Summary
Risedronate is a heterocyclic orally active aminobisphosphonate and it belongs to the bisphosphonate category: these drugs are powerful bone resorption inhibitors, thanks to their affinity for hydroxyapatite crystals at bone mineral matrix level and to their inhibiting effects on osteoclast activity, using the ability of inhibiting enzyme FPPS. Recent observations have reported that risedronate can decrease resorption entity, not only of the trabecular bone, but also of the cortical bone, modifying therefore the (bone compact) thickness and the cortical porosity entity, which is largely responsible of femoral fracture especially among elderly patients. Various controlled studies have proved the efficacy of risedronate in reducing fragility fracture risk significantly. In particular, it is able to lower in a very significant way the incidence of vertebral, non-vertebral and femoral fractures, with precocity of effects after only six months of therapy. The extension of protocols, moreover, has marked its efficacy even after seven years of treatment. Under the metabolic profile, these studies have also shown that risedronate activity can reduce bone resorption markers and increase bone density values at lumbar and femoral level. Results emerged from a group of women aged over 80 are relevant: risedronate has proved capable of decreasing femoral fracture risk. Also in male and steroidal osteoporosis, clinical controlled studies have shown that risedronate is effective in decreasing vertebral fracture incidence. Lastly, tolerability: the main side effects concern the gastrointestinal tract and they are usually rare, of minor entity and can be solved by sospending the treatment. Acute phase reaction is rare, due to risedronate oral administration; it is also valid for osteonecrosis of the jaw and atypical fractures.
Keywords: osteoporosis, bisphosphonates, risedronate, fractures, osteoclasts
Mechanism of action
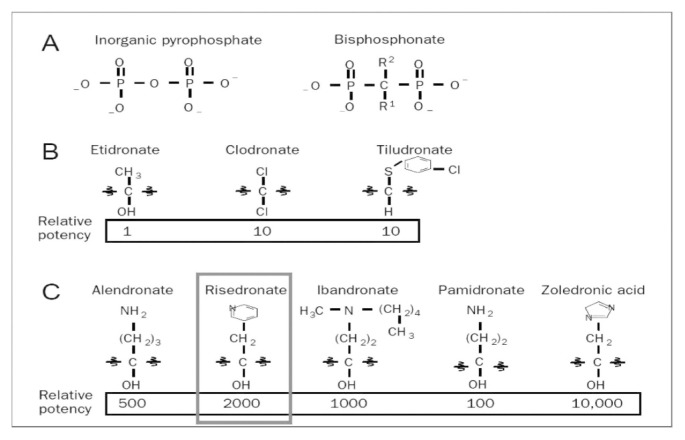
Risedronate is a heterocyclic orally active aminobisphosphonate (amino-BF). It belongs to the bisphosphonate category (BF), molecules discovered at the end of the ‘60s and structural analogues of inorganic pyrophosphate, a substance present in body fluids that prevents soft tissue calcification and plays a regulatory role in bone mineralization processes. BFs take their name after the presence of two carbon-phosphorus bonds and are currently used as first-choice drugs in the treatment of various bone metabolism diseases, such as osteoporosis, Paget’s disease, bone metastases, etc. (1). These drugs are powerful inhibitors of bone resorption, which is the mechanism at the basis of costant skeletal tissue turnover, known as remodeling. Bisphosphonate classic pharmacological effects are the result of their affinity for hydroxyapatite crystals at bone mineral matrix level and also of their inhibiting effects on osteoclast activity. In terms of antiresorptive ability, risedronate is characterized by a relative potency 2000 times higher than etidronate, which represents the reference bisphosphonate (Figure 1): compared to other BFs, only zoledronic acid, which is solely administered by venous infusion, has a higher biological relative potency. It has been demonstrated that bond ability at the matrix differs among various BFs and affect drug distribution at bone level, their potency and duration of action (2). The antiresorptive effect of heterocyclic amino BFs and therefore of risedronate is linked to the ability of inhibiting enzyme FPPS at osteoclastic level, key element of mevalonate cascade that leads to isoprenoid lipids production, used for post-translational modification of small GTP-binding proteins essential to osteoclast function.
Figure 1.
Molecular structure and biological potency of bisphosphonates (modified from Drake MT, 2008).
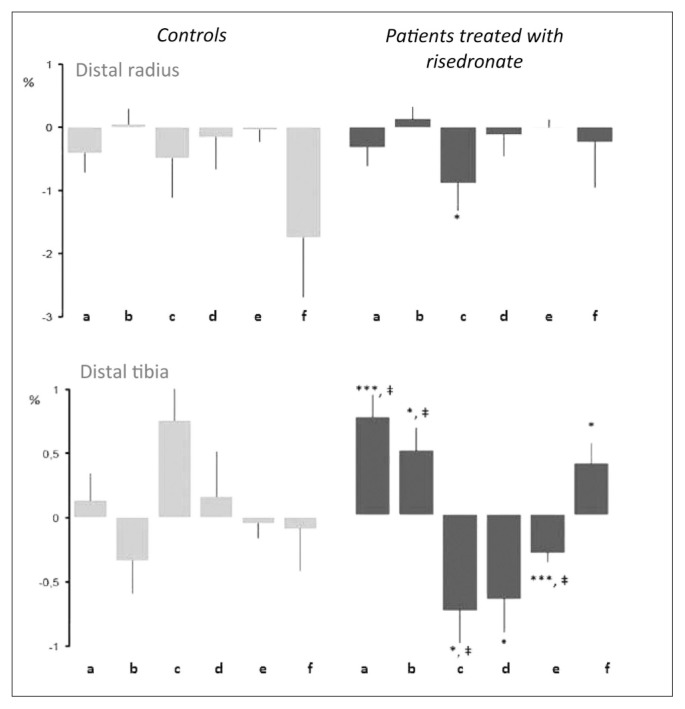
As for other similar compounds, risedronate inhibits selectively bone resorption induced by osteoclasts (3). After oral administration, around 50% of absorbed share (it’s a very low percentage because of poor bioavailability) sets on bones, where it stays for several weeks, whereas the rest is removed with urine within 24 hours and is intact because risedronate, as all bisphosphonates, is not metabolized. Risedronate presents a profile with great potential for inhibition of enzyme FPPS and moderate binding affinity for bone: among molecules, risedronate is one of strongest FPPS inhibitors, along with zoledronic acid (1, 2). On the contrary, as for the ability of interaction with hydroxyapatite crystals at mineral matrix level, risedronate has a significantly lower binding affinity compared to zoledronic acid and alendronate. Available information on binding ability between hydroxyapatite and risedronate, compared to other BFs suggest that also at saturation, risedronate attaches to hydroxyapatite surface to a lesser extent than alendronate, ibandrobate and zoledronic acid (1). This diminished binding activity gives risedronate the ability to best respect bone tissue quality, without interfering with antiresorptive activity (4). Risedronate is indeed effective with every kind of fracture, even though it reduces bone turnover to a lesser extent compared to other BFs. This apparent paradox is another example of the complex relationship between bone turnover reduction and anti-fracture efficacy. Risedronate effects, especially in terms of non-vertebral fractures, can be explained by the fact that less affinity with mineral matrix enables the compound to have a wider distribution inside bones and a better achievement of every area, including cortical region and osteocyte net (5). Recent researches give osteocytes a fundamental role in the regulation of metabolic turnover mechanism. Discover of osteocytes controlling bone formation through sclerostin secretion allowed to state that osteocytes can actually stimulate cellular precursors in bone marrow. In addition, osteocytes can stimulate osteoclast recruitment and activity through the activation of RANKL system. This complex mechanism happens thanks to the presence of a thick lacunar-canicular net, characteristic of osteocytes, which is predominantly set by mechanical loading (6). Rise-dronate strong inhibiting action in cellular sites is at the basis of its rapid anti-fracture action (2). Another important consideration in terms of reduction of remodeling activity comes from recent observations, showing that the drug can decrease resorption entity, not only of the trabecular bone, but also of the cortical one, modifying therefore compact bone thickness, which is largely responsible of femoral fracture specifically among elderly patients (7). In particular, rise-dronate has proven to be able to positively modify, in women over 55, total and cortical volumetric BMD at distal radius and distal tibia level, reducing at the same time specific cortical porosity indexes (cortical porosity, internal and external transitional zone porosity) (Figure 2). In this perspective, using three-dimensional microCT, it is possible to show through bone biopsies in women who suffer from postmenopausal osteoporosis that risedronate can decrease number and dimensions of intracortical cavities, reducing therefore bone remodeling and consequently non-vertebral fracture risk, thanks to bone resistance maintenance (8).
Figure 2.
Microstructural effects of risedronate in osteoporotic women aged over 55.
a: total volumetric BMD; b: cortical volumetric BMD; c: total cortical porosity; d: external cortical porosity; e: internal cortical porosity; f: volumetric trabecular BMD (modified from Bala Y, 2014).
Clinical efficacy
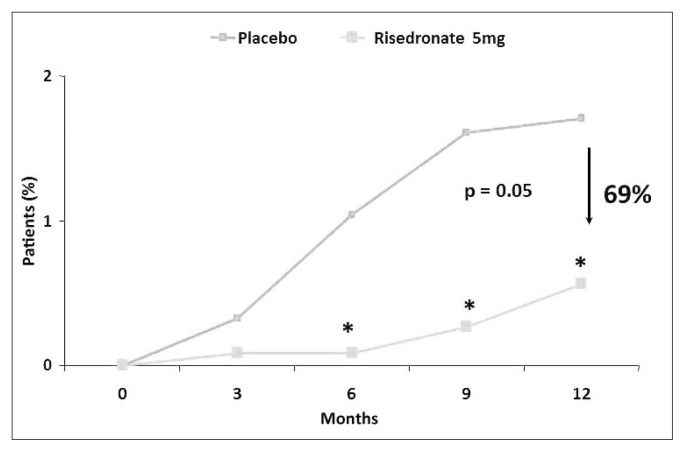
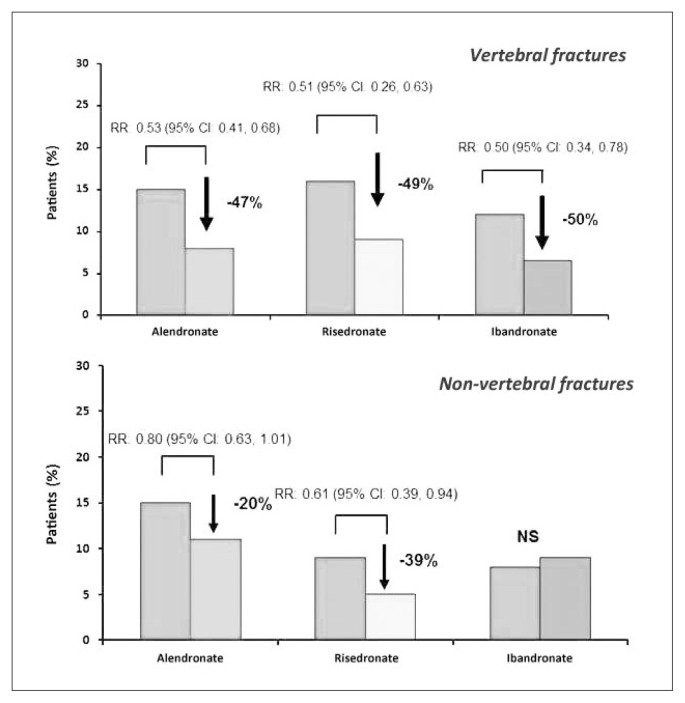
In postmenopausal osteoporosis, many random controlled and double-blind studies have proven risedronate ability to increase bone mineral density (BMD). In a trial conducted on 111 non- osteoporotic women in early menopause (average age: 51), risedronate (5 mg/die) increased after two years bone vertebral density of 1.4% and 2.6% femoral trochanteris level, against a diminution in both sites observed in placebo group. After one year of treatment suspension, vertebral bone density was 2.3% lower compared to baseline in women who had previously taken risedronate, against a 5.6% diminution in those treated with placebo (9). At a 30 mg weekly dosage in women with osteoporosis or osteopenia, risedronate has shown an increase of 5.7% and 2.9% respectively at lumbar and femoral level, after 12 months (10). In a further study conducted on 543 postmenopausal patients with bone vertebral density of 2 DS below average and treated with risedronate (2.5 or 5 mg/day) or with placebo, after 2 years vertebral bone mass rised by 4% compared to initial levels in women treated with 5 mg/day of risedronate and by 1.4% in those treated with 2.5 mg/day, against no registered variation in placebo group (11). It is known that the main endpoint in the study of drugs used in osteoporosis treatment is the ability of reducing fragility fracture risk significantly. For this reason, specific random and double-blind studies have been conducted, aiming to specifically assess risedronate efficacy in decreasing osteoporotic fracture incidence, compared to placebo. In a multicenter trial, 939 postmenopausal women (average age: 69) with reduced bone density (−2 DS below average) and a past vertebral fracture completed the three-year risedronate treatment (5mg/day): at the end, cumulative incidence of new vertebral fractures was of 11.3% in patients treated with risedronate, compared to 16.3% in those assigned to placebo group, with a reduction of relative risk of 41%, whereas non-vertebral fracture incidence was respectively of 5.2% against 8.4% and a reduction of relative risk of 39% (12). It is to report that precocity of anti-fracture effect of risedronate in this trial, with a reduction of relative risk for vertebral fractures of 65%, after 12 months of treatment. An analogue study conducted in Europe and Australia on 1226 patients suffering from full-blown osteoporosis confirmed risedronate efficacy, showing a significant reduction of relative risk of 49% for new vertebral fractures (13). In addition, a subsequent sub-analysis of this study confirmed precocity of anti-fracture effect of risedronate, with a statistically significant difference already after 6 months, compared to placebo group, proving a reduction of relative risk for vertebral fractures of 69% after 12 months (Figure 3) (14). Data confirmed also in non-vertebral fractures, where, compared to statistically significant difference already after 6 months, incidence of non-vertebral fractures decreased of 74% in the group of patients on risedronate treatment (15). In both trials, risedronate was administered along with calcium and vitamin D just like placebo and proved to be able to increase mineral density at lumbar and femoral level significantly, and at the same time to reduce bone resorption markers (12, 13). It emerges from a comparative analysis of random controlled studies conducted on bisphosphonates oral administered for osteoporosis therapy that both risedronate and alendronate can reduce incidence of vertebral, femoral and non-vertebral fractures significantly, in contrast to what happens for ibandrobate, whose clinical anti-fracture efficacy emerges solely for vertebral fractures (16). As shown in Figure 4, risedronate can significantly decrease relative risk (RR) for vertebral and non-vertebral fractures (−49% and −39% respectively); the same thing happens for alendronate as well, even if in smaller numbers (−47% and −20%), whereas it doesn’t happen for ibandrobate (17).
Figure 3.
Precocity of anti-fracture effect of risedronate (6 months) expressed as relative risk (RR) reduction (modified from Roux C, 2004).
Figure 4.
Reduction of relative risk (RR) of vertebral fracture (up) and non-vertebral (down) in women with postmenopausal osteoporosis after three years of therapy with bisphosphonates, oral administration (modified from Boonen S, 2007).
As for long-term efficacy and tolerability, in a first study designed like an extention of a previous trial lasted 3 years, it was possible to observe in 220 women that the risk of new vertebral fractures decreased even more (−59%) during 4th and 5th year in randomized group with risedronate treatment (18): furthermore, increase of vertebral and femoral bone mineral density and reduction of bone turnover markers observed at 3rd year were confirmed at 5th year. Another study conducted for 7 years confirmed long-term anti-fracture efficacy of the drug, showing that annual incidence of new vertebral fractures in patients treated with risedronate does not statistically differ from previous period, in conjunction with an excellent profile of tolerability (19). It is appropriate to notice that, despite extended periods of use, residronate did not induce to significant modifications of parameters linked to bone tissue quality. In a first histomorphometric analysis conducted after 3 years in fact, along with a significant reduction of remodeling indexes, no difference emerged compared to control group as for structural parameters, such as BV/TV, Ct.Th, Ct.Por, Tbn, Tb.Sp, Tb.Th (20). A further study conducted after 5 years of treatment using micro-CT allowed to appreciate bone volume and trabecular architecture maintenance, in conjunction with mineralization degree maintenance (21).
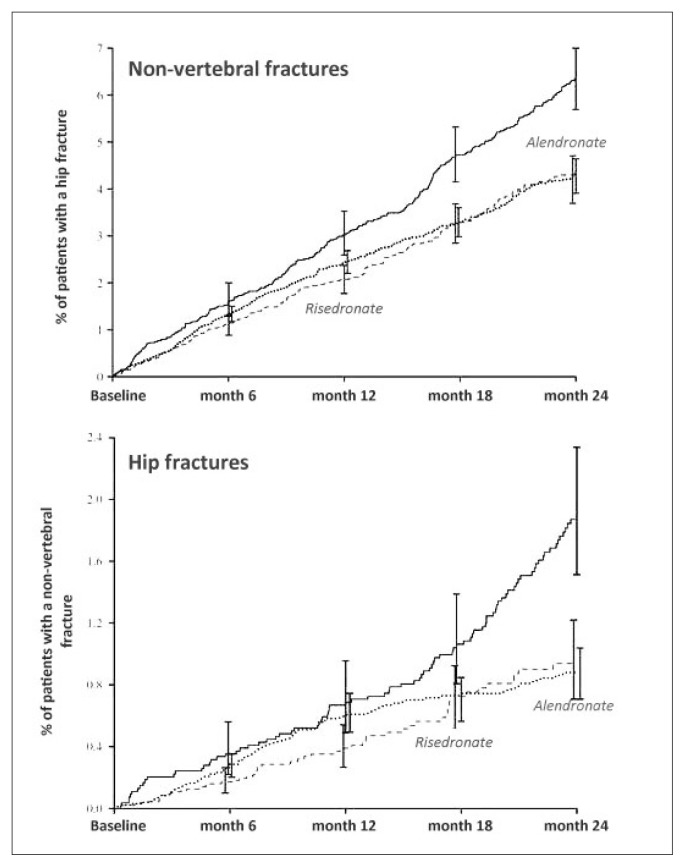
In a post-hoc analysis, in which data from three-year VERT studies were compared to results obtained from residronate dose of 35 mg weekly, risk of new vertebral fractures decreased after 1 year of 71% compared to placebo, by just more of what observed in the two VERT studies (61–65%) (22). In order to validate efficacy and tolerability of risedronate weekly administrated, a study conducted on a total of 1,456 women aged = / > 50, in postmenopause for 5 years, with reduced bone mineral density (lumbar or femoral T-score < / = 2.5 or T-score < 2 and at least one vertebral fracture), comparing 3 dosing schedules (risedronate 35 mg weekly, 50 mg weekly, 5 mg/day in conjunction with calcium and vitamin D) underlined that three dosing schedules showed comparable densitometric efficacy after one year of treatment, without detecting significant differences about incidence of side-effects (23). As for oral monthly administration in order to optimize adherence to the treatment, a comparative study between 150 mg monthly vs 5 mg/day was conducted: after one year, increase of two formulas was substantially similar (+3.5% vs +3.4%), underlining efficacy of monthly formula through statistical analysis of non-inferiority (24). Results from spontaneous observational studies are very interesting. In a retrospective study conducted on more than 33,000 patients aged over 65 aiming to analyze incidence of non-vertebral and femoral fractures specifically after 12 months of risedronate and alendronate treatment, has proven that cohort of patients treated with risedronate had a marked and precocious reduction of non-vertebral fracture incidence (19% and 18% after 6 and 12 months, respectively) compared to the group in therapy with alendronate: in particular for femoral fractures, reduction already reached 46% after 6 months and it remained so even at the end of observation period (43% after 12 months) (25). More recently, another observational study, lasted 2 years and conducted on osteoporotic women aged over 65, compared weekly treatment of risedronate and alendronate and could show that both treatments led to a reduction of incidence of femoral and non-vertebral fractures after 24 months, respectively of 45% and 30% (26). Data analysis after 6 and 12 months allowed nevertheless to point out that risedronate has a more premature ability of reducing incidence of both femoral and non-vertebral fractures (Figure 5).
Figure 5.
Cumulative curves concerning incidence of non-vertebral (up) and hip (down) fractures in patients suffering from osteoporosis and in treatment with risedronate (dashed line) or alendronate (dotted line) compared to a control group (modified from Lindsay R, 2013).
Data published in relation to osteoporosis management in elderly population are very interesting. A recent publication on this point underlined the importance of nutritional factors, in particular of calcium, vitamin D and proteins in preventing falls and it specifically identifies as major cause of inadequate prescribed medications, the perception that a long-term therapy is necessary in order to get an effective answer in terms of reduction of fracture risk. It is remarked how in the age group over 80, data are largely available, indicating that efficacy and safety of osteoporotic drugs are essentially excellent, identifying in poor adherence the main factor of a successful treatment (27). As for risedronate efficacy specifically, a post-hoc analysis of data obtained from pivotal studies allowed to appreciate the fact that after one and three years incidence of new vertebral fractures was of 2.5% and 10.9% in risedronate group and of 18.2% and 24.6% in control group, with a reduction of relative risk of 81% and 44% (28). Beside this important fact, it means that after just one year of treatment with risedronate, risk of new vertebral fractures in elderly people reduced of 81%; authors point out that NNT (Number Needed to Treat) is just 12 after 12 months, and it turns to 16 at the end of the three-year period. NNT indicates number of patients to treat in order to reduce of one unit a pathological event and therefore it indirectly evaluates theraupetic ability of drugs. As for risedronate, results of the study underline that it is necessary to treat only 12 patients during one year to get a reduction of a new vertebral fracture event, emphasizing efficacy and precocity of effect of the drug.
Another controlled study, specifically aimed at evaluate risedronate efficacy in elderly population, was conducted on a wide cohort of patients (5,445 women with full-blown osteoporosis, aged between 70 and 79). Results obtained after 3 years of treatment proved that incidence of new femoral fractures was of 1.9% in patients who had taken risedronate (2.5 mg/day or 5 mg/day) against 3.2% in those treated with placebo, with a reduction of fracture risk of 41% and a statistically significant difference (29). In addition, always in the same study, in a population of patients aged over 80 selected on a basis of a risk factor unlinked to bone mineral density (history of a recent fall, for example), incidence of femoral fracture was lower in the group treated with risedronate, compared to placebo group (4.2% vs 5.1%).
We have already discussed that from a pathophysiologic point of view recent studies have shown that risedronate slows microstructural deterioration at remodeling unit level (BMU) in women right after menopause, whereas in elderly women it acts so that it reduces BMU number (7, 8). Well, these histomorphometric data represent the rational basis of drug clinical efficacy: risedronate ability of reducing the number of intracortical cavities and therefore porosity in patients suffering from involutional osteoporosis ensures a better resistance and a reduction of fracture risk in elderly people, always respecting bone quality.
Osteoporosis therapy provides not only usage of antiresorptive drugs such as bisphosphonates, but also drugs characterized by a stimulating action on osteoblastic component and therefore on neoplasm, like teriparatide. In many occasions it is appropriate to rotate these two treatments, with the aim of optimize the effect on fracture risk. It has been proven that anabolic answer to teriparatide can be attenuated or delayed in patients previously treated with alendronate, able to markedly affect resorption mechanisms. A study, which was designed with the aim of monitoring response after a previous antiresorptive treatment, showed a more precocious answer of neoplasm markers after only 3 months in group of pre-treatment with risedronate, compared to the one with alendronate, with a significant increase of lumbar BMD after 12 months (5.1% vs 3.6%, p < 0.05) and of trabecular spine content with QCT (24.1% vs 13.7%, p < 0.05) (30, 31).
Male osteoporosis is a primitive form of multifactorial pathogenesis disease, in which increase of fracture risk is linked to an acceleration of osteoclast activity and to a lesser extent to a reduced osteoblast formation activity. Risedronate has proven to be effective even in this variety of osteoporosis. In a group of 316 men suffering from primitive and secondary osteoporosis, the drug produced an increase of lumbar and femoral BMD of 6.6 vs 2.2% and 4.4 vs 0.4% respectively after 2 years, compared to control group (32), with a reduction of fracture risk of 61% for new vertebral fractures and of 47% for non-vertebral fractures. In a study lasted 2 years, lumbar BMD increased after 12 and 24 months of 4.6% and 4.5% respectively, compared to placebo and in conjunction with a significant diminution of metabolic turnover indexes (33). In a following study, conducted as randomized and double-blind for the first two years and the next two as an open-label, risedronate at 35mg weekly dose promoted a lumbar BMD increase of 7.87% in the group treated for 4 years, and of 6.25% in the group that had placebo for the first 2 years and risedronate only in the following two (34). In both trials drug tolerability was excellent, with a full overlap of side-effects between study group and control group. Data from usage of risedronate in secondary male osteoporosis are interesting (35): positive results in terms of BMD increases were found in patients suffering from secondary osteoporosis to stroke, hyperthyroidism, inflammatory bowel diseases and post-transplant.
Steroid osteoporosis is a secondary variety of osteoporosis, of complex pathogenesis, in which predominates an increase of bone resorption processes, emphasis of bone mineralization and increase of fracture risk. Two clinical, controlled with placebo, randomized and double-blind trials, lasting one year, proved efficacy of risedronate (2.5 mg/day or 5 mg/day) in prevent loss of vertebral bone mass induced by corticosteroid therapy at high dose (>/= 7.5 mg/day of prednisone or equivalent compounds) for protracted periods (> 3 months) in 514 patients, both men and women (36–38). A third study, lasted 2 years and conducted on 120 women in post-menopause with rheumatoid arthritis, who took prednisolone at doses above 2.5 mg/day, confirmed risedronate ability of preserving bone mineral density in patients in protracted corticosteroid treatment (39). Furthermore, risedronate induced positive effects also in men with steroid osteoporosis.
As for skeletal involvement for malignant disorders, a multi-center, randomized, open-label study in fase II/III evaluated potential advantage of adding risedronate to docetaxel in patients with metastatic prostatic neoplasia, resistant to surgical castration. This study proved that adding risedronate to docetaxel in these patients, even if well tolerated, did not have any effect on disease progression, PSA levels, response to pain and survival (40). On the contrary, LHRH-agonist treatment for advanced prostate tumor produces an increase of bone turnover and a fast bone loss within the first 6 months of therapy, and it can be prevented thanks to weekly treatment with risedronate (41). In women in postmenopause at risk of fragility fracture in adjuvant treatment with aromatase inhibitors, such as anastrozole for breast neoplasia with receptors for estrogen receptor positive, addition of therapy with risedronate 35 mg/weekly induce a significant bone mass increase, both at spinal level and femoral one up to 24 months (42).
Risedronate is in general well tolerated. The main side effects concern the gastrointestinal tract: dyspepsia, abdominal pain, gastritis and oesophagitis. Also reported: headache, musculoskeletal pain, rarely glossitis, edemas, skin rashes. During clinical studies conducted on patients suffering from Paget’s disease, treated with risedronate 30 mg/day, three cases of iritis were reported. In some patients treated with risedronate a slight and transient diminution of serum levels of calcium and phosphate was observed: rare cases of abnormality in hepatic cytolysis tests were reported. Use of risedronate is however contraindicated in patients with hypocalcaemia, known hypersensitivity to a product component, inability of standing or sitting upright for at least 30 minutes, oesophagus disorders that lead to delayed oesophageal emptying, such as stenosis or achalasia, renal creatinine clearance < 30 ml/minute (43). As for risedronate, symptoms of “acute phase-like” reaction generally appear within 5 days from first dose intake. In comparative studies with risedronate 75 mg, 2 tablets monthly vs risedronate 5 mg/day, incidence of acute phase reactions was 7.6% of patients, compared to 3.6% respectively and duration of symptoms was 7 days maximum (34, 43). In clinical controlled studies conducted on women in post-menopause with osteoporosis, incidence of adverse events was similar to the group treated with risedronate, compared to placebo. In a combined analysis of 9 clinical studies, in which risedronate was once daily administered, incidence of adverse events of upper gastrointestinal tract was similar to patients randomized with risedronate and those treated with placebo; no significant difference emerged between risedronate 5 mg once daily and risedronate 35 mg once weekly as for adverse events of upper gastrointestinal tract (18, 19, 43). In recent years, report in literature of possible association between bisphosphonates and osteonecrosis of the jaw (ONJ) raised interest. Although many publications on BFs and ONJ have been produced, there are no prospective studies that prove that bisphosphonates substantially help pathogenesis of this condition. Even if some authors suggested that oral bisphosphonates can cause ONJ in patients with osteoporosis, data are very conflicting to date. In fact, recent meta-analysis indicate BFs use for osteoporosis is associated to ONJ incidence, lower than 1 on 100,000 patients every year of exposure (44). Another adverse event that has been linked to extended treatment with bisphosphonates is the occurrence of atypical diaphyseal subtrochanteric fractures. However, there are no sure facts about a possible link between use of risedronate and occurrence of subtrochanteric fractures (45). Efficacy and safety of risedronate in patients with renal impairment have been evaluated in a retrospective analysis that used data from 9 clinical trials (43). Incidence of vertebral fractures was significantly lower in patients treated with risedronate, compared to placebo group, for all sub-groups of renal impairment (mild, moderate, severe). Incidence of vertebral fractures in patients treated with risedronate was similar among various groups of renal impairment, whereas in patients treated with placebo, incidence of fracture increased along with the degree of renal dysfunction. Frequency of adverse events and renal adverse events linked to organ function was similar in placebo group and risedronate (5 mg/day) independently of blood creatinine values. A more recent retrospective analysis evaluated influence of different dosages (5 and 15 mg daily, 35 and 50 mg weekly, 75 mg 2 tablets monthly) of risedronate on renal function in patients with or without baseline renal impairment or risk factors without observing significant differences among groups (46).
References
- 1.Russell RGG, Watts NB, Ebetino FH, Rogers MJ. Mechanisms of action of bisphosphonates: similarities and differences and their potential influence on clinical efficacy. Osteoporos Int. 2008;19:733–759. doi: 10.1007/s00198-007-0540-8. [DOI] [PubMed] [Google Scholar]
- 2.Rogers MJ, Crockett J, Coxon F, Monkkonen J. Biochemical and molecular mechanism of action of bisphosphonates. 2011;49:34–41. doi: 10.1016/j.bone.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 3.Crandall C. Risedronate. A clinical review. Arch Intern Med. 2001;161:353–60. doi: 10.1001/archinte.161.3.353. [DOI] [PubMed] [Google Scholar]
- 4.Dufresne TE, Chmielewski PA, Manhart MD, Johnson TD, Borah B. Risedronate preserves bone architecture in early postmenopausal women in 1 year as measured by three-dimensional microcomputed tomography. Calcif Tissue Int. 2003;73:423–432. doi: 10.1007/s00223-002-2104-4. [DOI] [PubMed] [Google Scholar]
- 5.Papapoulos S. Primer on the metabolic bone diseases and disorders of mineral metabolism. Eight Edition. 2013. Bisphosphonates for postmenopausal osteoporosis; pp. 412–419. [Google Scholar]
- 6.Xiong J, O’Brien C. Osteocyte RANKL: new insights into the control of bone remodeling. J Bone Min Res. 2012;27:499–505. doi: 10.1002/jbmr.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bala Y, Chapurlat R, Cheung AM, Felsenberg D, LaRoche M, Morris E, Reeve J, Thomas T, Zanchetta J, Bock O, Ghasem-Zadeh A, Zebaze Djoumessi RM, Seeman E, Rizzoli R. Risedronate slows or partly reverses cortical and trabecular microarchitectural deterioration in postmenopausal women. J Bone Min Res. 2014;29(2):380–388. doi: 10.1002/jbmr.2101. [DOI] [PubMed] [Google Scholar]
- 8.Borah B, Dufresne T, Nurre J, Phipps R, Chmielewski P, Wagner L, Lundy M, Bouxsein M, Zebaze R, Seeman E. Risedronate reduces intracortical porosity in women with osteoporosis. J Bone Min Res. 2010;25:41–47. doi: 10.1359/jbmr.090711. [DOI] [PubMed] [Google Scholar]
- 9.Mortensen L, Charles P, Bekker PJ, Digennaro J, Johnston CC., Jr Risedronate increases bone mass in an early postmenopausal population: two years of treatment plus one year of follow-up. J Clin Endocrinol Metab. 1998;83:396–402. doi: 10.1210/jcem.83.2.4586. [DOI] [PubMed] [Google Scholar]
- 10.Gordon MS, Gordon MB. Response of bone mineral density to once-weekly administration of risedronate. Endocr Pract. 2002;8:202–207. doi: 10.4158/EP.8.3.202. [DOI] [PubMed] [Google Scholar]
- 11.Fogelman I, Ribot C, Smith R, Ethgen D, Sod E, Reginster JY. Risedronate reverses bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind placebo-controlled trial. J Clin Endocrinol Metab. 2000;85:1895–1900. doi: 10.1210/jcem.85.5.6603. [DOI] [PubMed] [Google Scholar]
- 12.Harris ST, Watts NB, Genant HK, McKeever CD, Hangartner T, Keller M, Chesnut CH, 3rd, Brown J, Eriksen EF, Hoseyni MS, Axelrod DW, Miller PD for the Vertebral Efficacy With Risedronate Therapy (VERT) Study Group (VERT) Study Group. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis. JAMA. 1999;282:1344–52. doi: 10.1001/jama.282.14.1344. [DOI] [PubMed] [Google Scholar]
- 13.Reginster I, Minne HW, Sorensen OH, Hooper M, Roux C, Brandi ML, Lund B, Ethgen D, Pack S, Roumagnac I, Eastell R. Randomized trial of the effects of risedronate on vertebral fractures in women with established postmenopausal osteoporosis. Vertebral Efficacy with Risedronate Therapy (VERT) Study Group. Osteoporos Int. 2000;11:83–91. doi: 10.1007/s001980050010. [DOI] [PubMed] [Google Scholar]
- 14.Roux C, Seeman E, Eastell R, Adachi J, Jackson RD, Felsenberg D, Songcharoen S, Rizzoli R, Di Munno O, Horlait S, Valent D, Watts NB. Efficacy of risedronate on clinical vertebral fractures within 6 months. Curr Med Res Opin. 2004;20:433–439. doi: 10.1185/030079903125003125. [DOI] [PubMed] [Google Scholar]
- 15.Harrington J, Ste-Marie L, Brandi ML, Civitelli R, Fardellone P, Grauer A, Barton I, Boonen S. Risedronate Rapidly Reduces the Risk for Nonvertebral Fractures in Women with Postmenopausal Osteoporosis. Calcif Tissue Int. 2004;74:129–135. doi: 10.1007/s00223-003-0042-4. [DOI] [PubMed] [Google Scholar]
- 16.Boonen S. Bisphosphonate efficacy and clinical trials for postmenopausal osteoporosis: similarities and differences. Bone. 2007;40:S26–S31. [Google Scholar]
- 17.Chesnut C, III, Skag A, Christiansen C, Recker R, Stakkestad JA, Hoiseth A, et al. Oral Ibandronate Osteoporosis Vertebral Fracture Trial in North America and Europe (BONE) Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19:1241–9. doi: 10.1359/JBMR.040325. [DOI] [PubMed] [Google Scholar]
- 18.Sorensen O, Crawford G, Mulder H, Hosking D, Gennari C, Mellstrom D, Pack S, Wenderoth D, Cooper C, Reginster J-Y. Long-term efficacy of risedronate: a 5-year placebo-controlled clinical experience. Bone. 2003;32:120–126. doi: 10.1016/s8756-3282(02)00946-8. [DOI] [PubMed] [Google Scholar]
- 19.Mellstrom D, Sorensen O, Goemaere S, Roux C, Johnson T, Chines A. Seven years of treatment with residronate in women with postmenopausal osteoporosis. Calcif Tissue Int. 2004;75:462–468. doi: 10.1007/s00223-004-0286-7. [DOI] [PubMed] [Google Scholar]
- 20.Eriksen E, Melsen F, Sod E, Barton I, Arton C, Chines A. Effects of long-term risedronate on bone quality and bone turnover in women with post-menopausal osteoporosis. Bone. 2002;31:620–625. doi: 10.1016/s8756-3282(02)00869-4. [DOI] [PubMed] [Google Scholar]
- 21.Borah B, Dufresne TE, Ritman EL, Jorgensen SM, Liu S, Chmielewski PA, Phipps RJ, Zhou Xiaojie, Sibonga JD, Turner RT. Long-term risedronate treatment normalizes mineralization and continues to preserve trabecular architecture: Sequential triple biopsy studies with micro-computed tomography. Bone. 2006;39:345–352. doi: 10.1016/j.bone.2006.01.161. [DOI] [PubMed] [Google Scholar]
- 22.Watts NB, Lindsay R, Li Z, Kasibhatla C, Brown J. Use of matched historical controls to evaluate the anti-fracture efficacy of once-a-week risedronate. Osteoporos Int. 2003;14:437–441. doi: 10.1007/s00198-003-1401-8. [DOI] [PubMed] [Google Scholar]
- 23.Harris S, Watts N, Li Z, Chines AA, Hanley D, Brown J. Two-year efficacy and tolerability of risedronate once a week for the treatment of women with postmenopausal osteoporosis. Curr Med Res Opin. 2004 May;20(5):757–64. doi: 10.1185/030079904125003566. [DOI] [PubMed] [Google Scholar]
- 24.Delmas PD, McClung MR, Zanchetta JR, Racewicz A, Roux C, Benhamou C, Man Z, Eusebio RA, Beary JF, Burgio DE, Matzkin E, Boonen S. Efficacy and safety of risedronate 150 mg once a month in the treatment of post-menopausal osteoporosis. Bone. 2008;42:36–42. doi: 10.1016/j.bone.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 25.Silverman SL, Watts NB, Delmas PD, Lange JL, Lindsay R. Effectiveness of bisphosphonates on nonvertebral and hip fractures in the first year of therapy: The risedronate and alendronate (REAL) cohort study. Osteoporos Int. 2007;18:25–34. doi: 10.1007/s00198-006-0274-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindsay R, Watts NB, Lange JL, Delmas PD, Silverman SL. Effectiveness of risedronate and alendronate on nonvertebral fractures: an observational study through 2 years of therapy. Osteoporos Int. 2013;24:2345–2352. doi: 10.1007/s00198-013-2332-7. [DOI] [PubMed] [Google Scholar]
- 27.Rizzoli R, Branco J, Brandi ML, Boonen S, Bruyere O, et al. Management of osteoporosis of the oldest old. Osteoporosis Int. 2014 Jul 15; doi: 10.1007/s00198-014-2755-9. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 28.Boonen S, McClung MR, Eastell R, El-Hajj Fuleihan G, Barton IP, Delmas P. Safety and Efficacy of Risedronate in Reducing Fracture Risk in Osteoporotic Women Aged 80 and Older: Implications for the Use of Antiresorptive Agents in the Old and Oldest Old. JAGS. 2004;52:1832–1839. doi: 10.1111/j.1532-5415.2004.52506.x. [DOI] [PubMed] [Google Scholar]
- 29.McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, Adami S, Fogelman I, Diamond T, Eastell R, Meunier PJ, Reginster JY for the Hip Intervention Program Study Group. Effect of risedronate on the risk of hip fracture in elderly women. N Engl J Med. 2001;344:333–40. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 30.Delmas P, Watts N, Miller P, et al. Bone turnover markers demonstrate greater earlier responsiveness to teriparatide following treatment with risedronate compared with alendronate: the OPTAMISE study. J Bone Miner Res. 2007;22(Suppl 1):S27. [Google Scholar]
- 31.Miller PD, Delmas PD, Lindsay R, Watts NB, Luckey M, Adachi J, Saag K, Greenspan SL, Seeman E, Boonen S, Meeves S, Lang TF, Bilezikian JP. Early Responsiveness of Women with Osteoporosis to Teriparatide After Therapy with Alendronate or Risedronate. J Clin Endocrinol Metab. 2008;93(10):3785–3793. doi: 10.1210/jc.2008-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ringe J, Farahmand P. Sustained efficacy of residronate in men with primary and secondary osteoporosis: results of a 2-year study. Rheumatol Int. 2009;29:311–315. doi: 10.1007/s00296-008-0689-2. [DOI] [PubMed] [Google Scholar]
- 33.Boonen S, Orwoll ES, Wenderoth D, Stoner KJ, Eusebio R, Delmas PD. Once-Weekly Risedronate in Men With Osteoporosis: Results of a 2-Year, Placebo-Controlled, Double-Blind, Multicenter Study. J Bone Miner Res. 2009;24:719–725. doi: 10.1359/jbmr.081214. [DOI] [PubMed] [Google Scholar]
- 34.Boonen S, Lorenc RS, Wenderoth D, Stoner KJ, Eusebio R, Orwoll ES. Evidence for safety and efficacy of risedronate in men with osteoporosis over 4 years of treatment: Results from the 2-year, open-label, extension study of a 2-year, randomized, double-blind, placebo-controlled study. Bone. 2012;51:383–388. doi: 10.1016/j.bone.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 35.Bobba R, Adachi JD. Review of the safety and efficacy of risedronate for the treatment of male osteoporosis. Clinical Interventions in Aging. 2007;2(3):275–282. [PMC free article] [PubMed] [Google Scholar]
- 36.Reid DM, Hughes RA, Laan RF, Sacco-Gibson NA, Wenderoth DH, Adami S, Eusebio RA, Devogelaer JP. Efficacy and safety of daily risedronate in the treatment of corticosteroid-induced osteoporosis in men and women: a randomized trial. European Corticosteroid-Induced Osteoporosis Treatment Study. J Bone Miner Res. 2000;15:1006–13. doi: 10.1359/jbmr.2000.15.6.1006. [DOI] [PubMed] [Google Scholar]
- 37.Dougherty JA. Risedronate for the prevention and treatment of corticosteroid-induced osteoporosis. Ann Pharmacotherapy. 2002;36(3):512–6. doi: 10.1345/aph.1A114. [DOI] [PubMed] [Google Scholar]
- 38.Cohen S, Levy RM, Keller M, Boling E, Emkey RD, Greenwald M, Zizic TM, Wallach S, Sewell KL, Lukert BP, Axelrod DW, Chines AA. Risedronate therapy prevents corticosteroid-induced bone loss a twelve-month, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Arthritis & Rheumatism. 1999;42:2309–18. doi: 10.1002/1529-0131(199911)42:11<2309::AID-ANR8>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 39.Eastell R, Devogelaer JP, Peel NF, Chines AA, Bax DE, Sacco-Gibson N, Nagant de Deuxchaisnes C, Russell RG. Prevention of bone loss with risedronate in glucocorticoid-treated rheumatoid arthritis patients. Osteoporos Int. 2000;11:331–7. doi: 10.1007/s001980070122. [DOI] [PubMed] [Google Scholar]
- 40.Meulenbeld HJ, van Werkhoven ED, Coenen JLLM, Creemers GJ, Loosveld OJL, de Jong PC, ten Tije AJ, Fossa SD, Gerritsen W, Dalesio O, de Wit R, Polee M. Randomised phase II/III study of docetaxel with or without risedronate in patients with metastatic Castration Resistant Prostate Cancer (CRPC), the Netherlands Prostate Study (NePro) European Journal of Cancer. 2012;48:2993–3000. doi: 10.1016/j.ejca.2012.05.014. [DOI] [PubMed] [Google Scholar]
- 41.Taxel P, Dowsett R, Richter L, Fall P, Klepinger A, Albertsen P. Risedronate prevents early bone loss and increased bone turnover in the first 6 months of luteinizing hormone-releasing hormone-agonist therapy for prostate cancer. BJU International. 2010;106:1473–1476. doi: 10.1111/j.1464-410X.2010.09329.x. [DOI] [PubMed] [Google Scholar]
- 42.Van Poznak C, Hannon RA, Mackey JR, Campone M, Apffelstaedt JP, Clack G, Barlow D, Makris A, Eastell R. Prevention of Aromatase Inhibitor–Induced Bone Loss Using Risedronate: The SABRE Trial. J Clin Oncol. 2010;28:967–975. doi: 10.1200/JCO.2009.24.5902. [DOI] [PubMed] [Google Scholar]
- 43.Taggart H, Bolognese MA, Lindsay R, Ettinger MP, Mulder H, Josse RG, Roberts A, Zippel H, Adami S, Ernst TF, Stevens KP. Upper gastrointestinal tract safety of risedronate: a pooled analysis of 9 clinical trials. Mayo Clin Proc. 2002;77:262–270. doi: 10.4065/77.3.262. [DOI] [PubMed] [Google Scholar]
- 44.Lee SH, Chang SS, Lee M, Chan RC, Lee CC. Risk of osteonecrosis in patients taking bisphosphonates for prevention of osteoporosis: a systematic review and meta-analysis. Osteoporos Int. 2014 Mar;25(3):1131–9. doi: 10.1007/s00198-013-2575-3. [DOI] [PubMed] [Google Scholar]
- 45.Rizzoli R, Akesson K, Bouxsein M, Kanis JA, Napoli N, Papapoulos S, Reginster JY, Cooper C. Subtrochanteric fractures after long-term treatment with bisphosphonates: a European Society on Clinical and Economic Aspects of Osteoporosis and Osteoarthritis, and International Osteoporosis Foundation Working Group Report. Osteoporos Int. 2011 Feb;22(2):373–90. doi: 10.1007/s00198-010-1453-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pazianas M, Cooper C, Ebetino FH, Russell RG. Long-term treatment with bisphosphonates and their safety in postmenopausal osteoporosis. Therapeutics and Clinical Risk Management. 2010;6:325–343. doi: 10.2147/tcrm.s8054. [DOI] [PMC free article] [PubMed] [Google Scholar]