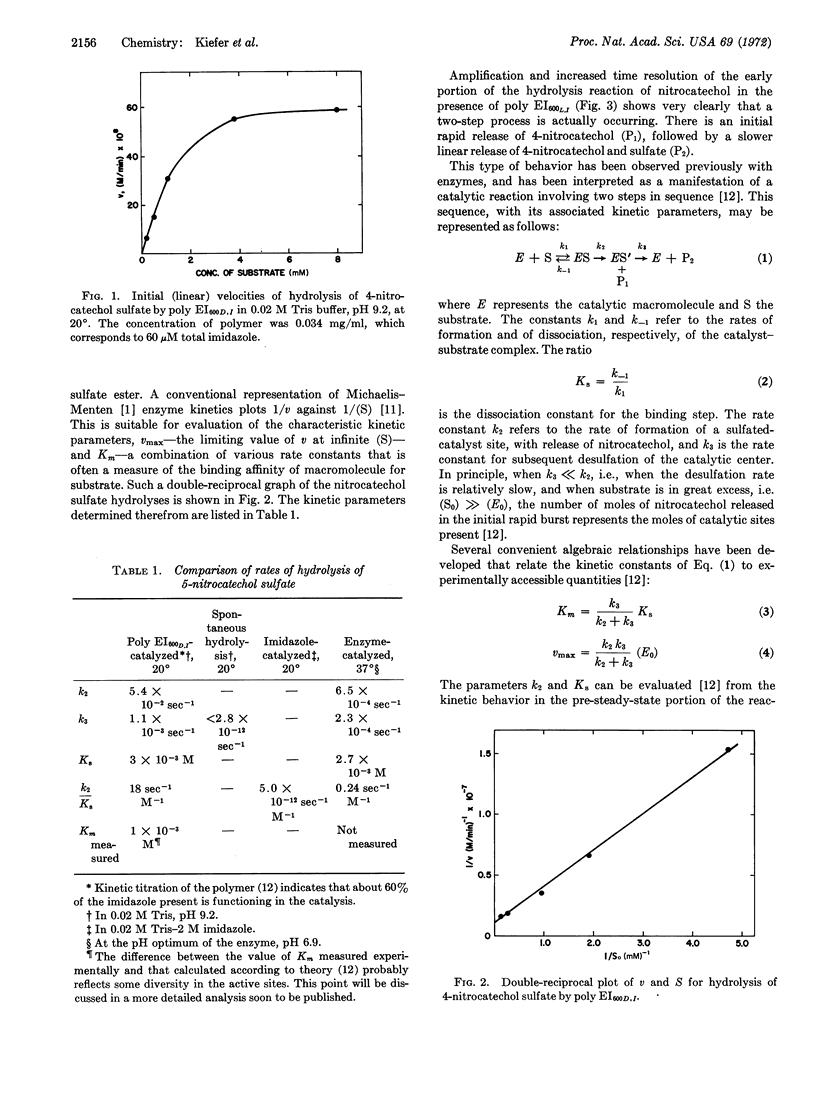
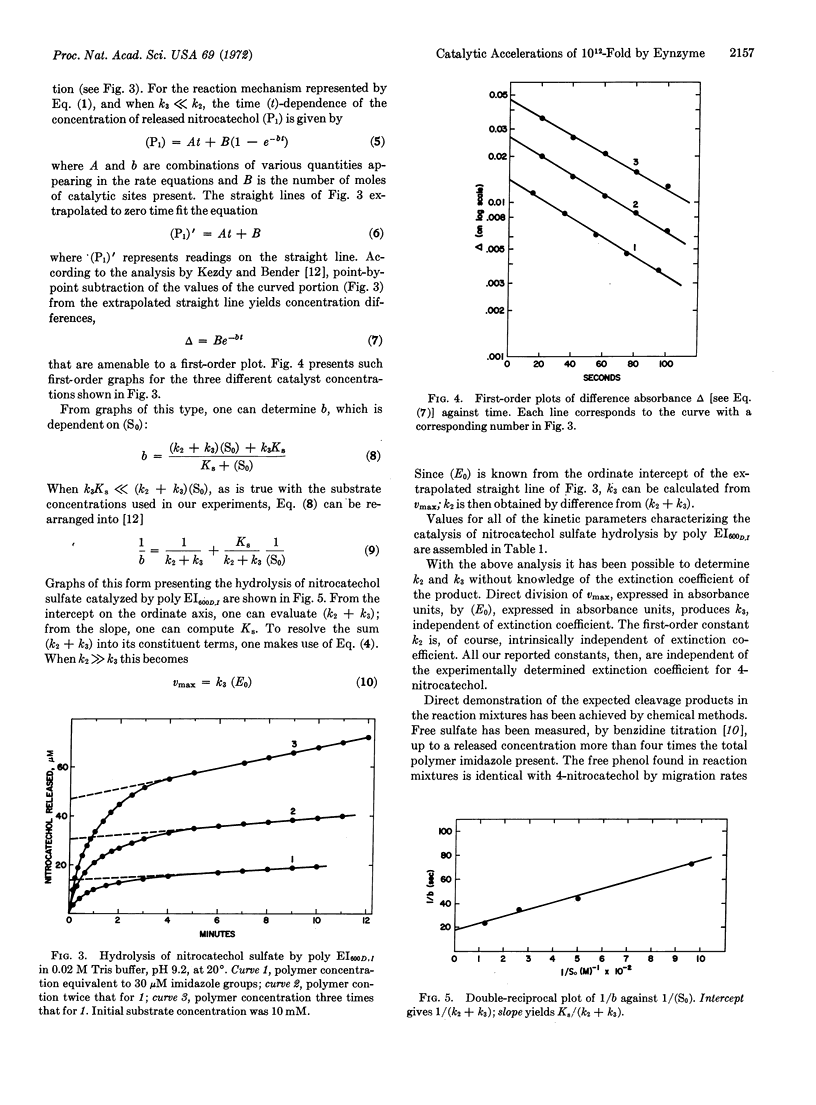
Abstract
A synthetic polymer, polyethyleneimine containing dodecyl (C12H25)-chains for binding sites and imidazole moieties for functional catalytic groups, catalyzes the hydrolysis of phenolic sulfate esters by a two-step mechanism resembling that of the corresponding natural enzyme. Quantitative rate studies give kinetic parameters that show that the polymer (synzyme) accelerates the rate 1012-fold compared to unbound imidazole, and 102-fold compared to a type IIA aryl sulfatase enzyme.
Keywords: Michaelis-Menten kinetics, aryl sulfatase, nitrocatechol sulfate
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BENDER M. L., KEZDY J. MECHANISM OF ACTION OF PROTEOLYTIC ENZYMES. Annu Rev Biochem. 1965;34:49–76. doi: 10.1146/annurev.bi.34.070165.000405. [DOI] [PubMed] [Google Scholar]
- Jerfy A., Roy A. B. The sulphatase of ox liver. XII. The effect of tyrosine and histidine reagents on the activity of sulphatase A. Biochim Biophys Acta. 1969 Mar;175(2):355–364. doi: 10.1016/0005-2795(69)90013-0. [DOI] [PubMed] [Google Scholar]
- KEZDY F. J., BENDER M. L. The kinetics of the alpha-chymotrypsin-catalyzed hydrolysis of p-nitrophenyl acetate. Biochemistry. 1962 Nov;1:1097–1106. doi: 10.1021/bi00912a021. [DOI] [PubMed] [Google Scholar]
- Klotz I. M., Harris J. U. Macromolecule-small molecule interactions. Strong binding by intramolecularly cross-linked polylysine. Biochemistry. 1971 Mar 16;10(6):923–926. doi: 10.1021/bi00782a001. [DOI] [PubMed] [Google Scholar]
- Klotz I. M., Royer G. P., Scarpa I. S. Synthetic derivatives of polyethyleneimine with enzyme-like catalytic activity (synzymes). Proc Natl Acad Sci U S A. 1971 Feb;68(2):263–264. doi: 10.1073/pnas.68.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz I. M., Royer G. P., Sloniewsky A. R. Macromolecule--small molecule interactions. Strong binding and cooperativity in a model synthetic polymer. Biochemistry. 1969 Dec;8(12):4752–4756. doi: 10.1021/bi00840a015. [DOI] [PubMed] [Google Scholar]
- ROBINSON D., SMITH J. N., SPENCER B., WILLIAMS R. T. Studies in detoxication. XXXXIII. A study of the arylsulphatase activity of takadiastase towards some phenolic ethereal sulphates. Biochem J. 1952 May;51(2):202–208. doi: 10.1042/bj0510202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A. B. The type II arylsulphatases of the red kangaroo. Biochim Biophys Acta. 1971 Jan 13;227(1):129–138. doi: 10.1016/0005-2744(71)90174-4. [DOI] [PubMed] [Google Scholar]
- SPENCER B. The ultramicro determination of inorganic sulphte. Biochem J. 1960 Jun;75:435–440. doi: 10.1042/bj0750435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigler P. B., Blow D. M., Matthews B. W., Henderson R. Structure of crystalline -chymotrypsin. II. A preliminary report including a hypothesis for the activation mechanism. J Mol Biol. 1968 Jul 14;35(1):143–164. doi: 10.1016/s0022-2836(68)80043-9. [DOI] [PubMed] [Google Scholar]


