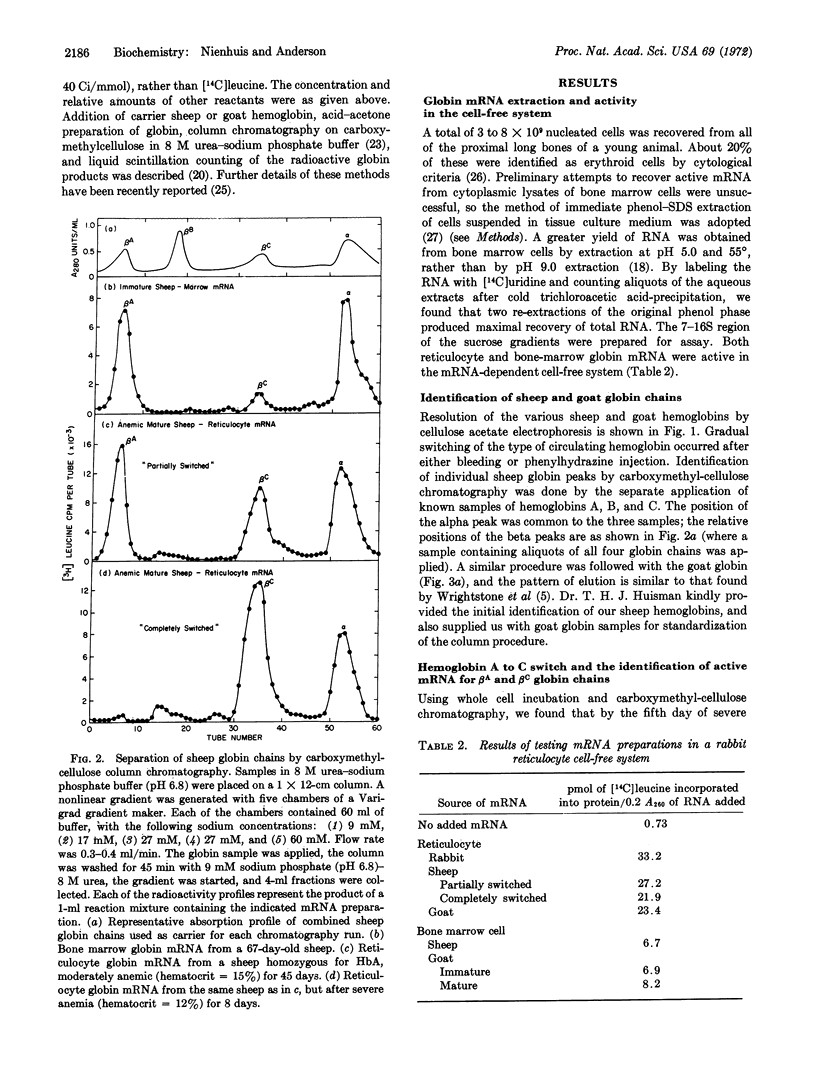
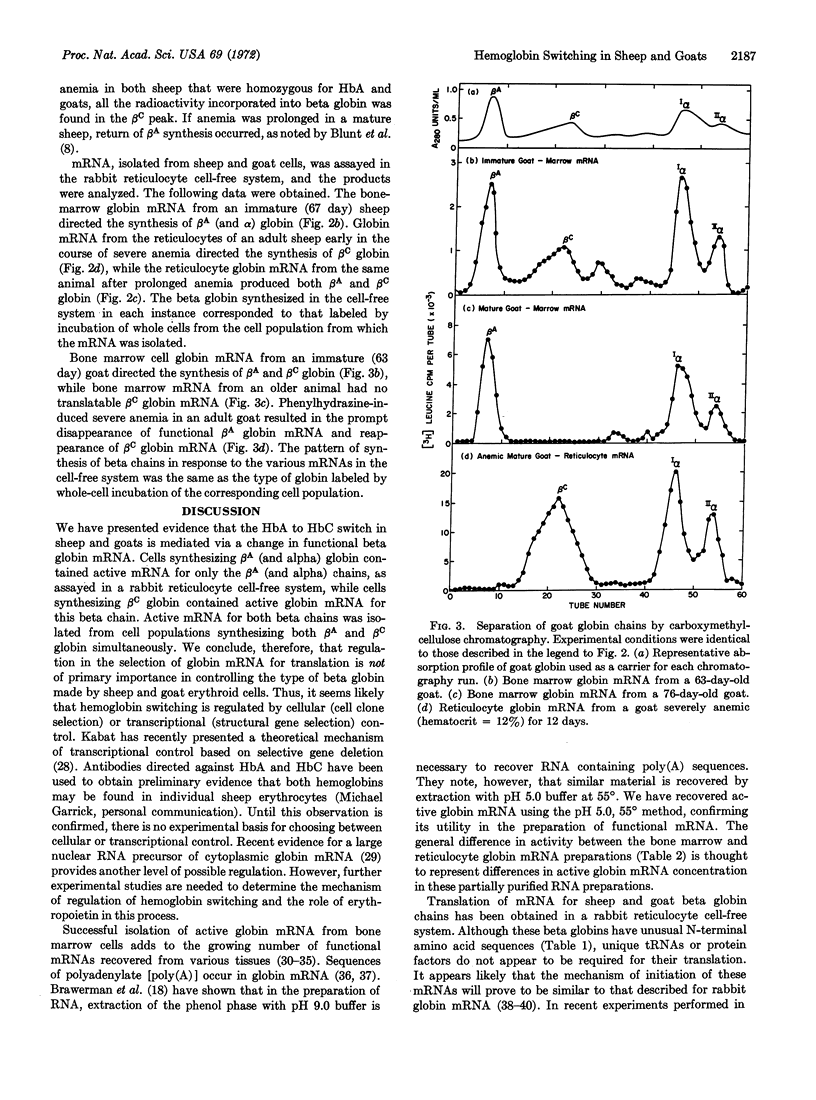
Abstract
Anemia causes a change in the type of circulating hemoglobin in goats and certain sheep: HbA (α2β2A) is replaced by HbC (α2β2C). We have isolated globin mRNA from erythroid cells of anemic and nonanemic animals to investigate the mechanism whereby anemia causes this switch. To study several stages in transition from βA to βC synthesis, active globin mRNA was isolated from bone marrow cells, as well as from reticulocytes. By assaying these globin mRNAs in a rabbit reticulocyte cell-free system, we have demonstrated that the switch from βA to βC globin synthesis is mediated via a change in functional globin mRNA.
Keywords: cell culture, β chains, mRNA isolation, anemia, erythroid
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blunt M. H., Huisman T. H., Lewis J. P. The production of haemoglobin C in adult sheep and goats. Aust J Exp Biol Med Sci. 1969 Oct;47(5):601–611. doi: 10.1038/icb.1969.154. [DOI] [PubMed] [Google Scholar]
- Boyer S. H., Hathaway P., Pascasio F., Orton C., Bordley J., Naughton M. A. Hemoglobins in sheep: multiple differences in amino acid sequences of three beta-chains and possible origins. Science. 1966 Sep 23;153(3743):1539–1543. doi: 10.1126/science.153.3743.1539. [DOI] [PubMed] [Google Scholar]
- Brawerman G., Mendecki J., Lee S. Y. A procedure for the isolation of mammalian messenger ribonucleic acid. Biochemistry. 1972 Feb 15;11(4):637–641. doi: 10.1021/bi00754a027. [DOI] [PubMed] [Google Scholar]
- Clegg J. B., Naughton M. A., Weatherball D. J. Abnormal human haemoglobins. Separation and characterization of the alpha and beta chains by chromatography, and the determination of two new variants, hb Chesapeak and hb J (Bangkok). J Mol Biol. 1966 Aug;19(1):91–108. doi: 10.1016/s0022-2836(66)80052-9. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Anderson W. F. Initiation of hemoglobin synthesis: comparison of model reactions that use artificial templates with those using natural messenger RNA. Proc Natl Acad Sci U S A. 1972 Mar;69(3):706–711. doi: 10.1073/pnas.69.3.706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Evans M. J., Lingrel J. B. Hemoglobin messenger ribonucleic acid. Synthesis of 9S and ribosomal ribonucleic acid during erythroid cell development. Biochemistry. 1969 Jul;8(7):3000–3005. doi: 10.1021/bi00835a048. [DOI] [PubMed] [Google Scholar]
- Fowler J. H., McCulloch E. A., Till J. E., Siminovitch L. An improved method for radioautography of erythropoietic cells labeled with Fe 55 or Fe 59. J Lab Clin Med. 1966 Sep;68(3):523–530. [PubMed] [Google Scholar]
- Gabuzda T. G., Schuman M. A., Silver R. K., Lewis H. B. Erythropoietic kinetics in sheep studied by means of induced changes in hemoglobin phenotype. J Clin Invest. 1968 Aug;47(8):1895–1904. doi: 10.1172/JCI105880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert J. M., Anderson W. F. Cell-free hemoglobin synthesis. II. Characteristics of the transfer ribonucleic acid-dependent assay system. J Biol Chem. 1970 May 10;245(9):2342–2349. [PubMed] [Google Scholar]
- Gross M., Goldwasser E. On the mechanism of erythropoietin-induced differentiation. V. Characterization of the ribonucleic acid formed as a result of erythropoietin action. Biochemistry. 1969 May;8(5):1795–1805. doi: 10.1021/bi00833a003. [DOI] [PubMed] [Google Scholar]
- HUISMAN T. H., REYNOLDS C. A., DOZY A. M., WILSON J. B. THE STRUCTURE OF SHEEP HEMOGLOBINS. THE AMINO ACID COMPOSITIONS OF THE ALPHA AND BETA CHAINS OF THE HEMOGLOBINS, A, B, AND C. J Biol Chem. 1965 Jun;240:2455–2460. [PubMed] [Google Scholar]
- Heywood S. M. Specificity of mRNA binding factor in eukaryotes. Proc Natl Acad Sci U S A. 1970 Dec;67(4):1782–1788. doi: 10.1073/pnas.67.4.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huisman T. H., Lewis J. P., Blunt M. H., Adams H. R., Miller A., Dozy A. M., Boyd E. M. Hemoglobin C in newborn sheep and goats: a possible explanation for its function and biosynthesis. Pediatr Res. 1969 May;3(3):189–198. doi: 10.1203/00006450-196905000-00001. [DOI] [PubMed] [Google Scholar]
- Kabat D. Gene selection in hemoglobin and in antibody-synthesizing cells. Science. 1972 Jan 14;175(4018):134–140. doi: 10.1126/science.175.4018.134. [DOI] [PubMed] [Google Scholar]
- Lim L., Canellakis E. S. Adenine-rich polymer associated with rabbit reticulocyte messenger RNA. Nature. 1970 Aug 15;227(5259):710–712. doi: 10.1038/227710a0. [DOI] [PubMed] [Google Scholar]
- Lockard R. E., Lingrel J. B. Identification of mouse haemoglobin messenger RNA. Nature. 1971 Oct 13;233(5320):204–206. [PubMed] [Google Scholar]
- Melli M., Pemberton R. E. New method of studying the precursor-product relationship between high molecular weight RNA and messenger RNA. Nat New Biol. 1972 Apr 12;236(67):172–174. doi: 10.1038/newbio236172a0. [DOI] [PubMed] [Google Scholar]
- Nienhuis A. W., Anderson W. F. Isolation and translation of hemoglobin messenger RNA from thalassemia, sickle cell anemia, and normal human reticulocytes. J Clin Invest. 1971 Nov;50(11):2458–2460. doi: 10.1172/JCI106745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhuis A. W., Laycock D. G., Anderson W. F. Translation of rabbit haemoglobin messenger RNA by thalassaemic and non-thalassaemic ribosomes. Nat New Biol. 1971 Jun 16;231(24):205–208. doi: 10.1038/newbio231205a0. [DOI] [PubMed] [Google Scholar]
- Parish J. H., Kirby K. S. Reagents which reduce interactions between ribosomal RNA and rapidly labelled RNA from rat liver. Biochim Biophys Acta. 1966 Dec 21;129(3):554–562. doi: 10.1016/0005-2787(66)90070-0. [DOI] [PubMed] [Google Scholar]
- Pemberton R. E., Baglioni C. Duck hemoglobin messenger RNA contains a polynucleotide sequence rich in adenylic acid. J Mol Biol. 1972 Apr 14;65(3):531–535. doi: 10.1016/0022-2836(72)90207-0. [DOI] [PubMed] [Google Scholar]
- Pemberton R. E., Housman D., Lodish H. F., Baglioni C. Isolation of duck haemoglobin messenger RNA and its translation by rabbit reticulocyte cell free system. Nat New Biol. 1972 Jan 26;235(56):99–102. doi: 10.1038/newbio235099a0. [DOI] [PubMed] [Google Scholar]
- Prichard P. M., Gilbert J. M., Shafritz D. A., Anderson W. F. Factors for the initiation of haemoglobin synthesis by rabbit reticulocyte ribosomes. Nature. 1970 May 9;226(5245):511–514. doi: 10.1038/226511a0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld G. C., Comstock J. P., Means A. R., O'Malley B. W. Estrogen-induced synthesis of ovalbumin messenger RNA and its translation in a cell-free system. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1695–1703. doi: 10.1016/0006-291x(72)90805-4. [DOI] [PubMed] [Google Scholar]
- SCHERRER K., DARNELL J. E. Sedimentation characteristics of rapidly labelled RNA from HeLa cells. Biochem Biophys Res Commun. 1962 Jun 4;7:486–490. doi: 10.1016/0006-291x(62)90341-8. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Anderson W. F. Factor dependent binding of methionyl-tRNAs to reticulocyte ribosomes. Nature. 1970 Aug 29;227(5261):918–920. doi: 10.1038/227918a0. [DOI] [PubMed] [Google Scholar]
- Shafritz D. A., Anderson W. F. Isolation and partial characterization of reticulocyte factors M1 and M2. J Biol Chem. 1970 Nov 10;245(21):5553–5559. [PubMed] [Google Scholar]
- Stavnezer J., Huang R. C. Synthesis of a mouse immunoglobulin light chain in a rabbit reticulocyte cell-free system. Nat New Biol. 1971 Apr 7;230(14):172–176. doi: 10.1038/newbio230172a0. [DOI] [PubMed] [Google Scholar]
- Thurmon T. F., Boyer S. H., Crosby E. F., Shepard M. K., Noyes A. N., Stohlman F., Jr Hemoglobin switching in nonanemic sheep. 3. Evidence for presumptive identity between the A--C factor and erythropoietin. Blood. 1970 Nov;36(5):598–606. [PubMed] [Google Scholar]
- Wrightstone R. N., Wilson J. B., Miller A., Huisman T. H. The structure of goat hemoglobins. IV. A third beta chain variant (betaE) with three apparent amino acid substitutions. Arch Biochem Biophys. 1970 Jun;138(2):451–456. doi: 10.1016/0003-9861(70)90368-1. [DOI] [PubMed] [Google Scholar]