Summary
The incidence of chronic pain is estimated to be 20–25% worldwide. Although major improvements in pain control have been obtained, more than 50% of the patients reports inadequate relief. It is accepted that chronic pain, if not adequately and rapidly treated, can become a disease in itself, often intractable and maybe irreversible. This is mainly due to neuroplasticity of pain pathways. In the present review I will discuss about pain depicting the rational for the principal pharmacological interventions and finally focusing on opioids, that represent a primary class of drug to treat pain.
Keywords: chronic pain, opioids, tapentadol, multi-target pharmacology
Pain is a physiological process
Pain has been defined by IASP as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage”. This definition implies a physiological role for pain that works as an alarm ring to avoid or limit tissue damage, thus serving an essential vital defensive function. Pain processing is organized in five essential steps: transduction, conduction, modulation, transmission and perception.
Transduction is the process that converts mechanic, thermic (cold or heat), and chemical (low or high pH) energies with high intensity, and thus potentially dangerous, in an electrical signal, the generator potential, that, in turn, will trigger the onset of the action potential.
This phenomenon occurs in the peripheral terminals of primary afferent somatic and visceral nociceptive fibers that have their bodies in the dorsal root ganglia. These high threshold primary sensory neurons that are specialized to respond to strong, potentially tissue-damaging stimuli are known as nociceptors. Their peripheral terminals contain highly specialized transducer proteins, most of which are channels, that open in response to specific high energy stimuli, becoming permeable to calcium and sodium ions that are in turn responsible for terminal depolarization. Most of these transducers have been characterized at molecular level, and their function has immediately appeared to be more complex. For instance the transducer TRPV1 which is a member of Transient Receptor Potential family (1) becomes activated in response to heat in excess of 43° C, but also in response to vanilloid chemical ligands such as capsaicin, the pungent ingredient in chili peppers. Many other transducers, such as TRPM8 that can be activated by menthol, possess the precious property of responding also to chemicals, making them suitable for drug development, as actually it happened for capsaicin which is an approved drug for chronic pain.
Conduction is the step in which action potentials travel along axons to reach the spinal cord. For conduction to occur, voltage-gated sodium ion channels must convert generator potential at the peripheral terminals into an action potential. Different isoforms of voltage-gated sodium ion channels are known, but Nav 1.8 and Nav 1.9 are selectively expressed in nociceptors and are blocked by local anesthetic and antiepileptic drugs. Nociceptors can have unmyelinated (C-fiber) or thinly myelinated (Aδ-fiber) axons. C-fibers are probably involved in chronic pain.
When action potentials reach the nociceptor terminals in the dorsal horn of the spinal cord, N-type voltage-gated calcium channel open, promoting neurotransmitter release. These N-type calcium channel contain the α2δ subunit to which pregabalin and gabapentin bind, blocking calcium ions influx and neurotransmitter release (2). For this pharmacological property, these drugs are used in the treatment of neuropathic pain. Transmission step coincides with synaptic communication between first- and second-order neurons is the transmission step. The synapse between the nociceptor, or first-order neuron and the spinothalamic neuron, or the second-order neuron, is primarily glutammatergic. In acute nociceptive pain glutamate mainly binds to AMPA receptors, which are ligand-gated ion channels, highly permeable to sodium ions that in turn depolarize second-order neurons and trigger the action potential that will ascend to thalamus. More intense or sustained C-fiber nociceptor activation results in the release of neuropeptide modulators (substance P, CGRP) and in a sustained postsynaptic depolarization. This depolarization removes the voltage-dependent blockade by Mg2+ of NMDA receptors, the other class of glutamate receptors expressed in second-order neurons in the dorsal horn, thus causing a large Ca2+influx. NMDA receptor activation is an essential step in central sensitization that always accompanies chronic pain. The involvement of NMDA receptors in chronic pain justifies the use of drugs that block these receptors such as ketamine (3). However not all the impulses coming from the periphery will reach the thalamus because synaptic transmission in the spinal cord is regulated by the action of both local interneurons and projections that descend from the brainstem to the dorsal horn. The major inhibitory neurotransmitters in the dorsal horn are opioids, norepinephrine, serotonin, glycine and GABA. The role of norepinephrine and serotonin in spinal modulation of pain stimuli explains why antidepressant drugs that inhibit the reuptake of these neurotransmitters are used in the treatment neuropathic pain (4). Opioids produce analgesia because of their action in the brain, brainstem, spinal cord and, in some circumstances, also in peripheral terminals of primary afferents neurons. All endogenous opioid peptides, which include β-endorphin, the enkephalins and the dynorphins bind to seven-transmembrane G protein coupled receptors (GPCR), that traditionally fall into three classes: μ, δ and κ receptors (5).
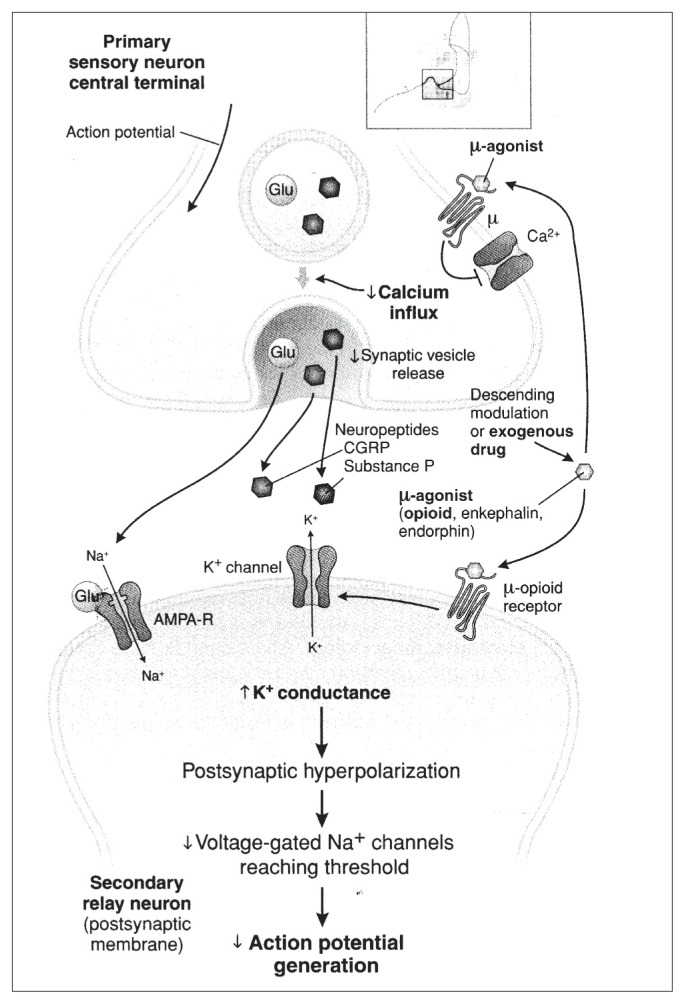
All opioid receptors are coupled to inhibitor G-proteins and receptor activation inhibits the adenylate cyclase and the intracellular generation of cAMP. However coupling of opioid receptors to K+ and Ca2+ ion channels is believed to be the more important mechanism by which both endogenous and exogenous opioids produce analgesia. In particular, in the dorsal horn of the spinal cord, the overwhelming majority of opioid receptors are μ receptors, at a predominantly presynaptic location (more than 70%) on the central terminals of nociceptors (C and Aδ fibers). The remaining 30% of opioid receptors are located post-synaptically on dendrites of second-order spinothalamic neurons and on interneurons. Endogenous opioids β-enkefalin and endorphins, acting on μ receptors in the dorsal horn of the spinal cord, are predominantly released by interneurons, whose activation depends on the activity of descending pathways, or directly by descending fibers. The activation of presynaptic μ receptors causes the inhibition of calcium ion channel, preventing the release of neurotransmitters. The activation of postsynaptic μ receptors causes potassium ion channels activation with consequent efflux of potassium ions and hyperpolarization of the projecting cell. Thus, μ opioid receptor stimulation in the spinal cord is a very efficacious mechanism to block synaptic transmission, limiting the number of nociceptive stimuli that reach thalamus and eventually the cortex where conscious perception of pain occurs (6) (Figure 1).
Figure 1.
Mechanisms of analgesic action of opioids.
AMPA-R = alphaamino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; CGRP = calcitonin gene-related peptide. (From Fornasari D. (6) Reproduced with permission from Golan DE, Tashjan AH, Armstrong EJ. Principles of Pharmacology. The Pharmaphysiologic Basis of Drug Therapy, 2nd Edition. Baltimore: Wolters Kluwer Health, 2008).
Pain as a pathological process
Clinical pain is generally identified with chronic pain. Chronic pain persists beyond the expected normal time for healing and serves no useful physiological purpose. In the case of nonmalignant conditions, the definition of chronic pain implies that it may persist for longer than 3 months. Chronic pain may be nociceptive, neuropathic or functional and all forms share some common characteristics. Inflammatory pain is sustained by tissue injury, as occurs after trauma, surgery or during chronic inflammatory diseases, such as rheumatoid arthritis, or degenerative diseases of the joints such as osteoarthritis. In these conditions, damaged cells and inflammatory cells recruited to the site of damage release substances that activate, and sensitize, peripheral nociceptors (7). Neuropathic pain is defined as spontaneous pain and hypersensitivity to pain associated with damage to or pathological changes in the peripheral nervous system, as occurs in diabetic peripheral neuropathy, AIDS, post-herpetic neuralgia, or pain originating in the central nervous system (CNS), as occurs with spinal cord injury, multiple sclerosis and stroke (7–9). Functional pain, a relatively new concept, is pain sensitivity caused by an abnormal processing or function of the CNS in response to normal stimuli, as may occur in fibromyalgia and irritable bowel syndrome (7).
In the last few years a new fundamental concept emerged that the neural substrates that mediate pain are plastic, that is, they modify on the basis of their use or modulatory influences. Dynamic changes in these structure can occur over several temporal scales (acute to chronic) and on the molecular, synaptic, cellular and network levels (10). These modifications have their counterparts at clinical level, as pain acquires new semeiotic features: it can be spontaneous, evoked by innocuous and normally non-painful stimuli (allodynia), or perceived as more intense and long-lasting than usual when painful stimuli are applied (hyperalgesia). A quite rapid modification of pain neural structures occurs in acute and, especially, chronic inflammatory pain in which nociceptors, at the periphery, lower their activation threshold and increase their responsiveness. This phenomenon is known as peripheral sensitization and is mainly the result of the phosphorylation of two molecular structures peripherally involved in pain processing: the transducer TRPV1 and the Nav 1.8 channel. Upon phosphorylation, TRPV1 becomes activated at 37°C, instead of 43°C, and Nav 1.8 decreases its activation threshold and allows the passage of a greater amount of sodium ions when it opens. Phosphorylation of these substrates is the consequence of the activation of different kinases by sensitizing agents that work through their own membrane receptors, such as prostaglandins, bradikinin, adenosine, released by inflammatory cells.
Peripheral sensitization is an important target for pain pharmacology and the NSAIDs are mainly used just to reduce the peripheral presence of an important component of sensitizing molecules.
If the inflammatory process underlying pain resolve in few days, all the plastic modifications in the nociceptors revert to normality. On the contrary persistency of inflammation, as well as of any other pathological condition that causes chronic pain such as peripheral neuropathic pain, provoking repetitive high-intensity synaptic transmission determines plastic modifications in the spinal cord, affecting second-order spinothalamic neurons, but also interneurons, some of which could also die as a consequence of the abnormal stimulation from periphery. This phenomenon is also known as central sensitization. Plastic modifications of pain pathways are not restricted to spinal cord, but can interest supraspinal integrative structures such thalamus or cortex. Structural plasticity can occur at various anatomical and temporal scales. At the macroscopic anatomical level, long-term neuropathic pain in humans has widespread effects on brain anatomy, depending on the duration and the intensity of pain. Local morphological alterations in the brain, mostly representing a decrease in the brain gray matter, have been reported in people with phantom pain, chronic back pain, irritable bowel syndrome, fibromyalgia and headaches (11). The important question is whether neuroplastic modifications of neural structures involved in pain processing are always reversible or whether some of them become irreversible, depending on the duration and the intensity of pain. This may help to explain the large number of patients encountered in the clinical practice who suffer from almost intractable pain and raises the question if analgesic drugs may be distinguished in drugs that only alleviate the pain symptoms and drugs that also may revert pain-induced neuroplastic changes.
Opioid receptor agonists
Opioid receptor agonist are primary drugs for the management of acute and chronic pain with intensity from moderate to severe. In chronic pain, they are used both for cancer and non-cancer pain. They are primarily agonists at μ receptors, with mechanisms and sites of action already described for their endogenous counterparts involved in analgesia (12). Opioids receptor agonist, now simply opioids, are classified according to their efficacy, or intrinsic activity, in full agonist and partial agonist, although a traditional way to classify these drugs in weak and strong opioids is still widely used (Table 1).
Table 1.
Classification of opioids.
| World Health Organization | Functional classification |
|---|---|
| Weak Opioids | Full agonists |
| Codeine, Dihydrocodeine, Destropropoxyphene, Tramadol | Morphine, Methadone, Fentanyl, Hydromorphone, Oxycodone, Buprenorphine, Tapentadol, Tramadol |
| Strong opioids | Partial agonists |
| Morphine, Methadone, Fentanyl, Hydromorphone, Oxycodone, Buprenorphine, Tapentadol | Buprenorphine, Pentazocine, Buturphanol |
| Agonist-Antagonist | |
| Nalbuphine, Nalorphine | |
| Full antagonist | |
| Naloxone, Naltrexone, Metylnatrexone, Alvimopan |
Beside differences in efficacy and potency, basically the pharmacodynamic properties of opioids, with few exceptions, are quite similar, that explains why they also produce similar adverse effects. On the contrary, pharmacokinetics properties can be relevant and determine specific uses of these drugs. For instance, the high lipid solubility, the low molecular weight and the high potency make fentanyl an ideal drug for transdermal and transmucosal administration. In particular transmucosal preparations of fentanyl are used to treat breakthrough cancer pain and represent a class of opioids known as ROO, rapid onset opioids, for their properties to produce a fast analgesia.
Metabolism is also relevant to distinguish opioids: morphine, hydromorphone and tapentadol are not metabolized by CYP450 enzymes, but directly undergo glucuronidation, limiting the probability of drug-drug interactions. Opioids share several adverse effects, some of which are common, such as nausea, vomiting, constipation, drowsiness, some occasional, such as allucinations or mood changes, others rare, such as respiratory depression, seizures or addiction.
Some common adverse effects can undergo tolerance and disappear after few days, such as nausea and vomiting. Thus it is important to inform patients about it, because these adverse effects are often the reason for withdrawal from treatment, especially in the case of non-cancer pain patients.
Other adverse effects never disappear or regress, such constipation; thus laxatives often need to be used continuously during opioid therapy. Because constipation is a peripheral phenomenon due to μ receptor activation in the bowel, peripheral opioid receptor antagonists, such as oral naloxone or subcutaneous metylnaltrexone and alvimopan, are currently used in different settings to reverse opioid-induced bowel dysfunction without compromising analgesia. For instance, a combination of oxycodone and naloxone at fixed ratio is available for the treatment of severe pain.
What’s new?
In the last few years, novel formulations of old opioids have been provided, surely representing steps forward in pain therapy. However, the great difficulty in treating chronic pain and especially neuropathic pain clearly indicates that new molecules with innovative pharmacodynamics are required. Recently one innovative molecule has become available: Tapentadol.
Tapentadol is an analgesic drug with a dual mechanism of action: it is a μ receptor full agonist, with an efficacy comparable to that of morphine, and it is an inhibitor of norepinephrine reuptake.
As mentioned before, norepinephrine released by descending pathways in the dorsal horn of the spinal cord plays a relevant role in pain modulation. Norepinephrine binds to α2-adrenergic receptors, localized on presynaptic (nociceptors) and postsynaptic (spinothalamic) neurons, thus inhibiting synaptic transmission with mechanisms identical to those described for μ receptors: blockade of presynaptic calcium ion channels and activation of postsynaptic potassium ion channels. Thus tapentadol allows norepinephrine to accumulate in the spinal cord synapses.
The concomitant stimulation of μ and α2 receptors has several advantages: a synergistic inhibitory effect on the spinal synapse, a sparing effect on μ receptors, with a consistent reduction of adverse events, such vomiting and constipation, and a better control of mixed pain, very frequent in several clinical conditions, such as low back pain, in which neuropathic and nociceptive components co-exist and better respond to norepinephrine and opioids respectively (13).
Conclusions
Pain is a very serious burden for patients, families, societies and physicians.
A better understanding of neuroplasticity processes underlying chronic pain is necessary to direct therapeutic intervention towards mechanisms responsible for the establishment of intractable and, perhaps, irreversible pain. New molecules are also needed to exploit new therapeutic targets and to be used especially in combination with available drugs. Multi-target pharmacology is probably the best way to treat pain, that allows to attack more than a single pathogenetic mechanism and to keep low the dosage of the single principles, by exploiting synergy. In this perspective, drugs with a dual mechanism of action, such as tapentadol, would be very useful (14).
Finally, physicians must become definitively aware that pain must be treated as soon as possible, first of all by diagnosing and treating the underlying disease responsible for pain, but also keeping in mind that changes occur during chronic pain that could transform pain into a disease in itself.
References
- 1.Patapoutian A, Tate S, Woolf CJ. Transient receptor potential channels: targeting pain at the source. Nat Rev Drug Discov. 2009;8:55–68. doi: 10.1038/nrd2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stahl SM, Porreca F, Taylor CP, Cheung R, Thorpe AJ, Clair A. The diverse therapeutic actions of pregabalin: is a single mechanism responsible for several pharmacological activities? Trends Pharmacol Sci. 2013 Jun;34(6):332–9. doi: 10.1016/j.tips.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Youn DH, Gerber G, Sather WA. Ionotropic glutamate receptors and voltage-gated Ca2+ channels in long-term potentiation of spinal dorsal horn synapses and pain hypersensitivity. Neural Plast. 2013;2013:654257. doi: 10.1155/2013/654257. Epub 2013 Oct 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartrick CT. Noradrenergic reuptake inhibition in the treatment of pain. Expert Opin Investig Drugs. 2012 Dec;21(12):1827–34. doi: 10.1517/13543784.2012.731393. [DOI] [PubMed] [Google Scholar]
- 5.Pathan H, Williams J. Basic opioid pharmacology: an update. British Journal of Pain. 2012;6:11–16. doi: 10.1177/2049463712438493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fornasari D. Pain mechanisms in patients with chronic pain. Clin Drug Investig. 2012 Feb;32(Suppl 1):45–52. doi: 10.2165/11630070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.Woolf CJ. Pain: moving from symptom control toward mechanism-specific pharmacologic management. Ann Intern Med. 2004;140(6):441–51. doi: 10.7326/0003-4819-140-8-200404200-00010. [DOI] [PubMed] [Google Scholar]
- 8.Malik B, Stillman M. Pain Syndromes. In: Savitz S, Ronthal M, editors. Neurology Review for Psychiatrists. 1st Edition ed. Philadelphia: Lippincott Williams & Wilkins; 2009. pp. 253–5. [Google Scholar]
- 9.Koltzenburg M, Scadding J. Neuropathic pain. Curr Opin Neurol. 2001;14(5):641–7. doi: 10.1097/00019052-200110000-00014. [DOI] [PubMed] [Google Scholar]
- 10.Kuner R. Central mechanisms of pathological pain. Nat Med. 2010 Nov;16(11):1258–66. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- 11.Apkarian AV, Sosa Y, Sonty S, Levy RM, Harden RN, Parrish TB, Gitelman DR. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004 Nov 17;24(46):10410–5. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Leon-Casasola OA. Opioids for chronic pain: new evidence, new strategies, safe prescribing. Am J Med. 2013 Mar;126(3 Suppl 1):S3–11. doi: 10.1016/j.amjmed.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Hartrick CT, Rozek RJ. Tapentadol in pain management: a μ-opioid receptor agonist and noradrenaline reuptake inhibitor. CNS Drugs. 2011 May;25(5):359–70. doi: 10.2165/11589080-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 14.Chaparro LE, Wiffen PJ, Moore RA, Gilron I. Combination pharmacotherapy for the treatment of neuropathic pain in adults. Cochrane Database Syst Rev. 2012 Jul 11;7:CD008943. doi: 10.1002/14651858.CD008943.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]