Abstract
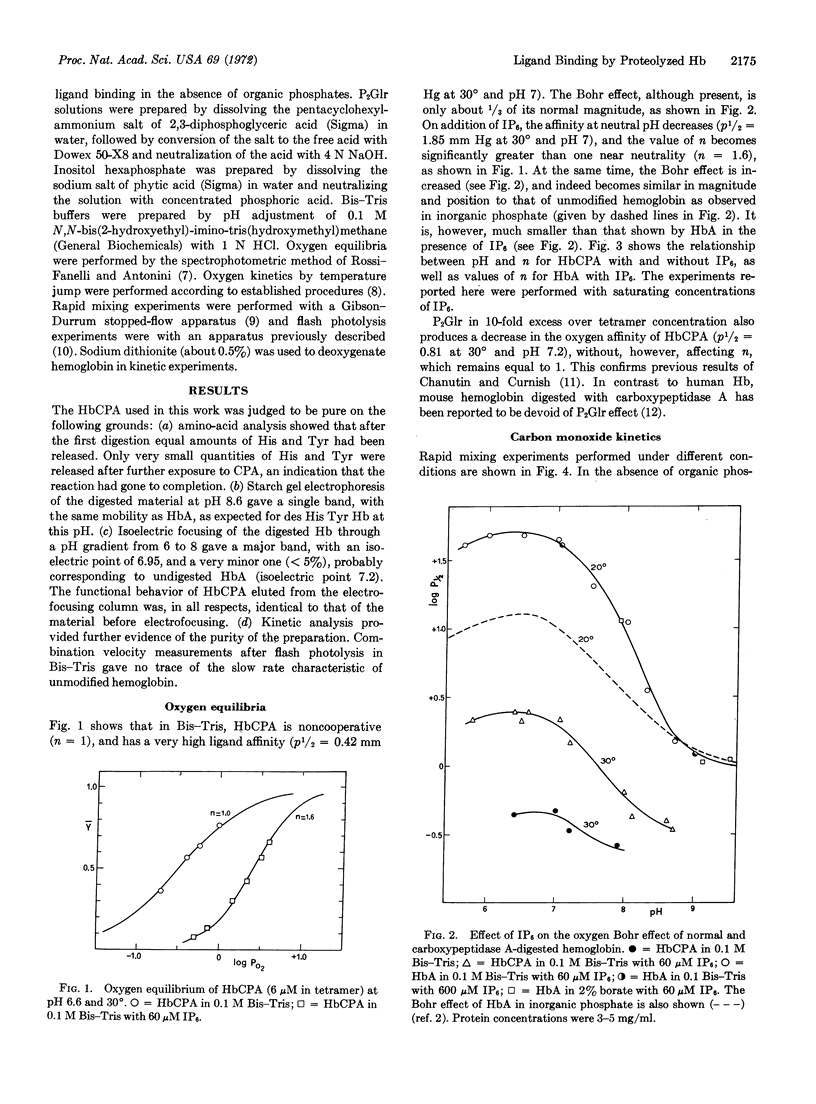
In the absence of organic phosphates human hemoglobin A digested with carboxypeptidase A (des His, Tyr β) has high ligand affinity, a greatly reduced Bohr effect, and no heme-heme interaction. Under these conditions, it shows the simple, homogeneous ligand-binding kinetics characteristic of noncooperative heme proteins in which the high combination velocity for both O2 and CO accounts, to a larger extent, for the increased affinity for both these ligands.
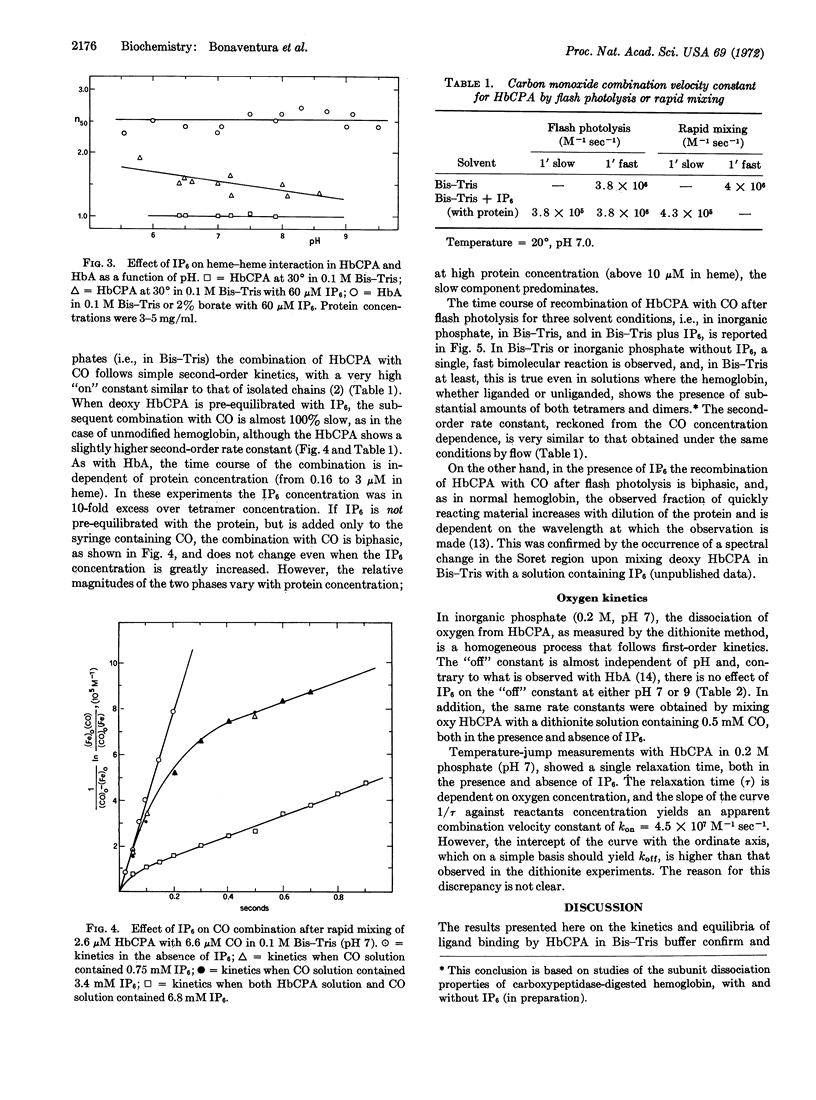
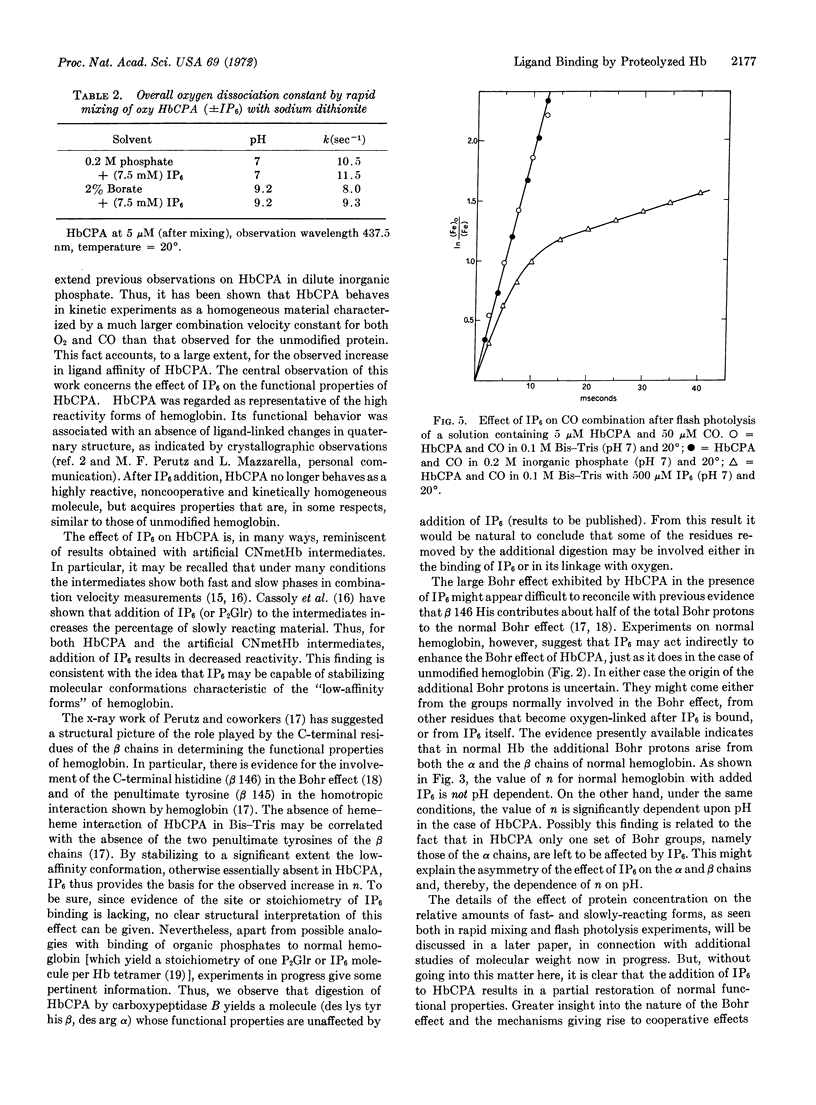
Addition of inositol hexaphosphate dramatically alters the functional properties of this digested hemoglobin. The Bohr effect is greatly increased, and at neutral pH the protein shows significant, though still reduced, heme-heme interaction, together with a 5-fold decrease in affinity. In the presence of saturating amounts of the organic phosphate, the value of n is pH dependent, dropping from 1.9 at pH 5.8 to 1.3 at pH 8.6. After inositol hexaphosphate addition, the combination of the deoxy form of the digested hemoglobin with CO is 10-times slower than that observed in the absence of the inorganic phosphate; also the combination with CO after flash photolysis is biphasic and is similar, in many respects, to that observed for unmodified hemoglobin. Besides these functional changes, addition of inositol hexaphosphate to the modified deoxyhemoglobin results in an increase in the extinction coefficient at 430 nm similar to that observed on mixing the isolated α and β chains of normal hemoglobin. The results are consistent with the idea that inositol hexaphosphate shifts an equilibrium between high- and low-affinity forms of the protein.
Keywords: heme proteins, ligand binding, inositol hexaphosphate, enzyme modifications
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTONINI E., WYMAN J., MORETTI R., ROSSI-FANELLI A. The interaction of bromthymol blue with hemoglobin and its effect on the oxygen equilibrium. Biochim Biophys Acta. 1963 Apr 2;71:124–138. doi: 10.1016/0006-3002(63)90991-0. [DOI] [PubMed] [Google Scholar]
- Antonini E., Anderson N. M., Brunori M. Properties of the product of partial photodissociation of carbon monoxide hemoglobin. J Biol Chem. 1972 Jan 10;247(1):319–321. [PubMed] [Google Scholar]
- BRUNORI M., ANTONINI E., WYMAN J., ZITO R., TAYLOR J. F., ROSSI-FANELLI A. STUDIES ON THE OXIDATION-REDUCTION POTENTIALS OF HEME PROTEINS. II. CARBOXYPEPTIDASE DIGESTS OF HUMAN HEMOGLOBIN. J Biol Chem. 1964 Jul;239:2340–2344. [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. Intracellular organic phosphates as regulators of oxygen release by haemoglobin. Nature. 1969 Feb 15;221(5181):618–622. doi: 10.1038/221618a0. [DOI] [PubMed] [Google Scholar]
- Brunori M., Amiconi G., Antonini E., Wyman J. Artificial intermediates in the reaction of haemoglobin. Functional and conformational properties of the cyanmet intermediates. J Mol Biol. 1970 Apr 28;49(2):461–471. doi: 10.1016/0022-2836(70)90257-3. [DOI] [PubMed] [Google Scholar]
- Brunori M., Bonaventura J., Bonaventura C., Antonini E., Wyman J. Carbon monoxide binding by hemoglobin and myoglobin under photodissociating conditions. Proc Natl Acad Sci U S A. 1972 Apr;69(4):868–871. doi: 10.1073/pnas.69.4.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunori M., Talbot B., Colosimo A., Antonini E., Wyman J. Studies on the reaction of isocyanides with haemproteins. II. Binding to normal and modified human haemoglobins. J Mol Biol. 1972 Apr 14;65(3):423–434. doi: 10.1016/0022-2836(72)90199-4. [DOI] [PubMed] [Google Scholar]
- Cassoly R., Gibson Q. H., Ogawa S., Shulman R. G. Effects of phosphate upon CO binding kinetics and NMR spectra of hemoglobin valency hybrids. Biochem Biophys Res Commun. 1971 Sep;44(5):1015–1021. doi: 10.1016/s0006-291x(71)80187-0. [DOI] [PubMed] [Google Scholar]
- Chanutin A., Curnish R. R. Effect of organic phosphates on the oxygen equilibrium of carboxypeptidase digests of human hemoglobin. Arch Biochem Biophys. 1968 Jan;123(1):163–165. doi: 10.1016/0003-9861(68)90114-8. [DOI] [PubMed] [Google Scholar]
- Gibson Q. H., Milnes L. Apparatus for rapid and sensitive spectrophotometry. Biochem J. 1964 Apr;91(1):161–171. doi: 10.1042/bj0910161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray R. D., Gibson Q. H. The effect of inositol hexaphosphate on the kinetics of CO and O 2 binding by human hemoglobin. J Biol Chem. 1971 Dec 10;246(23):7168–7174. [PubMed] [Google Scholar]
- Hänisch G., Engel J., Brunori M., Fasold H. Ligand induced conformational changes in various normal and modified hemoglobins as indicated by changes in optical rotatory dispersion. Eur J Biochem. 1969 Jun;9(3):335–342. doi: 10.1111/j.1432-1033.1969.tb00613.x. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Wootton J. F. Inhibition of Bohr effect after removal of C-terminal histidines from haemoglobin beta-chains. Nature. 1970 Nov 21;228(5273):766–767. doi: 10.1038/228766a0. [DOI] [PubMed] [Google Scholar]
- Perutz M. F. Stereochemistry of cooperative effects in haemoglobin. Nature. 1970 Nov 21;228(5273):726–739. doi: 10.1038/228726a0. [DOI] [PubMed] [Google Scholar]
- ROSSI-FANELLI A., ANTONINI E. Studies on the oxygen and carbon monoxide equilibria of human myoglobin. Arch Biochem Biophys. 1958 Oct;77(2):478–492. doi: 10.1016/0003-9861(58)90094-8. [DOI] [PubMed] [Google Scholar]
- Tomita S., Riggs A. Studies of the interaction of 2,3-diphosphoglycerate and carbon dioxide with hemoglobins from mouse, man, and elephant. J Biol Chem. 1971 Feb 10;246(3):547–554. [PubMed] [Google Scholar]


