, University of Rochester, School of Nursing, Rochester, New York.
James M. McMahon, University of Rochester, School of Nursing, Rochester, New York.
Margaret V. McDonald, Visiting Nurse Service of New York, Center for Home Care Policy and Research.
Wan Tang, University of Rochester, Department of Biostatistics and Computational Biology, Rochester, New York.
Wenjuan Wang, University of Rochester, Department of Biostatistics and Computational Biology, Rochester, New York.
Judith Brasch, University of Rochester, School of Nursing, Rochester, New York.
Eileen Fairbanks, University of Rochester, School of Nursing, Rochester, New York.
Shivani Shah, Visiting Nurse Service of New York, Center for Home Care Policy and Research.
Feng Zhang, University of Rochester, School of Nursing, Rochester, New York.
Ding-Geng (Din) Chen, University of Rochester, School of Nursing and Department of Biostatistics and Computational Biology, Rochester, New York.
References
- Agency for Healthcare Research and Quality. Eliminating CAUTI: Interim data report: A national patient safety imperative. Rockville, MD: Author; 2013. Retrieved from http://www.ahrq.gov/professionals/quality-patient-safety/cusp/cauti-interim/index.html. [Google Scholar]
- American Hospital Association. Trendwatch: Examining the drivers for readmissions and reducing unnecessary readmissions for better patient care. 2011 Retrieved from http://www.aha.org/research/reports/tw/11sep-tw-readmissions.pdf.
- Bandura A. Self-efficacy: The exercise of control. New York, NY: W. H. Freeman; 1997. [Google Scholar]
- Burke JP, Jacobson JA, Garibaldi RA, Conti MT, Alling DW. Evaluation of daily meatal care with poly-antibiotic ointment in prevention of urinary catheter-associated bacteriuria. Journal of Urology. 1983;129:331–334. doi: 10.1016/s0022-5347(17)52083-2. [DOI] [PubMed] [Google Scholar]
- Cottenden A, Bliss D, Buckley B, Fader M, Getliffe KC, Patterson J, Wilde MH. Management using continence products. In: Abrams P, Cardozo L, Khoury S, Wein AJ, editors. Incontinence: 5th international consultation on incontinence. Arnheim, The Netherlands: ICUD-EAU Publishers; 2013. pp. 1651–1786. [Google Scholar]
- Coyle ME, Francis K, Chapman Y. Self-management activities in diabetes care: A systematic review. Australian Health Review. 2013;37:513–522. doi: 10.1071/AH13060. [DOI] [PubMed] [Google Scholar]
- Diggle PJ, Heagerty P, Liang K-Y, Zeger S. Analysis of longitudinal data. Oxford, UK: Oxford University Press; 2002. [Google Scholar]
- Gastañaga VM, McLaren CE, Delfino RJ. Power calculations for generalized linear models in observational longitudinal studies: A simulation approach in SAS. Computer Methods and Programs in Biomedicine. 2006;84:27–33. doi: 10.1016/j.cmpb.2006.07.011. [DOI] [PubMed] [Google Scholar]
- Getliffe K. Managing recurrent urinary catheter blockage: Problems, promises, and practicalities. Journal of Wound, Ostomy and Continence Nursing. 2003;30:146–151. doi: 10.1067/mjw.2003.120. [DOI] [PubMed] [Google Scholar]
- Gray M, Krissovich M. Does fluid intake influence the risk for urinary incontinence, urinary tract infection, and bladder cancer? Journal of Wound, Ostomy and Continence Nursing. 2003;30:126–131. doi: 10.1067/mjw.2003.117. [DOI] [PubMed] [Google Scholar]
- Johnson JR, Kuskowski MA, Wilt TJ. Systematic review: Antimicrobial urinary catheters to prevent catheter-associated urinary tract infection in hospitalized patients. Annals of Internal Medicine. 2006;144:116–126. doi: 10.7326/0003-4819-144-2-200601170-00009. [DOI] [PubMed] [Google Scholar]
- Khan A, Housami F, Melotti R, Timoney A, Stickler D. Strategy to control catheter encrustation with citrated drinks: A randomized crossover study. Journal of Urology. 2010;183:1390–1394. doi: 10.1016/j.juro.2009.12.024. http://dx.doi.org/10.1016/j.juro.2009.12.024. [DOI] [PubMed] [Google Scholar]
- Lennon S, McKenna S, Jones F. Self-management programmes for people post stroke: A systematic review. Clinical Rehabilitation. 2013;27:867–878. doi: 10.1177/0269215513481045. [DOI] [PubMed] [Google Scholar]
- Niël-Weise BS, van den Broek PJ, da Silva EMK, Silva LA. Urinary catheter policies for long-term bladder drainage. Cochrane Database Systematic Review. 2012;8:CD004201. doi: 10.1002/14651858.CD004201.pub3. [DOI] [PubMed] [Google Scholar]
- Parker D, Callan L, Harwood J, Thompson DL, Wilde M, Gray M. Nursing interventions to reduce the risk of catheter-associated urinary tract infection: Part 1: Catheter selection. Journal of Wound, Ostomy and Continence Nursing. 2009;36:23–34. doi: 10.1097/01.WON.0000345173.05376.3e. [DOI] [PubMed] [Google Scholar]
- Thompson RL, Haley CE, Searcy MA, Guenthner SM, Kaiser DL, Gröschel DH, Wenzel RP. Catheter-associated bacteriuria: Failure to reduce attack rates using periodic instillations of a disinfectant into urinary drainage systems. JAMA. 1984;251:747–751. doi: 10.1001/jama.1984.03340300039025. [DOI] [PubMed] [Google Scholar]
- van Gaalen JL, Beerthuizen T, van der Meer V, van Reisen P, Redelijkheid GW, Snoeck-Stroband JB, Sont JK. Long-term outcomes of internet-based self-management support in adults with asthma: Randomized controlled trial. Journal of Medical Internet Research. 2013;15:e188. doi: 10.2196/jmir.2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washington EA. Instillation of 3% hydrogen peroxide or distilled vinegar in urethral catheter drainage bag to decrease catheter-associated bacteriuria. Biological Research for Nursing. 2001;3:78–87. doi: 10.1177/109980040200300203. [DOI] [PubMed] [Google Scholar]
- Wilde MH. Urine flowing: A phenomenological study of living with a urinary catheter. Research in Nursing & Health. 2002;25:14–24. doi: 10.1002/nur.10020. [DOI] [PubMed] [Google Scholar]
- Wilde MH. Life with an indwelling urinary catheter: The dialectic of stigma and acceptance. Qualitative Health Research. 2003;13:1189–1204. doi: 10.1177/1049732303257115. [DOI] [PubMed] [Google Scholar]
- Wilde MH, Brasch J. A pilot study of self-monitoring urine flow in people with long-term urinary catheters. Research in Nursing and Health. 2008a;31:490–500. doi: 10.1002/nur.20281. [DOI] [PubMed] [Google Scholar]
- Wilde MH, Brasch J. Teaching self-management to long-term urinary catheter users. International Journal of Urological Nursing. 2008b;2:62–71. [Google Scholar]
- Wilde MH, Brasch J, Getliffe K, Brown KA, McMahon JM, Smith JA, Tu X. Study on the use of long-term urinary catheters in community-dwelling individuals. Journal of Wound, Ostomy and Continence Nursing. 2010;37:301–310. doi: 10.1097/WON.0b013e3181d73ac4. [DOI] [PubMed] [Google Scholar]
- Wilde MH, Getliffe K, Brasch J, McMahon J, Anson E, Tu X. A new urinary catheter-related quality of life instrument for adults. Neurourology and Urodynamics. 2010;29:1282–1285. doi: 10.1002/nau.20865. [DOI] [PubMed] [Google Scholar]
- Wilde MH, Cameron BL. Meanings and practical knowledge of people with long-term urinary catheters. Journal of Wound, Ostomy and Continence Nursing. 2003;30:33–43. doi: 10.1067/mjw.2003.6. [DOI] [PubMed] [Google Scholar]
- Wilde MH, Dougherty MC. Awareness of urine flow in people with long-term urinary catheters. With Commentary from B. Roe and Response by Authors. Journal of Wound Ostomy and Continence Nursing. 2006;33(2):164–75. doi: 10.1097/00152192-200603000-00011. [DOI] [PubMed] [Google Scholar]
- Wilde MH, Garvin S. A concept analysis of self-monitoring. Journal of Advanced Nursing. 2007;57:339–350. doi: 10.1111/j.1365-2648.2006.04089. [DOI] [PubMed] [Google Scholar]
- Wilde MH, McDonald MV, Brasch J, McMahon JM, Fairbanks E, Shah S, Scheid E. Long-term urinary catheter users self-care practices and problems. Journal of Clinical Nursing. 2013;22:356–367. doi: 10.1111/jocn.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilde MH, Zhang F, Fairbanks E, Shah S, McDonald MV, Brasch J. Perceived value of a urinary catheter self-management program in the home. Home Healthcare Nurse. 2013;31:465–473. doi: 10.1097/NHH.0b013e3182a89791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan KH, Hayashi K. Bootstrap approach to inference and power analysis based on three test statistics for covariance structure models. British Journal of Mathematical and Statistical Psychology. 2003;56:93–110. doi: 10.1348/000711003321645368. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A figure displaying the percentage in each group reporting catheter problems at each timepoint during the trial.