Abstract
Reproductive experience (i.e. parturition and lactation) leads to persistent alterations in anxietylike behaviour that are influenced by the oestrous cycle. We recently found that repeated administration of the selective oestrogen receptors (ER)α agonist propyl-pyrazole triol (PPT) results in anxiolytic-like behaviours on the elevated plus maze (EPM) in primiparous (but not nulliparous) female rats. The present study examined the effects of the acute administration of PPT on EPM behaviour in primiparous and aged-matched, nulliparous female rats. In addition, corticosterone secretion, corticotrophin-releasing hormone (CRH) gene expression and expression of the immediate early gene product Fos in the paraventricular nucleus (PVN) and amygdala were measured either after EPM testing or in home cage controls. Acute PPT administration significantly modified EPM behaviour as a function of reproductive experience, with nulliparous females tending toward increased anxiety-like behaviours and primiparous females tending toward decreased anxiety-like behaviours. In home cage controls, PPT increased corticosterone secretion in all females; however, both vehicle- and PPT-treated, primiparous females had reduced corticosterone levels compared to their nulliparous counterparts. Significant effects of PPT on CRH mRNA within the PVN were observed after the administration of PPT but only in primiparous females tested on the EPM. PPT also increased Fos expression within the PVN of EPM-exposed females; however, both vehicle- and PPT-treated primiparous females had reduced Fos expression compared to nulliparous females. In the amygdala, PPT increased Fos immunore-activity in the central but not the medial or basolateral amygdala, although these effects were only observed in home cage females. Additionally, both vehicle- and PPT-treated home cage, primiparous females had increased Fos in the central nucleus of the amygdala compared to nullip-arous controls. Overall, these data demonstrate that reproductive experience alters the behavioural response to acute ERα activation. Moreover, the findings suggest that central regulation of the hypothalamic-adrenal-pituitary axis is modified as a consequence of reproductive experience.
Keywords: Corticotrophin-releasing hormone, anxiety, stress, Fos, PVN, amygdala
The physiological changes that accompany pregnancy, parturition and lactation/mothering result in long-term changes in neural and endocrine function (1–14). These persistent effects are observed in a number of species, including women, and several findings suggest a shift in the activation and/or expression of oestrogen receptors in parous females (15–19). Additionally, reproductive experience can alter both cognitive function and emotionality, with studies demonstrating improved learning and memory, as well as decreased anxiety as a function of parity (14,20–25). Given the influence of oestrogens on both cognition and affect (26–31), parity-induced changes in oestrogenic activity represents one possible mechanism underlying the long-term effects of reproductive experience on these parameters.
Oestrogen mediates its effects via the activation of two distinct receptor isoforms: oestrogen receptor α and β (ERα and ERβ, respectively). A number of studies in rodents suggest that ERβ may have anxiolytic effects (32–35), whereas ERα may have little impact on anxiety-like behaviour, or may potentially be anxiogenic (35,36). These studies, however, have uniformly been conducted in virgin females. By contrast, we recently observed significant anxiolysis in response to the ERα agonist, propyl-pyrazole triol (PPT) that was unique to primiparous females (37). Because PPT in that study was administered repeatedly (i.e. once daily for 3 days and then 30 min before testing), it is unclear whether the effects of PPT on anxietylike behaviour were the result of genomic effects induced over a number of days or the more rapid effects of the agonist. Indeed, several studies have demonstrated rapid effects of ERα activation mediated by membrane bound ERα receptors that activate signal transduction pathways resulting in phosphorylation (38–40). Thus, the differential effects of PPT on anxiety-like behaviour in reproductively experience females may be mediated by the acute, nonge-nomic effects of PPT.
In addition to differences in anxiety-like behaviour, primiparous females also demonstrated a more robust increase in corticotrophin-releasing hormone (CRH) mRNA within the paraventricular nucleus (PVN) after the administration of PPT (37). Because all of the animals were sacrificed after testing on the elevated plus maze (EPM), a known stressor, it is unclear whether a similar shift in CRH mRNA after PPT administration would be observed under basal conditions, or whether this increase represents an interaction between stress and reproductive experience. Interestingly, no difference in corticosterone secretion as a function of reproductive experience was observed, suggesting that increased CRH mRNA in the PVN did not translate into greater activation of the hypothalamic-pituitary-adrenal (HPA) axis.
CRH serves as a critical regulator of behavioural, endocrine, autonomic and immune responses to changes in both the internal and external milieu. Many of these effects are a result of the activity of CRH neurones in the PVN and their regulation of the HPA axis. However, CRH neurones originating in the PVN also project to a number of brain regions with effects mediated by activation of CRH receptor 1 and CRH receptor 2. In addition, a significant number of CRH neurones are localised in the central nucleus of the amygdala (CeA). Indeed, CRH activity in the CeA plays a significant role in modulating anxiety-like behaviour (41,42). Oestrogens can regulate CRH transcription via direct effects on gene transcription, as well as via indirect effects on CRH neuromodulators (43). Moreover, although ERα stimulates the transcription of CRH, ERβ has been shown to attenuate the effects of stress on CRH transcription. In the CeA, oestrogens significantly increase CRH mRNA, an effect associated with increased fear behaviour (44). Thus, the expression and/or functional response of ER subtypes in select neuronal populations influences the effect of oestrogens on CRH-mediated behaviour, such as anxiety. Furthermore, the response of CRH neurones in both the PVN and CeA to oestrogens is likely to be dependent upon a number of additional variables, including the physiological state of the animal, as well as their previous experiences.
The present study aimed to determine the effect of previous reproductive experience on the modulation of anxiety-like behaviour by the ERα agonist PPT. Specifically, the effect of acute PPT administration (1 mg/kg) on anxiety-like behaviour as measured on the EPM was determined in primiparous females and age-matched, nulliparous controls. Additional subjects were examined for their response to PPT under either a basal, nonstressed condition or as measured after EPM testing (i.e. stressed condition). Physiological parameters measured include corticosterone secretion, CRH transcription in the PVN and CeA, as well as Fos activation in the PVN and amygdala (medial, MeA; basolateral, BLA; central, CeA). The findings revealed significant effects of reproductive experience on anxiety-like behaviour after acute PPT, as well as differences in both basal and stress-induced Fos activation and CRH gene expression.
Materials and methods
Subjects and mating
Female Sprague–Dawley rats (201–225 g) were purchased from Charles River Laboratories (Kingston, NY, USA) and housed in our animal facility. One week after arrival, half of the females were mated with males from our colony. On post-partum day 1 (day after parturition), litters were culled to 10 pups (five males and five females). All litters were weaned on post-partum day 21. All other females were not mated and served as age-matched, nulliparous controls. For all experiments, animals were maintained under a 14 : 10 h light/dark cycle at 21–25° C with food and water available ad lib. Animals were maintained in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals and the methods were approved by the Institutional Animals Care and Use Committee of Tufts University, Cummings School of Veterinary Medicine.
Experimental procedures
Experiment 1: Acute effects of the selective ERα agonist PPT on anxiety-like behaviour in age-matched, nulliparous and primiparous rats
Beginning 5–6 weeks after primiparous female litters were weaned, primiparous and age-matched, nulliparous subjects were ovariectomised under isoflurane anaesthesia. One week later, females were tested on the EPM 30 min after a single injection of 4,4’,4”-(propyl-[(1)H]-pyrazole-1,3,5-triyl) trisphenol (PPT; 1.0 mg/kg; Tocris Bioscience, Bristol, UK) or vehicle (dimethyl sulphoxide; Sigma-Aldrich, St Louis, MO, USA). All injections were performed s.c. Ten minutes after being placed on the EPM (i.e. 40 min post-injection), all females were sacrificed by brief exposure to CO2, followed by rapid decapitation. Trunk blood was collected into heparinised tubes for the measurement of corticosterone. Brains were collected as described below and relative expression of CRH mRNA in the PVN and CeA were determined. Additional subjects were injected with either PPT or vehicle but were not tested and served as home cage controls for the measurement of corticosterone and CRH mRNA. All home cage control subjects were sacrificed 40 min post-injection. Briefly, animals were transported in their home cage to a procedure room, exposed to CO2 for < 30 s and rapidly decapitated to match the procedures of EPM-tested subjects. Time between transport and sacrifice was < 3 min to minimise the response of the HPA axis.
Experiment 2: Acute effects of the selective ERα agonist PPT on c-Fos expression in the PVN and amygdala in age-matched, nulliparous and primiparous rats
Procedures were similar to those described for Experiment 1, with half of the females tested on the EPM, whereas the other half were not tested and served as home cage controls; however, in this experiment, all females were perfused 2.5 h after injection.
EPM testing and corticosterone radioimmunoassay
All EPM testing was conducted in a quiet behavioural testing room. At the time of testing, animals were placed into the centre of a fully automated EPM (Hamilton-Kinder, Poway, CA, USA). Each test lasted 5 min, during which animals were able to explore the maze. Ethanol (70%) was used to clean the apparatus between individual test sessions. Measures included time (s), entries and distance travelled (cm) in different maze zones which included both the open and closed arms, as well as the intersection (i.e. the central platform or choice point). Increased exploration of the open arms was interpreted as reduced anxiety-like behaviour. Corticosterone secretion was determined using a standard radioimmunoassay kit (Coat-a-Count; Siemens AG, Munich, Germany).
Brain collection and processing
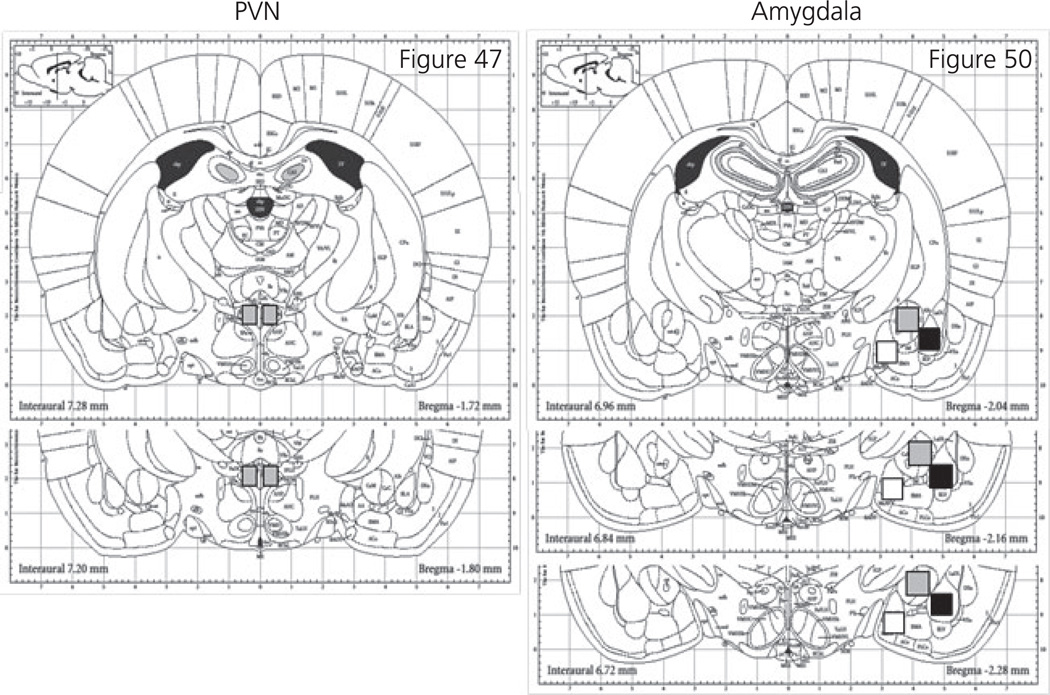
For Experiment 1, brains were removed and rapidly frozen in −20° C meth-ylbutane and stored at −80° C. Micropunches (0.5 × 0.5 mm) from the PVN and amygdala (MeA, BLA, CeA) were collected. Total RNA was extracted using RNAeasy (Qiagen, Valancia, CA, USA). Samples were then processed for cDNA and a quantitative polymerase chain reaction was used to determine the relative expression of CRH mRNA. For all samples, GAPDH was used as the housekeeping gene and relative expression was determined utilising the ΔΔCT method. Taqman® probes for both CRH and GAPDH were purchased from Applied Biosystems (Foster City, CA, USA). For Experiment 2, rats were deeply anaesthetised with ketamine/xylazine (25 mg/5 mg) and perfused with 4% paraformaldehyde. Brains were cryoprotected in 30% sucrose and subsequently frozen and stored at −80° C until immunohistochemical (IHC) processing. For cFos IHC, 30-µm coronal sections through the PVN and CeA were collected using a cryostat. Fos immunoreactivity (-IR) was measured using rabbit anti-c-Fos (dilution 1 : 2000; Santa Cruz Biotechnology, TX, USA) and the rabbit IgG vectastain elite avidin biotin complex kit (Vector Laboratories, Inc., Burlingame, CA, USA). Sections were mounted, dried overnight and coverslipped. Slides were randomised and coded, with the number of Fos-IR cells counted manually at × 200 by an investigator who was blind to the subject’s condition. The regions counted are shown in Fig. 1.
Fig. 1.
Schematic of brain regions quantified for Fos-immunoreactivity based on Paxinos and Watson [45]. Sections were quantified from the paraventricular nucleus (PVN; grey box), medial amygdala (white box), basolateral amygdala (black box) and central amygdala (grey box).
Statistical analysis
For EPM data, a three-way repeated measures ANOVA was conducted with maze location (open arm, central platform or closed arm); reproductive experience (nulliparous or primiparous) and drug (PPT or Vehicle) as factors. For all other data, a three-way ANOVA was conducted with testing condition (EPM or home cage), reproductive experience (nulliparous or primiparous) and drug (PPT or vehicle) as factors. Post-hoc significance was examined using Tukey’s test. P < 0.05 was considered statiatically significant.
Results
Experiment 1: EPM behaviour
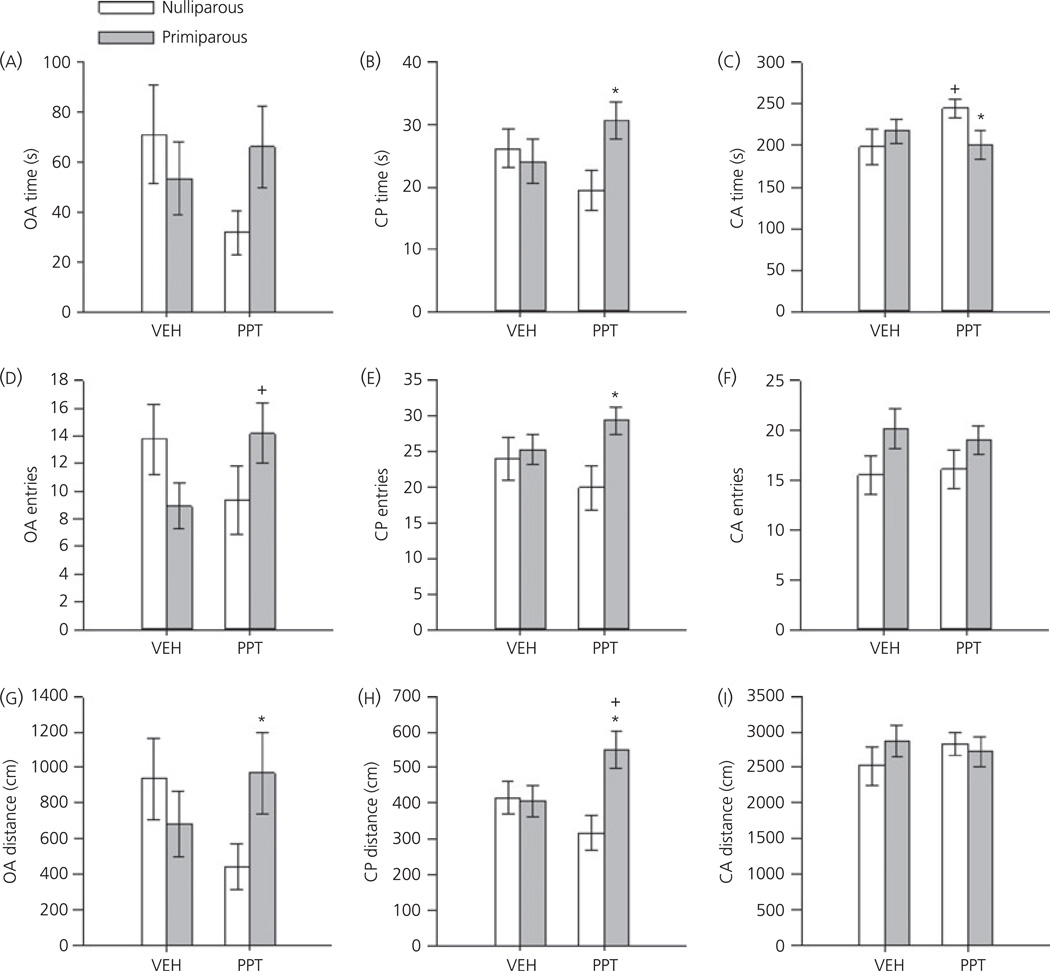
Significant differences in the acute effects of PPT on EPM behaviours were observed as a function of reproductive experience. Expected main effects of maze location were revealed for all three dependent measures (F2,94 = 168.8, 69.3, 194.1; all P < 0.001 for time, entries and distance, respectively). Additionally, significant three-way interactions between location × reproductive experience × drug were observed for each dependent measure (F2,94 = 3.58, 3.88, 2.99; all P ≤ 0.05 for time, entries and distance, respectively). As shown in Fig. 2, these effects were largely a result of the opposing effects of PPT on EPM behaviours in nulliparous and primiparous females. Overall, PPT-treated nulliparous females tended to decrease the time spent (P = 0.06) and distance travelled (P = 0.06) on the open arm compared to vehicle-treated controls. These females also significantly increased the time spent in the closed arms (P = 0.05) compared to vehicle-treated controls. By contrast, PPT-treated primiparous increased their entries into the open arm (P = 0.04) compared to vehicle-treated primiparous females. When compared with PPT-treated nulliparous females, PPT-treated primiparous animals spent significantly less time in the closed arms (P = 0.03), travelled greater distances on the open arm (P = 0.05) and tended to spend more time in the open arm (P = 0.07). In addition, PPT-treated primipa-rous females spent significantly more time (P = 0.02), made more entries (P = 0.02) and travelled greater distances (P < 0.01) at the choice point (i.e. central platform) compared to PPT-treated, nulliparous females. Of note, neither entries, nor distance travelled in the closed arms were altered by reproductive experience or PPT, indicating that the change in exploratory behaviour on the open arms and central platform was not the result of a general increase in activity.
Fig. 2.
Mean ± SEM percentage time spent on the open arm (OA; a, d, g), central platform (CP; b, e, h) and closed arm (CA; c, f, i) of the elevated plus maze in ovariectomised, nulliparous and age-matched, primiparous females after administration of either vehicle (VEH) or the oestrogen receptor α agonist propyl-pyrazole triol (PPT) (1 mg/kg). *P < 0.05 compared to PPT-treated nulliparous females. +P < 0.05 compared to vehicle-treated animals within reproductive experience condition. N = 12–14 per group.
Corticosterone
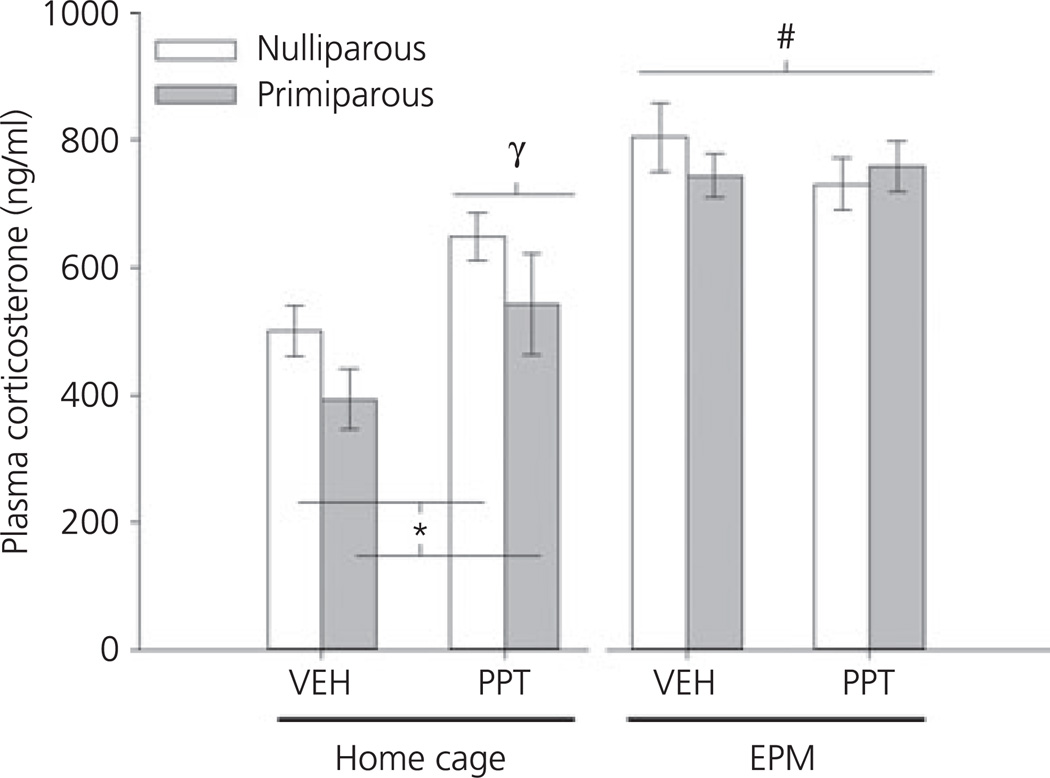
As expected, there was a significant main effect of EPM testing on corticosterone secretion (F1,92 = 33.01, P < 0.001), with levels significantly increased 10 min after testing in all groups (Fig. 3). There was also a main effect of reproductive experience (F1,92 = 4.11, P < 0.05), which was the result of the significantly lower cortico-sterone secretion observed in home cage, primiparous females regardless of their drug treatment (P = 0.05). A main effect of drug (F1,92 = 3.88, P = 0.05) and a drug × testing interaction was also observed (F1,92 = 7.52, P < 0.01). These effects were a result of the significant increase in corticosterone observed in home cage, PPT-administered females, regardless of reproductive experience. Similar effects were not observed after EPM testing.
Fig. 3.
Mean ± SEM corticosterone secretion (ng/ml) in ovariectomised, nulliparous and age-matched, primiparous females administered vehicle (VEH) or propyl-pyrazole triol (PPT). Home cage females were sacrificed 40 min post-injection. Elevated plus maze (EPM) tested females were sacrificed 5 min after completing EPM testing (i.e. 40 min post-injection and 10 min after being placed on the EPM). #P < 0.01 main effect of EPM testing; *P < 0.05 main effect of reproductive experience; γP < 0.01 main effect of drug. N = 12–13 per group.
CRH gene expression in PVN and CeA
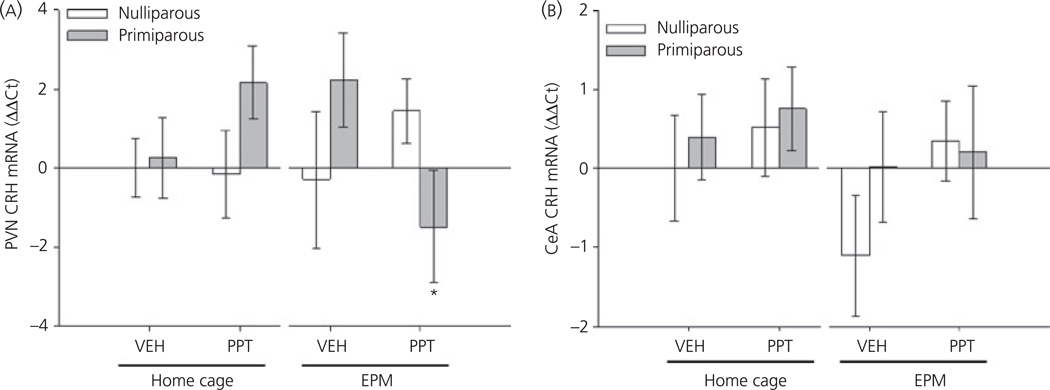
A significant three-way interaction between testing condition, reproductive experience and drug was observed with regard to CRH mRNA expression in the PVN (F1,48 = 5.63, P < 0.05). As shown in Fig. 4(A), PPT-treated, primiparous females tested on the EPM had decreased CRH mRNA expression compared to home cage, PPT-treated, primiparous females (P < 0.05) and tended to have reduced expression compared to vehicle-treated, primiparous (P = 0.06) and PPT-treated, nulliparous females (P = 0.07) after testing on the EPM. No significant effects of testing, reproductive experience or drug on CRH mRNA expression were observed in the CeA (all P > 0.2; Fig. 4B).
Fig. 4.
Relative expression of corticotrophin-releasing hormone (CRH) mRNA in the paraventricular nucleus (PVN) (A) and central amygdala (CeA) (B) mRNA of ovariectomised, nulliparous and age-matched, primiparous females administered vehicle (VEH) or propyl-pyrazole triol (PPT). Home cage females were sacrificed 40 min post-injection. Elevated plus maze (EPM) tested females were sacrificed 10 min after being placed on the EPM. *P < 0.05 compared to home cage, primiparous PPT-treated females. N = 5–7 per group.
Experiment 2: Fos immunoreactivity in the PVN and Amygdala
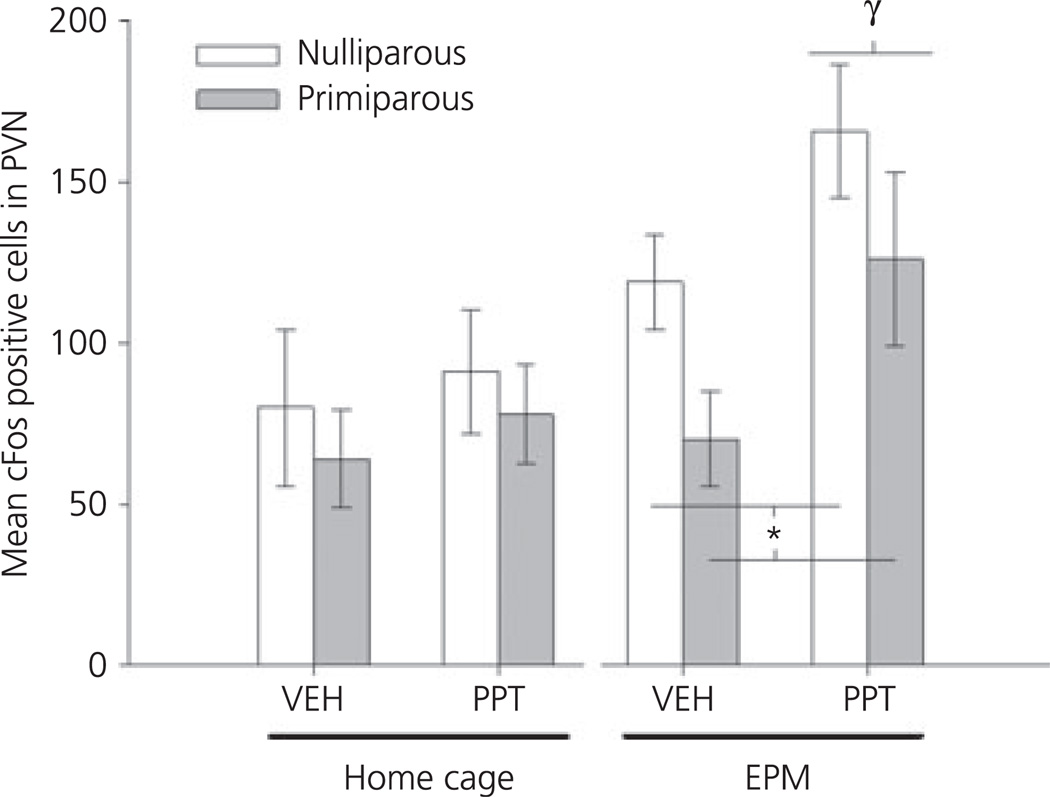
In the PVN, there was a significant main effect of both testing (F1,40 = 7.8, P < 0.01) and drug (F1,40 = 4.7, P < 0.05), as well as a trend toward a main effect of reproductive experience (F1,40 = 3.03, P = 0.09). As shown in Fig. 5, animals tested on the EPM had an increased number of Fos-IR positive cells in the PVN compared to home cage controls. When home cage and EPM-tested animals were analysed further, there were no significant group differences observed in home cage animals; however, significant main effects of both parity (F1,19 = 4.9, P < 0.05) and drug (F1,19 = 6.61, P < 0.05) were observed in females tested on the EPM. These effects were the result of increased Fos-IR after PPT-administration, regardless of reproductive experience, coupled with a reduced Fos-IR observed in both vehicle- and PPT-treated, primiparous females.
Fig. 5.
Mean ± SEM number of Fos-immunoreactive positive cells in the paraventricular nucleus (PVN) of ovariectomised, nulliparous and age-matched, primiparous females administered vehicle (VEH) or propyl-pyrazole triol (PPT). Females either remained in their home cage (top) or were tested on the elevated plus maze (EPM) (bottom). *P < 0.01 main effect of reproductive experience. γP < 0.05 main effect of drug. N = 5–7 per group.
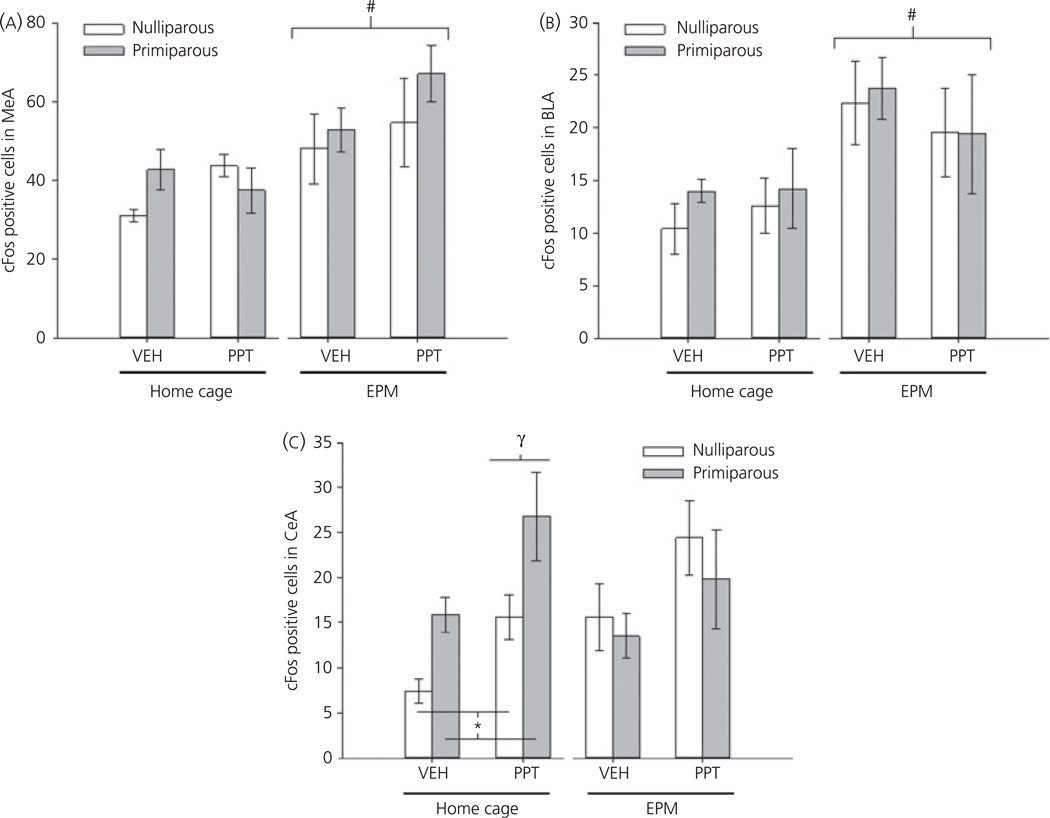
Initial analyses demonstrated significant regional differences in Fos-IR (main effect of brain region, F2,64 = 121, P < 0.001), as well as a region by testing interaction (F2,64 = 5.51, P < 0.01). As shown in Fig. 6, the highest number of Fos-positive cells was observed in the MeA. Moreover, both the MeA and BLA demonstrated a significant increase in Fos-IR after exposure to the EPM (both P < 0.01). Effects in the MeA and BLA were not further influenced by either reproductive experience or drug. By contrast, both a main effect of drug (F1,32 =11.45, P < 0.01) and a reproductive experience by testing interaction (F1,32 = 6.82, P = 0.01) were observed in the CeA. Subsequent analyses indicate that no significant effects of either reproductive experience or drug were observed in females tested on the EPM. There were, however, significant main effects of reproductive experience (F1,20 = 11.02, P < 0.01) and drug (F1,20 = 10.35, P < 0.01) observed in home cage females. These data, shown in Fig. 6(C), reveal increased Fos-IR in the CeA after PPT-administration, regardless of reproductive experience, coupled with increased Fos-IR in primiparous females compared to nulliparous females, regardless of drug treatment. Moreover, although vehicle- and PPT-treated, nulliparous females showed increased Fos-IR in the CeA after EPM testing (P < 0.05); no such effect was observed in primiparous females. Thus, reproductive experience modulates the activity of the CeA and alters the response to exposure to the EPM.
Fig. 6.
Mean ± SEM number of Fos positive cells in the medial amygdala (MeA) (A), basolateral amygdala (BLA) (B) and central amygdala (CeA) (C) of nulliparous and age-matched, primiparous females administered vehicle (VEH) or propyl-pyrazole triol (PPT). Females either remained in their home cage or were tested on the EPM. *P < 0.01 main effect of reproductive experience. γP < 0.05 main effect of drug. #P < 0.05 main effect of testing. N = 5 per group.
Discussion
The findings of the present study indicate that reproductive experience modifies both the behavioural and neuronal response to acute activation of the ERα subtype. When tested 30 min after the administration of PPT, behavioural differences between nulliparous and primiparous females emerged. Although PPT tended to be anxi-ogenic in nulliparous females, the opposite profile was observed in primiparous females. Thus, primiparous females increased their entries and exploration of the open arms of the EPM, at the same time as decreasing their time in the closed arms compared to nulliparous females. These findings are similar to the findings reported previously after four daily injections of PPT in primiparous females (37). Given that the present study used a single acute injection administered 30 min before testing, the observed effects of ERα activation on anxiety-like behaviour in both studies were likely mediated by activation of membrane, and not nuclear, oestrogen receptors.
To begin to identify neural and/or endocrine changes underlying these differences in behaviour in the present study, we first examined corticosterone secretion. Previous studies have suggested that reproductive experience may alter stress responsiveness (24). As a measure of the HPA axis response to mild psychological stress (46), we measured corticosterone secretion in untested, home cage females and in females sacrificed 10 min after their initial exposure to the EPM. In untested, vehicle- and PPT-administered females, primiparous females had significantly lower levels of corticosterone secretion. As expected, EPM exposure increased corticosterone and these effects were similar in nulliparous and primiparous females. PPT significantly enhanced corticosterone secretion in home cage animals regardless of reproductive experience, although no further effect of PPT could be discerned in EPM-tested females. These data suggest that reproductive experience reduces home cage corticosterone but does not appear to fundamentally alter either the response to PPT or EPM exposure. It is possible that experienced females actually demonstrate a more robust corticosterone response given their lower home cage levels. The use of a within-subject design to ascertain the effects of reproductive experience on the dynamic range of the acute stress response would help answer this question.
A number of studies have observed alterations in corticosterone levels during pregnancy and lactation as a function of previous reproductive experience (47,48). Thus, a shift in neuroendocrine regulation of the HPA axis as a result of a prior pregnancy has been demonstrated previously. The data obtained in the present study suggest that such parity-induced changes extend beyond future pregnancies and can be discerned even in the absence of ovarian hormones. Other forms of reproductive experience, such as sexual experience, have also been shown to influence corticosterone secretion in both males and females from a number of species (49–51). In addition, improved spatial memory coincident with changes in hippocampal morphology and neurogenesis has also been reported after reproductive experience (7,9,52–55). Such functional outcomes are hypothesised to be related to experience-induced modifications in hormonal milieu, including short-term increases in corticosterone release associated with both sexual activity and lactation. It does not appear to be the case, however, that differences in PPT-medi-ated behavioural responses in the present study correlate with the effects of this agonist on corticosterone secretion at the time of testing because both vehicle- and PPT-treated primiparous females have reduced corticosterone, whereas only those administered PPT showed reduced anxiety-like behaviour. Although the HPA axis is regulated by CRH activity in the PVN and the subsequent release of adrenocorticotrophin releasing hormone and corticosterone, CRH has also be shown to regulate anxiety-like behaviour via central, non-HPA mediated, actions. For example, increased CRH production in the CeA is anxiogenic (56), whereas decreased CRH activity in this region is anxiolytic (57). We previously observed effects of reproductive experience on CRH gene expression in both the PVN and lateral amygdala of females administered PPT repeatedly and sacrificed 30 min after exposure to the EPM. In that study, PPT increased CRH mRNA in the PVN of primiparous females compared to vehicle-treated controls, which is an effect that was not observed in nulliparous females. By contrast, only nulliparous, PPT-treated females had increased CRH mRNA in the lateral amygdala (37). Given the repeated nature of the PPT administration in that study, coupled with the potential effects of EPM exposure, it was unclear whether these changes reflected the effects of repeated PPT administration or an interaction between testing and PPT. With respect to the data obtained in the present study, no significant effects were observed in tissue punches from the CeA in either home cage or EPM exposed females after acute PPT administration, although the data were fairly variable, even in home cage females. In the PVN, there was a tendency for PPT-treated, home cage primiparous females to display increased CRH gene expression. This effect was in contrast to the significant decrease in CRH mRNA expression observed in PPT-treated, primiparous females tested on the EPM. Reconciling the differences between repeated versus acute administration, as well as effects that appear to interact with exposure to a stressor, will require additional studies. Nonetheless, these data continue to indicate that regulation of CRH gene transcription by ERα activation in both stress- and anxiety-related brain regions differs in reproductively experienced females compared to age-matched, virgin controls. Moreover, the nature of these effects appears to be dependent upon the duration of oestrogen receptor agonist treatment.
In addition to measuring CRH transcription, we also examined neuronal activity more broadly in both the PVN and amygdala using the immediate early gene cfos as a marker. Overall, in the PVN, Fos-IR was increased in animals tested on the EPM, a finding that has been previously reported (58,59). PVN Fos-IR, however, was significantly lower in primiparous females after EPM exposure compared to nulliparous controls. These effects were observed in both vehicle- and PPT-treated animals but were not observed in home cage controls. Moreover, PPT administration significantly increased Fos-IR in both nulliparous and primiparous females but only when coupled with EPM exposure. Because no significant differences in corticosterone levels were observed in PPT- compared to vehicle-treated females tested on the EPM, this suggests that the effects of PPT on Fos-IR in the PVN may be unrelated to activation of the HPA axis. Whether increased Fos-IR after PPT and exposure to the EPM occurs in similar cell types in nulliparous and primiparous females remains to be determined. The findings of the present study, however, suggest that the response of the PVN to a mild psychological stressor is blunted in primiparous females. Furthermore, because these effects are observed in ovariectomised females, these data suggest that some of the effects of reproductive experience on stress-sensitive parameters are independent of gonadal hormones.
Similar to other reports in the literature, exposure to the EPM increased Fos-IR in both the MeA and BLA but was without significant effect in the CeA (60–62). No significant effects of either acute PPT administration or reproductive experience were observed in either the MeA or BLA. Effects of both reproductive experience and acute PPT administration were, however, observed in the CeA. By contrast to the effects observed in the PVN, alterations in Fos-IR in the CeA were only observed in home cage females. Specifically, PPT increased Fos-IR in the CeA in all females; however, both vehicle- and PPT-treated, primiparous females had a greater number of Fos-IR positive cells in this nucleus compared to aged-matched, nulliparous controls. No significant effects were observed after EPM exposure, although there was a tendency toward increased Fos-IR after PPT administration.
In sum, the findings of the present study demonstrate the significant effects of reproductive experience that can be observed in the absence of circulating gonadal hormones and impact brain regions critical for the regulation of anxiety-, fear- and stress-related behaviours. Furthermore, the acute activation of the ERα subtype induces opposite effects on anxiety-like behaviour in nulliparous and primiparous females. These behavioural effects are most likely modulated by membrane bound ERα subtypes, given the time course for the observed change. It is also of interest that the effects of acute PPT at the level of the PVN appear to be regulated by exposure to mild psychological stress. Recent findings have documented significant effects of membrane-bound ERα receptors that appear to be associated with the suppression of inhibitory neurotransmission in the hippocampus, a brain region that regulates the PVN (63). To what extent any similar effect of ERα may be observed in other brain regions remains to be determined. These findings, however, suggest that rapid effects of membrane ERα receptors can influence the activity of neural regions critical for modulating the stress response. Of note, reproductive experience has been shown to alter ERα expression in a number of brain regions that regulate the HPA axis, including the amygdala, medial preoptic area and PVN (18,19,64). To what extent such experience-induced modifications reflect changes in membrane and/or nuclear ERα expression remains unknown.
Finally, in an attempt to fit these data within an experimental framework, we propose that changes in oxytocin (OT) projections from the PVN to the CeA may underlie some of the observed effects of reproductive experience. Recent studies identified magnocellular OT neurones that project from the PVN to the CeA, the activation of which increases OT release into the CeA. Consequently, this increased OT release activates GABA neurones within the CeA, which then inhibit CeA output, decreasing fear-related behaviours (65). Significant long-term changes in OT within the PVN after reproductive experience have been reported in a number of species, although those studies were typically conducted during a first versus second pregnancy (48,66). Thus, the increased Fos-IR observed in the CeA may represent decreased output from this nucleus and could be related to the lower corticosterone levels observed in home cage, primiparous females. In turn, the increased Fos-IR observed after exposure to the EPM in primiparous females may occur in OT neurones, which, when coupled with acute PPT, may converge to suppress CRH transcription. Certainly, numerous studies will be necessary to test this hypothesis, and it is unclear to what extent the changes in anxiety-like behaviour are related to these observed differences in Fos-IR or home cage corticosterone. Indeed, it remains unclear whether differences in anxiety-like behaviour in PPT-treated primiparous females are the result of changes in the perceived stress associated with the task or an increased motivation to explore. It is important to note that an increased motivation to explore is different from a general increase in activity because the later would affect behaviour in all parts of the maze, whereas the former may simply increase the likelihood of open arm exploration. The use of additional tests of anxiety-like behaviour, as well as fear-related behaviours, should be conducted to dissociate these effects.
In conclusion, the effects of pregnancy and lactation on the female brain continue to be observed after active parenting has subsided. These effects are not simply a result of differences in circulating gonadal hormones, although they certainly alter both the behavioural and neural response to selective activation of the ERα subtype. Given the long-term impact that modifications in stress and anxiety can have on health, understanding the neural mechanisms underlying reproductive experience may reveal potential targets for decreasing the negative effects of stress on mental health in women.
References
- 1.Kinsley CH, Franssen RA, Meyer EA. Reproductive experience may positively adjust the trajectory of senescence. Curr Top Behav Neurosci. 2012;10:317–345. doi: 10.1007/7854_2011_123. [DOI] [PubMed] [Google Scholar]
- 2.Franssen RA, Rzucidlo AM, Franssen CL, Hampton JE, Benkovic SA, Jr, Bardi M, Kinsley CH, Lambert KG. Reproductive experience facilitates recovery from kainic acid-induced neural insult in female Long-Evans rats. Brain Res. 2012;1454:80–89. doi: 10.1016/j.brainres.2012.03.028. [DOI] [PubMed] [Google Scholar]
- 3.Bridges RS, Scanlan VF, Lee JO, Byrnes EM. Reproductive experience alters prolactin receptor expression in mammary and hepatic tissues in female rats. Biol Reprod. 2011;85:340–346. doi: 10.1095/biolreprod.111.091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carvalho-Freitas MI, Anselmo-Franci JA, Teodorov E, Nasello AG, Palermo-Neto J, Felicio LF. Reproductive experience modifies dopaminer-gic function, serum levels of prolactin, and macrophage activity in female rats. Life Sci. 2007;81:128–136. doi: 10.1016/j.lfs.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 5.Byrnes EM, Lee JO, Bridges RS. Alterations in GABA(A) receptor alpha2 subunit mRNA expression following reproductive experience in rats. Neuroendocrinology. 2007;85:148–156. doi: 10.1159/000102535. [DOI] [PubMed] [Google Scholar]
- 6.Byrnes EM, Bridges RS. Reproductive experience and expression of dopamine D(2) receptor mRNA: a possible mechanism for reduced pro-lactin secretion in primiparous rats. J Neuroendocrinol. 2007;19:773–778. doi: 10.1111/j.1365-2826.2007.01586.x. [DOI] [PubMed] [Google Scholar]
- 7.Pawluski JL, Walker SK, Galea LA. Reproductive experience differentially affects spatial reference and working memory performance in the mother. Horm Behav. 2006;49:143–149. doi: 10.1016/j.yhbeh.2005.05.016. [DOI] [PubMed] [Google Scholar]
- 8.Pawluski JL, Vanderbyl BL, Ragan K, Galea LA. First reproductive experience persistently affects spatial reference and working memory in the mother and these effects are not due to pregnancy or ‘mothering’ alone. Behav Brain Res. 2006;175:157–165. doi: 10.1016/j.bbr.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 9.Pawluski JL, Galea LA. Hippocampal morphology is differentially affected by reproductive experience in the mother. J Neurobiol. 2006;66:71–81. doi: 10.1002/neu.20194. [DOI] [PubMed] [Google Scholar]
- 10.Byrnes EM, Bridges RS. Reproductive experience reduces the sedative, but not anxiolytic effects of diazepam. Psychoneuroendocrinology. 2006;31:988–996. doi: 10.1016/j.psyneuen.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Bridges RS, Byrnes EM. Reproductive experience reduces circulating 17beta-estradiol and prolactin levels during proestrus and alters estrogen sensitivity in female rats. Endocrinology. 2006;147:2575–2582. doi: 10.1210/en.2005-0917. [DOI] [PubMed] [Google Scholar]
- 12.Anderson GM, Grattan DR, van den Ancker W, Bridges RS. Reproductive experience increases prolactin responsiveness in the medial preoptic area and arcuate nucleus of female rats. Endocrinology. 2006;147:4688–4694. doi: 10.1210/en.2006-0600. [DOI] [PubMed] [Google Scholar]
- 13.Macbeth AH, Luine VN. Changes in anxiety and cognition due to reproductive experience: a review of data from rodent and human mothers. Neurosci Biobehav Rev. 2010;34:452–467. doi: 10.1016/j.neubiorev.2009.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Macbeth AH, Scharfman HE, Maclusky NJ, Gautreaux C, Luine VN. Effects of multiparity on recognition memory, monoaminergic neuro-transmitters, and brain-derived neurotrophic factor (BDNF) Horm Behav. 2008;54:7–17. doi: 10.1016/j.yhbeh.2007.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Butt S, Borgquist S, Anagnostaki L, Landberg G, Manjer J. Parity and age at first childbirth in relation to the risk of different breast cancer subgroups. Int J Cancer. 2009;125:1926–1934. doi: 10.1002/ijc.24494. [DOI] [PubMed] [Google Scholar]
- 16.Kass L, Durando M, Ramos JG, Varayoud J, Powell CE, Luque EH, Munoz-de-Toro M. Association of increased estrogen receptor beta2 expression with parity-induced alterations in the rat mammary gland. J Steroid Biochem Mol Biol. 2004;91:29–39. doi: 10.1016/j.jsbmb.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Arslan AA, Zeleniuch-Jacquotte A, Lukanova A, Afanasyeva Y, Katz J, Levitz M, Del Priore G, Toniolo P. Effects of parity on pregnancy hormonal profiles across ethnic groups with a diverse incidence of breast cancer. Cancer Epidemiol Biomarkers Prev. 2006;15:2123–2130. doi: 10.1158/1055-9965.EPI-06-0470. [DOI] [PubMed] [Google Scholar]
- 18.Meurisse M, Gonzalez A, Delsol G, Caba M, Levy F, Poindron P. Estradiol receptor-alpha expression in hypothalamic and limbic regions of ewes is influenced by physiological state and maternal experience. Horm Behav. 2005;48:34–43. doi: 10.1016/j.yhbeh.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Byrnes EM, Babb JA, Bridges RS. Differential expression of oestrogen receptor alpha following reproductive experience in young and middle-aged female rats. J Neuroendocrinol. 2009;21:550–557. doi: 10.1111/j.1365-2826.2009.01874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Byrnes EM, Bridges RS. Reproductive experience alters anxiety-like behavior in the female rat. Horm Behav. 2006;50:70–76. doi: 10.1016/j.yhbeh.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 21.Walf AA, Frye CA. Parity and estrogen-administration alter affective behavior of ovariectomized rats. Physiol Behav. 2008;93:351–356. doi: 10.1016/j.physbeh.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Love G, Torrey N, McNamara I, Morgan M, Banks M, Hester NW, Glasper ER, Devries AC, Kinsley CH, Lambert KG. Maternal experience produces long-lasting behavioral modifications in the rat. Behav Neurosci. 2005;119:1084–1096. doi: 10.1037/0735-7044.119.4.1084. [DOI] [PubMed] [Google Scholar]
- 23.Gatewood JD, Morgan MD, Eaton M, McNamara IM, Stevens LF, Macbeth AH, Meyer EA, Lomas LM, Kozub FJ, Lambert KG, Kinsley CH. Motherhood mitigates aging-related decrements in learning and memory and positively affects brain aging in the rat. Brain Res Bull. 2005;66:91–98. doi: 10.1016/j.brainresbull.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 24.Wartella J, Amory E, Lomas LM, Macbeth A, McNamara I, Stevens L, Lambert KG, Kinsley CH. Single or multiple reproductive experiences attenuate neurobehavioral stress and fear responses in the female rat. Physiol Behav. 2003;79:373–381. doi: 10.1016/s0031-9384(03)00150-1. [DOI] [PubMed] [Google Scholar]
- 25.Zimberknopf E, Xavier GF, Kinsley CH, Felicio LF. Prior parity positively regulates learning and memory in young and middle-aged rats. Comp Med. 2011;61:366–377. [PMC free article] [PubMed] [Google Scholar]
- 26.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010;62:155–198. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller KJ, Conney JC, Rasgon NL, Fairbanks LA, Small GW. Mood symptoms and cognitive performance in women estrogen users and nonusers and men. J Am Geriatr Soc. 2002;50:1826–1830. doi: 10.1046/j.1532-5415.2002.50511.x. [DOI] [PubMed] [Google Scholar]
- 28.Savonenko AV, Markowska AL. The cognitive effects of ovariectomy and estrogen replacement are modulated by aging. Neuroscience. 2003;119:821–830. doi: 10.1016/s0306-4522(03)00213-6. [DOI] [PubMed] [Google Scholar]
- 29.Maki PM, Dumas J. Mechanisms of action of estrogen in the brain: insights from human neuroimaging and psychopharmacologic studies. Semin Reprod Med. 2009;27:250–259. doi: 10.1055/s-0029-1216278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Small GW. Estrogen effects on the brain. J Gend Specif Med. 1998;1:23–27. [PubMed] [Google Scholar]
- 31.Fink G, Sumner BE, Rosie R, Grace O, Quinn JP. Estrogen control of central neurotransmission: effect on mood, mental state, and memory. Cell Mol Neurobiol. 1996;16:325–344. doi: 10.1007/BF02088099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walf AA, Frye CA. ERbeta-selective estrogen receptor modulators produce antianxiety behavior when administered systemically to ovariecto-mized rats. Neuropsychopharmacology. 2005;30:1598–1609. doi: 10.1038/sj.npp.1300713. [DOI] [PubMed] [Google Scholar]
- 33.Bodo C, Rissman EF. New roles for estrogen receptor beta in behavior and neuroendocrinology. Front Neuroendocrinol. 2006;27:217–232. doi: 10.1016/j.yfrne.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 34.Walf AA, Koonce CJ, Frye CA. Estradiol or diarylpropionitrile decrease anxiety-like behavior of wildtype, but not estrogen receptor beta knockout, mice. Behav Neurosci. 2008;122:974–981. doi: 10.1037/a0012749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tomihara K, Soga T, Nomura M, Korach KS, Gustafsson JA, Pfaff DW, Ogawa S. Effect of ER-beta gene disruption on estrogenic regulation of anxiety in female mice. Physiol Behav. 2009;96:300–306. doi: 10.1016/j.physbeh.2008.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lund TD, Rovis T, Chung WC, Handa RJ. Novel actions of estrogen receptor-beta on anxiety-related behaviors. Endocrinology. 2005;146:797–807. doi: 10.1210/en.2004-1158. [DOI] [PubMed] [Google Scholar]
- 37.Byrnes EM, Casey K, Bridges RS. Reproductive experience modifies the effects of estrogen receptor alpha activity on anxiety-like behavior and corticotropin releasing hormone mRNA expression. Horm Behav. 2012;61:44–49. doi: 10.1016/j.yhbeh.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yang JZ, O’Flatharta C, Harvey BJ, Thomas W. Membrane ERalpha-dependent activation of PKCalpha in endometrial cancer cells by estra-diol. Steroids. 2008;73:1110–1122. doi: 10.1016/j.steroids.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Grove-Strawser D, Boulware MI, Mermelstein PG. Membrane estrogen receptors activate the metabotropic glutamate receptors mGluR5 and mGluR3 to bidirectionally regulate CREB phosphorylation in female rat striatal neurons. Neuroscience. 2010;170:1045–1055. doi: 10.1016/j.neuroscience.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Christensen A, Dewing P, Micevych P. Membrane-initiated estradiol signaling induces spinogenesis required for female sexual receptivity. J Neurosci. 2011;31:17583–17589. doi: 10.1523/JNEUROSCI.3030-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonfiglio JJ, Inda C, Refojo D, Holsboer F, Arzt E, Silberstein S. The corti-cotropin-releasing hormone network and the hypothalamic-pituitary-adrenal axis: molecular and cellular mechanisms involved. Neuroendocri-nology. 2011;94:12–20. doi: 10.1159/000328226. [DOI] [PubMed] [Google Scholar]
- 42.Lalmansingh AS, Uht RM. Estradiol regulates corticotropin-releasing hormone gene (crh) expression in a rapid and phasic manner that parallels estrogen receptor-alpha and -beta recruitment to a 3’,5’-cyclic adeno-sine 5’-monophosphate regulatory region of the proximal crh promoter. Endocrinology. 2008;149:346–357. doi: 10.1210/en.2007-0372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Handa RJ, Mani SK, Uht RM. Estrogen receptors and the regulation of neural stress responses. Neuroendocrinology. 2012;96:111–118. doi: 10.1159/000338397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Horm Behav. 2006;49:197–205. doi: 10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 45.Paxinos G, Watson C. The Rat Brain. Compact 6th Edition. London: Elsevier; 2009. pp. 4–50. [Google Scholar]
- 46.Aoki M, Shimozuru M, Kikusui T, Takeuchi Y, Mori Y. Sex differences in behavioral and corticosterone responses to mild stressors in ICR mice are altered by ovariectomy in peripubertal period. Zoolog Sci. 2010;27:783–789. doi: 10.2108/zsj.27.783. [DOI] [PubMed] [Google Scholar]
- 47.Pawluski JL, Charlier TD, Lieblich SE, Hammond GL, Galea LA. Reproductive experience alters corticosterone and CBG levels in the rat dam. Physiol Behav. 2009;96:108–114. doi: 10.1016/j.physbeh.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 48.Nephew BC, Bridges RS, Lovelock DF, Byrnes EM. Enhanced maternal aggression and associated changes in neuropeptide gene expression in multiparous rats. Behav Neurosci. 2009;123:949–957. doi: 10.1037/a0016734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Riechert J, Chastel O, Becker PH. Why do experienced birds reproduce better? Possible endocrine mechanisms in a long-lived seabird, the common tern. Gen Comp Endocrinol. 2012;178:391–399. doi: 10.1016/j.ygcen.2012.06.022. [DOI] [PubMed] [Google Scholar]
- 50.Bonilla-Jaime H, Vazquez-Palacios G, Arteaga-Silva M, Retana-Marquez S. Hormonal responses to different sexually related conditions in male rats. Horm Behav. 2006;49:376–382. doi: 10.1016/j.yhbeh.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 51.Angelier F, Shaffer SA, Weimerskirch H, Chastel O. Effect of age, breeding experience and senescence on corticosterone and prolactin levels in a long-lived seabird: the wandering albatross. Gen Comp Endocrinol. 2006;149:1–9. doi: 10.1016/j.ygcen.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 52.Pawluski JL, Galea LA. Reproductive experience alters hippocampal neurogenesis during the postpartum period in the dam. Neuroscience. 2007;149:53–67. doi: 10.1016/j.neuroscience.2007.07.031. [DOI] [PubMed] [Google Scholar]
- 53.Glasper ER, Kozorovitskiy Y, Pavlic A, Gould E. Paternal experience suppresses adult neurogenesis without altering hippocampal function in Peromyscus californicus. J Comp Neurol. 2011;519:2271–2281. doi: 10.1002/cne.22628. [DOI] [PubMed] [Google Scholar]
- 54.Leuner B, Glasper ER, Gould E. Sexual experience promotes adult neuro-genesis in the hippocampus despite an initial elevation in stress hormones. PLoS ONE. 2010;5:e11597. doi: 10.1371/journal.pone.0011597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Leuner B, Mirescu C, Noiman L, Gould E. Maternal experience inhibits the production of immature neurons in the hippocampus during the postpartum period through elevations in adrenal steroids. Hippocampus. 2007;17:434–442. doi: 10.1002/hipo.20278. [DOI] [PubMed] [Google Scholar]
- 56.Flandreau EI, Ressler KJ, Owens MJ, Nemeroff CB. Chronic overexpres-sion of corticotropin-releasing factor from the central amygdala produces HPA axis hyperactivity and behavioral anxiety associated with gene-expression changes in the hippocampus and paraventricular nucleus of the hypothalamus. Psychoneuroendocrinology. 2012;37:27–38. doi: 10.1016/j.psyneuen.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liebsch G, Landgraf R, Gerstberger R, Probst JC, Wotjak CT, Engelmann M, Holsboer F, Montkowski A. Chronic infusion of a CRH1 receptor anti-sense oligodeoxynucleotide into the central nucleus of the amygdala reduced anxiety-related behavior in socially defeated rats. Regul Pept. 1995;59:229–239. doi: 10.1016/0167-0115(95)00099-w. [DOI] [PubMed] [Google Scholar]
- 58.Ebner K, Muigg P, Singewald G, Singewald N. Substance P in stress and anxiety: NK-1 receptor antagonism interacts with key brain areas of the stress circuitry. Ann NY Acad Sci. 2008;1144:61–73. doi: 10.1196/annals.1418.018. [DOI] [PubMed] [Google Scholar]
- 59.Viltart O, Mairesse J, Darnaudery M, Louvart H, Vanbesien-Mailliot C, Catalani A, Maccari S. Prenatal stress alters Fos protein expression in hippocampus and locus coeruleus stress-related brain structures. Psy-choneuroendocrinology. 2006;31:769–780. doi: 10.1016/j.psyneuen.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 60.Butler RK, White LC, Frederick-Duus D, Kaigler KF, Fadel JR, Wilson MA. Comparison of the activation of somatostatin- and neuropeptide Y-containing neuronal populations of the rat amygdala following two different anxiogenic stressors. Exp Neurol. 2012;238:52–63. doi: 10.1016/j.expneurol.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Duncan GE, Knapp DJ, Breese GR. Neuroanatomical characterization of Fos induction in rat behavioral models of anxiety. Brain Res. 1996;713:79–91. doi: 10.1016/0006-8993(95)01486-1. [DOI] [PubMed] [Google Scholar]
- 62.Troakes C, Ingram CD. Anxiety behaviour of the male rat on the elevated plus maze: associated regional increase in c-fos mRNA expression and modulation by early maternal separation. Stress. 2009;12:362–369. doi: 10.1080/10253890802506391. [DOI] [PubMed] [Google Scholar]
- 63.Huang GZ, Woolley CS. Estradiol acutely suppresses inhibition in the hippocampus through a sex-specific endocannabinoid and mGluR-dependent mechanism. Neuron. 2012;74:801–808. doi: 10.1016/j.neuron.2012.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song Z, Tai F, Yu C, Wu R, Zhang X, Broders H, He F, Guo R. Sexual or paternal experiences alter alloparental behavior and the central expression of ERalpha and OT in male mandarin voles (Microtus mandarinus) Behav Brain Res. 2010;214:290–300. doi: 10.1016/j.bbr.2010.05.045. [DOI] [PubMed] [Google Scholar]
- 65.Knobloch HS, Charlet A, Hoffmann LC, Eliava M, Khrulev S, Cetin AH, Osten P, Schwarz MK, Seeburg PH, Stoop R, Grinevich V. Evoked axonal oxytocin release in the central amygdala attenuates fear response. Neuron. 2012;73:553–566. doi: 10.1016/j.neuron.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 66.Broad KD, Levy F, Evans G, Kimura T, Keverne EB, Kendrick KM. Previous maternal experience potentiates the effect of parturition on oxytocin receptor mRNA expression in the paraventricular nucleus. Eur J Neurosci. 1999;11:3725–3737. doi: 10.1046/j.1460-9568.1999.00782.x. [DOI] [PubMed] [Google Scholar]