Summary
Sarcopenia is the age-associated loss of skeletal muscle mass and function. It is a major clinical problem for older people and research in understanding of pathogenesis, clinical consequences, management, and socioeconomic burden of this condition is growing exponentially. The causes of sarcopenia are multifactorial, including inflammation, insulin resistance, changing endocrine function, chronic diseases, nutritional deficiencies and low levels of physical activity. Operational definition of sarcopenia combines assessment of muscle mass, muscle strength and physical performance. The diagnosis of sarcopenia should be based on having a low appendicular fat free mass in combination with low handgrip strength or poor physical functioning. Imaging techniques used for estimating lean body mass are computed tomography, magnetic resonance imaging, bioelectrical impedance analysis and dual energy X-ray absorptiometry, the latter considered as the preferred method in research and clinical use.
Pharmacological interventions have shown limited efficacy in counteracting the age-related skeletal muscle wasting. Recent evidence suggests physical activity and exercise, in particular resistance training, as effective intervention strategies to slow down sarcopenia.
The Italian Society of Orthopaedics and Medicine (Or-toMed) provides this position paper to present the update on the role of exercise on sarcopenia in the elderly.
Keywords: physical exercise, sarcopenia, physical activity
Introduction
The term “sarcopenia” was coined by Irwin Rosenberg in 1989. He suggested, from the Greek, the terms sarcomalacia (‘softening of the flesh’) or sarcopenia (‘lack of flesh’), to describe the decline in lean body mass with age (1). The International Working Group on Sarcopenia in Older People (IWGS), in 2011, defined sarcopenia as “the age-associated loss of skeletal muscle mass and function” (2). In 2009, the European Working Group on Sarcopenia in Older People (EWGSOP) had already defined sarcopenia as a syndrome characterized by progressive and generalized loss of skeletal muscle mass and strength with a risk of adverse outcomes such as physical disability, poor quality of life and death (3).
The European Group proposed the coexistence of the two following factors to characterize sarcopenia: low skeletal muscle mass and low muscle function (strength or performance). Furthermore EWGSOP defined 3 clinical conditions: 1) pre-sarcopenia that is characterized by low muscle mass without impact on muscle strength or physical performance; 2) sarcopenia, characterized by low muscle mass, plus low muscle strength or low physical performance; 3) severe sarcopenia that is identified when all three criteria of the definition are met (low muscle mass, low muscle strength and low physical performance). Sarcopenia can be considered ‘primary’(or age-related) when no other cause is evident but ageing itself; ‘secondary’ when it is caused by specific medical conditions. Secondary sarcopenia can be further divided into: activity-related (bedridden, sedentary lifestyle, deconditioning or zero-gravity conditions), disease-related (advanced organ failure, inflammatory disease, malignancy or endocrine disease) and nutrition-related (inadequate dietary intake of energy and/or protein, malabsorption, gastrointestinal disorders or use of medications that cause anorexia) (3). Although cachexia may be a component of sarcopenia, the two conditions are not the same (2).
Several mechanisms might be involved in the onset and progression of sarcopenia. It was estimated that, after the age of 50, the reduction in muscle cross-sectional area (CSA) is ~1%/yr (4, 5), related to a decline in both muscle fiber size and number (6). Type I muscle fibers are generally recruited for most of the activities of daily living (ADLs) and during submaximal exercise (e.g. walking); type II muscle fibers are instead above all recruited during high-intensity activities. Lexell et al., examining the cross-sections (15 micron) of autopsied whole vastus lateralis muscle, showed the selective atrophy of type II fibers (with a preservation of type I fibers) with aging, probably because older people usually reduce their high intensity activities (5, 7). Within the muscle, there is a decrease in non-contractile area along with a decrease in myosin-actin cross-bridging. Single-fiber intrinsic force is decreased. There is a decline in the number of T-tubule di-hydropyridine receptors and an increase in uncoupled Ryanodine receptors. Twitch contraction time and maximum shortening speed are lower (2).
In a review of 2006, Narici suggested that, at whole muscle level, intrinsic force reduction is the result of the combined effect of changes in muscle architecture, tendon mechanical properties, neural drive (reduced agonist and increased antagonist muscle activity), and single fiber-specific tension. The alterations in both muscle architecture and tendon mechanical properties have also been shown to contribute to the loss of intrinsic muscle force with aging. Indeed, sarcopenia of the human plantarflexors, defined as a 25% reduction in muscle volume, was found to be associated with a 10% reduction in fiber fascicle length and 13% reduction in pennation angle. These architectural alterations were accompanied by a 10% decrease in tendon stiffness (8).
Kandarian et al. has shown that, at a molecular level, sarcopenia results from a disproportionate decrease in skeletal muscle protein synthesis and/or an increased protein breakdown. Several studies conducted on molecular pathways of muscle hypertrophy demonstrated that anabolic hormones and muscular activity drive the system through activation of the phosphatidyl inositol 3 kinase/serine-threonine kinase Akt system (PI3K/Akt) (9). This system stimulates muscle protein synthesis through the activation of the mammalian target of rapamycin (mTOR) and p70 S6 kinase (SGKI) and inhibits atrophy by phosphorylating the forkhead transcription factors FOXO. Phosphorylated FOXO is inactive, thus reducing the expression of the muscle-specific E3 ubiquitin ligases, Muscle Atrophy F-box (MAFbx/atrogin I) and muscle Ring finger-1 (MuRF-1), and subsequently preventing protein degradation by the ubiquitin-proteasome system. A greater expression of MuRF-1 and MAFbx/atrogin I has been observed in aged rodent muscles compared with young ones along with a 90% higher level of ubiquitin conjugates (10). Cytokines stimulate MuRF-1, which activates the ubiquitin-proteasome system (11). Skeletal muscle also contains a key autocrine signal to limit muscle growth: myostatin (MSTN), a member of the transforming growth factor β family, that has been shown to play a significant role in muscle growth as an inhibitor of hypertrophy. MSTN signaling is mediated through its receptor activin IIB (ACVR2B), which conducts a signal to the nucleus through the SMAD proteins, that activate downstream gene transcription (SMAD pathways) (12).
Epidemiology
A progressive loss of muscle mass occurs from approximately 50 years of age. This loss has been estimated at about 8% per decade until the age of 70 years, after which the loss increases to 15% per decade (13). This loss causes a 40% decrease in muscle circumference from 30 to 60 years of age. Goodpaster showed that the annual rates of leg strength decline is of 3.4% in white men and 2.6% in white women and it is about three times greater than the rates of loss of leg lean mass (approximately 1% per year). The loss of lean mass is independent from leg strength decline in both men and women, as well as the gain of lean mass seems not to be accompanied by strength maintenance or gain. A 10–15% loss of leg strength per decade is seen until 70 years of age, after which a faster loss, ranging from 25 to 40% by decade, occurs (14). Muscle mass loss is greater in men as compared to women (15). The presence of functional impairment in the elderly is associated with increased morbidity and mortality. It is estimated that approximately 14% of people aged 65–75 years require help in basic activities of daily living, a proportion that increases to 45% in people over 85 years of age (16). Healthcare costs attributable to sarcopenia in the United States (US) in 2000 were estimated at 18.5 billion dollars (17).
Definition
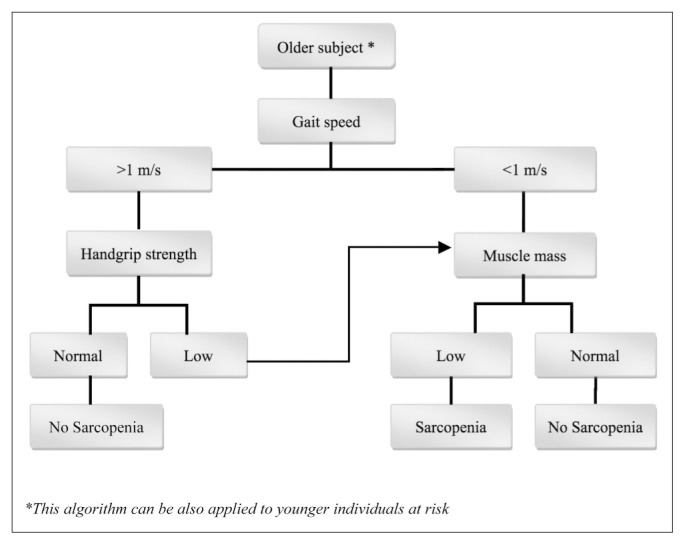
There is unanimous agreement that the presence of sarcopenia should be evaluated in older patients who present a decline in physical functioning, strength, or health status. Clinicians should also investigate sarcopenia in patients with difficulties in performing activities of daily living (ADLs), history of recurrent falls, recent weight loss, recent hospitalization, or suffering from chronic conditions associated with muscle loss (e.g., type 2 diabetes, chronic heart failure, chronic obstructive pulmonary disease, chronic kidney disease, rheumatoid arthritis and malignancies). Sarcopenia should be always considered in bedridden or nonambulatory patients, or in subjects unable to rise from a chair unassisted. In addition, for ambulatory patients and subjects that are able to rise from a chair without any help, the gait speed during a 4-meter walking should be assessed. Patients with a gait speed lower than 1 m/s should be referred for body composition assessment using total body less head dual energy x-ray absorptiometry (TBLH DXA). Sarcopenia can be diagnosed with TBLH DXA when the ratio appendicular fat-free mass to the square of height (Appendicular Skeletal Muscle mass Index = ASMMI) is <7.23 kg/m2 for males and <5.67 kg/m2 for females (18), which corresponds to the 20th percentile of the lean body mass of healthy young adults population (2).
Recommendations.
Sarcopenia is a universal phenomenon with a multifactorial etiology. The presence of sarcopenia should be investigated from 50 years of age. To differentiate primary and secondary forms of sarcopenia is necessary to get information about nutritional status (protein intake, energy intake and vitamin D status), level of physical activity, hormonal changes (declines in serum testosterone and growth hormone), insulin resistance and the presence of chronic inflammatory conditions (2).
Sarcopenia should be always evaluated in patients with declines in physical functioning, strength or health status.
Assessment techniques
Body imaging techniques
The most common imaging techniques for estimating muscle mass or lean body mass are: computed tomography (CT scan), magnetic resonance imaging (MRI) and dual energy X-ray absorptiometry (DXA). CT and MRI can effectively separate fat from other soft tissues, and therefore represent the gold standards for estimating muscle mass. High cost, limited access to equipment and concerns about radiation exposure limit the use for routine clinical practice. DXA is an attractive and reliable method both for research and clinical use, giving the chance to perform a valid assessment of lean body mass, with minimal radiation exposure (19).
Bioimpedance analysis
Bioimpedance analysis (BIA) estimates fat and lean body mass. The body composition estimation by BIA is inexpensive, readily reproducible and appropriate for both ambulatory and bedridden patients. BIA measurements under standard conditions have been found to well correlate with MRI evaluation. There are validated predictive values for adults, including older subjects (20). Jansenn published reference data for white men and women. Furthermore BIA is a good portable alternative to DXA (21).
Anthropometric features
There are relatively few studies validating anthropometric measures in older and obese people; several confounders make anthropometric measures vulnerable to error and questionable for individual use (22). Age-related changes in fat deposits and loss of skin elasticity contribute to errors of estimation in older people.
Handgrip strength
Muscle strength assessment correlates with handgrip strength. Isometric handgrip strength is strongly related with lower extremity muscle power, knee extension torque and calf cross-sectional muscle area and represents a clinical marker of poor mobility and a better predictor of clinical outcomes (23). There is also a strong relationship between baseline handgrip strength and limitations in the activities of daily living (24).
Leg extension
Knee extensor muscles strength can be measured with isometric or isokinetic manners, the latter being a closer reflection of muscle function in everyday activities. Isometric strength test can be done measuring the maximum resistance applied to the ankle when the subject is seated with the lower leg hanging down and the knee flexed to 90° (25). Modern isokinetic dynamometers allow both isometric and isokinetic strength evaluations as concentric torque at various angular velocities (26), although their use in clinical practice is limited by the need for special equipment and training.
Physical performance evaluation
Outcomes of physical function might be performed by the use of the Short Physical Performance Battery (SPPB) that is a simple test to measure lower extremity function using tasks that mimic daily activities. The SPPB examines three areas: static balance, gait speed and getting in and out of a chair (27). It has been recently recommended by an international working group its use as a functional outcome measure in clinical trials in frail older persons (28). Recently, Cesari et al. suggested the importance of gait speed as a predictor of adverse health events (severe mobility limitation, mortality) (29) and it can also be used as a single parameter for clinical practice and research (3).
Timed up-and-go test
The timed up-and-go (TUG) test measures the time that a person takes to rise from a chair, walk 3 meters, turn around, walk back to the chair and sit down. The TUG, used in geriatric assessment, is an useful performance measurement (3).
Recommendations.
CT and MRI are the most sensitive and specific methods for the measurement of muscle mass, but the high cost and the high dose of radiation, limit their use in clinical practice (3).
DXA is the recommended technique for estimating the lean body mass in clinical practice (3).
BIA can be used as an alternative to DXA for bedridden patients (3).
Anthropometric measurements are not recommended for diagnosis of sarcopenia (3).
Handgrip strength is a good, simple and reliable measure of muscle strength (3).
Knee extension strength is suitable for research studies (3).
The Short Physical Performance Battery is the recommended rating scale for the assessment of physical performance in clinical practice (3).
Gait speed and TUG can also be used as indicators of physical performance in clinical practice (3) (Figure 1).
Physical exercise
Physical activity
It is a common belief that physical activity (PA) can slow the loss of skeletal muscle mass and function. Raguso et al. (30) recently conducted a longitudinal study on body composition and PA in older adults concluding that leisure activities did not prevent the changes in body composition. In this study the average time spent in moderate to intense activities reported by the active subjects was 90 minutes a day, with approximately 70% reporting over 60 minutes a day. Examples of moderate-to intense activities, for Raguso et al., were walking up stairs, running, biking, playing tennis, skiing, and swimming. None of the subjects performed resistance training. Although this level of physical activity meets or exceeds the standards recommended by American Heart Association for cardiovascular exercise (31) it doesn’t seem to be sufficient to maintain lean mass or decrease fat mass. One of the explanations to this contradiction is the extremely overestimation of the self-reported PA. Mitchell et al. (32) also reported similar findings from a large cross-sectional study. They showed that lean body mass was not related to level of PA or dietary intake. Kent-Braun et al. reported that high levels of PA slow the loss of skeletal muscle oxidative capacity and sarcopenia (33). They suggest that decrease in skeletal muscle oxidative capacity is not a necessary consequence of aging, but it could be related to both quality and quantity of PA. These conflicting results are likely due to different study designs, activities, and challenges of self reported PA. It should be emphasized that few studies have included very older people (over 80 years) that have the highest prevalence of sarcopenia (34). There is a growing interest in research on the effectiveness of the resistance training on the improvement of strength and muscle function in elderly.
Aerobic exercise training
Among other impairments, aging is characterized by a progressive decline in aerobic exercise capacity (i.e., maximal oxygen consumption) related to, at organ or system level, the reduction of cardiovascular efficiency and, at a cellular level, to reduced quantity or quality of skeletal muscle mitochondria (35–38). It is well known that aerobic exercise induces an increase in skeletal muscle mitochondria and this is particularly true in aging muscle. Muscle mitochondrial adaptations to aerobic training appear to be the result of exercise-induced increases in the transcription of mitochondrial genes. Various signals, including Ca2+ and adenosine monophosphate (AMP), produced within working skeletal muscle during acute exercise, activate intracellular signaling pathways [e.g., calcium/calmodulin-dependent protein kinase (CaMK), AMP-activated protein kinase (AMPK)] leading to an increased transcription of target mitochondrial genes. Aerobic training causes the cumulative effect of these acute pulses in gene transcription resulting in the synthesis and incorporation of new mitochondrial proteins (38). The transcriptional co-activator peroxisome proliferator activated receptor gamma co-activator 1-α (PGC-1α) is a pivotal regulator of this process because of its ability to co-activate several transcription factors thus regulating mitochondrial biogenesis (39). Exercise induces transient transcriptional activation of the PGC-1α gene in skeletal muscle, preserving muscle quality and aerobic capacity in aging (40). Increased muscle mitochondrial content and/or improved mitochondrial function following aerobic exercise lead to improved metabolic control, resulting in reduced oxidative stress and optimized exercise capacity (41,42). Muscle-specific PGC-1α knockout mice, which display impaired mitochondrial biogenesis, experience accelerated sarcopenia with aging (43). On the contrary, mice that overexpress muscle-specific PGC-1α, have an increased muscle mitochondrial volume and are resistant to muscle atrophy induced by denervation and fasting (44). It is also known that aerobic exercise increases skeletal muscle insulin sensitivity, but the mechanisms underlying are not well understood yet (45). Holloszy suggests that this action depends on insulin signaling, increased muscle glucose transporters, and mitochondrial function (46). Insulin resistance is a common feature of aging (47) and may precipitate loss of skeletal muscle mass by reducing the physiological effects of insulin or insulin-like growth factors (IGFs). It has been postulated that the primary role of insulin in the regulation of muscle mass is to inhibit in a dose-dependent manner the muscle protein breakdown (48).
Resistance training
It is well established that traditional, slow-velocity resistance exercise (RE) (i.e., performing the concentric and eccentric phase of each muscle contraction in 2–3 sec) is a safe, feasible, and effective intervention to induce muscle hypertrophy and increase strength in older adults (49). RE seems to increase muscle protein synthesis (50), satellite cell activation and proliferation (51), anabolic hormone production and decrease in catabolic cytokine activity (52). RE has been shown to increase both type I and II muscle fiber cross-sectional areas and whole-body lean tissue mass in older adults leading to an increase in muscle strength (53). Candow et al. (54) showed that 22 weeks of whole-body RE training (3 days per week) in healthy older males (60–71 years) was sufficient to overcome the age-related deficits in whole-body lean tissue mass, regional muscle size, and upper and lower limbs strength. This RE training protocol restores these parameters to the level observed in untrained young males. There is common agreement that RE is effective to improve muscle function and the overall quality of life.
A meta-analysis by Peterson et al. (55) on 49 randomized controlled trials (RCTs) and non-randomized studies, including 1,328 adults over 50 years, concludes that RE is effective in eliciting gains in lean body mass among older people, particularly if they performed higher volume programs. Furthermore the authors suggest that older people should consider to start a resistance exercise program as early as possible to optimize its effectiveness.
A systematic review by Latham et al. (56) reported that most resistance training programs last 8–12 weeks, using 2–3 sets of 8–10 repetitions at 65% of 1-repetition maximum (1 RM), and are performed 2–3 days per week. This type of resistance training focuses on concentric muscle contraction and has lower influence on eccentric muscle strength. Most of the reviewed studies reported an increase in strength, but found limited changes in functional tests such as chair stand and timed up and go.
A recent Cochrane review included 121 trials with 6,700 participants assessing the effects of progressive resistance training (PRT) on physical function (57). In most trials, PRT was performed 2–3 times weekly at a high intensity. The PRT programs had a large positive effect on muscle strength and a small but significant improvement in physical ability. There was a modest improvement in gait speed, but a larger effect in getting out of a chair. Authors’ conclusions are that “PRT is an effective intervention for improving strength and physical functioning in older people, including functional performance of some simple and complex tasks”. They warn that adverse events were not sufficiently reported; thus, transferring these exercises to clinical populations should be approached with caution.
An intriguing hypothesis about the mechanism of action of RE on muscle is provided by Raue et al. (58) who evaluated the effects of a single bout of RE, a program of PRT for 12 weeks, and aging on human skeletal muscle transcriptome in young and older individuals. The transcriptome is the set of all RNA molecules, including mRNA, rRNA, tRNA, and other non-coding RNA produced in one cell or a population of cells. The authors generated a transcriptome signature of RE adaptations including 661 genes responsive to RE and correlated with the increase in whole muscle size and strength after PRT. The fiber type specific microarray analysis suggested that RE-induced gene response is specifically targeted to the fast-twitch muscle fibers and to skeletal muscle of young adults. Transcriptome data highlight how much potent an exercise stimulus is to the molecular milieu of skeletal muscle and provide further insight into the molecular basis of sarcopenia and the impact of RE at the fiber type specific level.
Power training
Until recently, research has focused on the impact of resistance training on muscular strength rather than power, which is the product of force and speed. The decline in muscular power is much steeper (3–4% per year after 60 years of age) than strength and results in a decreased ability to produce force rapidly (59). Muscle power is a significant predictor of performing activities of daily living (i.e., gardening, carrying groceries, climbing stairs) and is reduced with age at both slow and fast velocities (60).
Fast-velocity RE (i.e., performing the concentric phase as quickly as possible and taking 2 sec to perform the eccentric phase of each muscle contraction) appears to be a novel intervention for older adults to enhance muscle power (61). With the subsequent atrophy of type II fibers with aging, fast-velocity movements are important for preserving aging muscle health. Several studies have shown a significant increase in muscle power with fast velocity RE in older adults, because of greater motor unit recruitment of type II fibers (62–64). Therefore, fast-velocity contractions should be considered when designing RE training programs for older adults.
Henwood et al. reported data about power training, consisting of 3–4 sets at 20–80% of 1 RM, 2–3 times per week for 8–16 weeks, reporting significant improvement in strength, power, and global physical functioning (65, 66). A RCT comparing strength and power training reported similar improvements in muscle strength but larger improvements in muscle power (67). These data confirm the strong relationship in the physiological response to muscular strength and power exercise training. InChianti study have reported that physical function, particularly in the lower limbs, has a stronger relationship with muscular power than strength (68). In order to achieve and preserve muscular strength and power, resistance training needs to use a progressively increasing load to maintain the desired range of repetitions per set of exercise. The American College of Sports Medicine recently put forward a position statement on progressive resistance training in healthy adults (69). They recommended a 2–10% increase in load when the individual can perform the current workload for 1–2 repetitions over the desired number. They also recommended that progression in power training using 2 loading stages: the first stage is strength training and the second stage is light loads (0–60% of 1 RM for lower body exercises; 30–60% of 1 RM for upper body exercises) performed at a fast contraction velocity with 3–5 minutes of rest between sets for multiple sets per exercise (3–5 sets).
Recommendations.
Physical exercise approach to sarcopenic patients requires a clear definition in terms of both quality and quantity.
Older adults should have a plan for obtaining recommended levels of physical exercise, in terms of intensity, frequency and duration, through a gradual (or stepwise) approach over time (31) (Table 1).
For older adults, PA includes recreational or leisure-time activities, transportation (e.g. walking or cycling), occupational (if the person is still engaged in work), household chores, sports, family and community activities (70).
Aerobic exercise improves metabolic control, reduces oxidative stress and optimizes exercise capacity.
Older adults should perform moderate-intensity aerobic exercise for a minimum of 30 min on five days each week or vigorous-intensity aerobic activity for a minimum of 20 min on three days each week (31).
Moderate-intensity aerobic exercise. On an absolute scale, moderate intensity refers to activity that is performed at 3.0–5.9 times the intensity of rest. On a scale relative to an individual’s personal capacity, moderate-intensity PA is usually a 5 or 6 on a scale of 0–10 (70).
Vigorous-intensity aerobic exercise. On an absolute scale, vigorous intensity refers to activity that is performed at 6.0 or more times the intensity of rest. On a scale relative to an individual’s personal capacity, vigorous intensity PA is usually a 7 or 8 on a scale of 0–10 (70).
Aerobic exercise should be performed in bouts of at least 10 minutes duration (70).
RE is a safe, feasible, and effective intervention to induce muscle hypertrophy and increase strength and should be started as early as possible, to optimize its effectiveness, in older adults.
For improvements in muscle mass and muscle strength in older adults, the use of both multiple- and single-joint exercises (free weights and machines), with slow-to-moderate lifting velocity, for 1–3 sets per exercise, with 60–80% of 1 RM, for 8–12 repetitions, with 1–3 min of rest among sets, for 2–3 days/week, is recommended (69).
Resistance training needs to use a progressively increasing load to maintain the desired range of repetitions per set of exercise.
Older adults should perform muscle strengthening activities using the major muscles groups to maintain or increase muscular mass and strength (31).
Fast-velocity contractions should be considered when designing RE training programs for older adults.
PRT is effective for improving strength and physical functioning in older people, but this approach requires caution.
Increasing power in older adults requires the performance of both single- and multiple-joint exercises for 1–3 sets per exercise using light to moderate loading (30–60% of 1 RM) for 6–10 repetitions with high repetition velocity (69).
To reduce risk of injuries and other adverse events, older people should consult their health-care provider about the types and amounts of PA and exercise appropriate for them (71).
Conclusions
Sarcopenia represents a major cause of disability and increased health care costs in elderly. Although very common, it is underdiagnosed and undertreated. Sarcopenia should be investigated from 50 years of age and above all in older patients with a decline in physical functioning, strength or health status. The identification of sarcopenic patients can be performed using: assessment of mobility and muscle strength (postural balance, gait speed, handgrip strength, leg extension), measures of body composition (DXA or BIA) and global physical function (SPPB).
Considering the multifactorial nature of the sarcopenic process, comprehensive interventions are needed. In addition to nutritional approach and pharmacological treatments, physical activity plays a key role. Certainly, the best physical exercise intervention to maintain or enhance muscular strength and power is the resistance training, above all in elderly. The feasibility, sustainability, and safety of aerobic exercise, resistance and power training in older adults need to be confirmed by larger longitudinal trials.
Figure 1.
EWGSOP-suggested algorithm for sarcopenia case findings in older individuals, modified according to the recommendations of IWGS that proposed the gait speed threshold of 1 m/s.
Table 1.
Recommendations for physical exercise in sarcopenic older people.
| Type of Training | Frequency | Intensity | Duration/Set |
|---|---|---|---|
| Aerobic Exercise | A minimum of 5 days/week, for moderate intensity, or 3 days/week for vigorous intensity | Moderate intensity at 5–6 on a 10-point scale; Vigorous intensity at 7–8 on 10-point scale | Accumulate at least 30 min/day of moderate-intensity activity, in bouts of at least 10 min each; continuous vigorous activity for at least 20 min/day |
| Resistance Exercise involving the major muscle groups (free weights and machines) | At least 2 days/week | Slow-to-moderate lifting velocity 60–80% of 1 RM | 8–10 exercises 1–3 sets per exercise 8–12 repetitions (1–3 min of rest among set) |
| Power Training to practice only after the resistance training | 2 days/week | Light to moderate loading (30–60% of 1 RM) High repetition velocity |
1–3 sets per exercise, 6–10 repetitions |
References
- 1.Rosenberg I. Summary comments: epidemiological and methodological problems in determining nutritional status of older persons. Am J Clin Nutr. 1989;50:1231–3. [Google Scholar]
- 2.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, Abellan vanKan G, Andrieu S, Bauer J, Breuille D, Cederholm T, Chandler J, De Meynard C, Donini L, Harris T, Kannt A, Keime Guibert F, Onder G, Papanicolaou D, Rolland Y, Rooks D, Sieber C, Souhami E, Verlaan S, Zamboni M. Sarcopenia: An Undiagnosed Condition in Older Adults. Current Consensus Definition: Prevalence, Etiology, and Consequences. International Working Group on Sarcopenia. J Am Med Dir Assoc. 2011;12:249–256. doi: 10.1016/j.jamda.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Finbarr C, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M. Sarcopenia: European consensus on definition and diagnosis Report of the European Working Group on Sarcopenia in Older People. Age and Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frontera WR, Hughes VA, Fielding RA, et al. Aging of skeletal muscle: A 12-yr longitudinal study. J Appl Physiol. 2000;88:1321–1326. doi: 10.1152/jappl.2000.88.4.1321. [DOI] [PubMed] [Google Scholar]
- 5.Lexell J, Taylor CC, Sjostrom M. What is the cause of the ageing atrophy? Total number, size and proportion of different fiber types studied in whole vastus lateralis muscle from 15- to 83-year-old men. J Neurol Sci. 1988;84:275–294. doi: 10.1016/0022-510x(88)90132-3. [DOI] [PubMed] [Google Scholar]
- 6.Lexell J, Henriksson-Larsen K, Wimblod B, Sjostrom M. Distribution of different fiber types in human skeletal muscles: Effects of aging studied in whole muscle cross sections. Muscle Nerve. 1983;6:588–595. doi: 10.1002/mus.880060809. [DOI] [PubMed] [Google Scholar]
- 7.Porter MM, Vandervoort AA, Lexell J. Aging of human muscle: Structure, function and adaptability. Scand J Med Sci Sports. 1995;5:129–142. doi: 10.1111/j.1600-0838.1995.tb00026.x. [DOI] [PubMed] [Google Scholar]
- 8.Narici MV, Maganaris CN. Adaptability of elderly human muscles and tendons to increased loading. J Anat. 2006;208:433–443. doi: 10.1111/j.1469-7580.2006.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kandarian SC, Jackman RW. Intracellular signaling during skeletal muscle atrophy. Muscle Nerve. 2006;33:155–165. doi: 10.1002/mus.20442. [DOI] [PubMed] [Google Scholar]
- 10.Clavel S, Coldefy AS, Kurkdjian E, et al. Atrophy-related ubiquitin ligases, atrogin-1 and MuRF1 are up-regulated in aged rat tibialis anterior muscle. Mech Ageing Dev. 2006;127:794–801. doi: 10.1016/j.mad.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Lecker SH, Jagoe RT, Gilbert A, et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 2004;18:39–51. doi: 10.1096/fj.03-0610com. [DOI] [PubMed] [Google Scholar]
- 12.Serrano AL, Munoz-Canoves P. Regulation and dysregulation of fibrosis in skeletal muscle. Exp Cell Res. 2010;316:3050–3058. doi: 10.1016/j.yexcr.2010.05.035. [DOI] [PubMed] [Google Scholar]
- 13.Grimby G, Saltin B. The ageing muscle. Clin Physiol. 1983;3:209. doi: 10.1111/j.1475-097x.1983.tb00704.x. [DOI] [PubMed] [Google Scholar]
- 14.Goodpaster BH, Park SW, Harris TB, et al. The loss of skeletal muscle strength, mass, and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61A:1059–64. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- 15.Newman AB, Lee JS, Visser M, et al. Weight change and the conservation of lean mass in old age: the health, aging and body composition study. Am J Clin Nutr. 2005;82(4):872–8. doi: 10.1093/ajcn/82.4.872. [DOI] [PubMed] [Google Scholar]
- 16.Lamberts SW, van den Beld AW, van der Lely AJ. The endocrinology of aging. Science. 1997;278:419–424. doi: 10.1126/science.278.5337.419. [DOI] [PubMed] [Google Scholar]
- 17.Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R. The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc. 2004;52(1):80–5. doi: 10.1111/j.1532-5415.2004.52014.x. [DOI] [PubMed] [Google Scholar]
- 18.Newman AB, Kupelian V, Visser M, et al. Sarcopenia: Alternative definitions and associations with lower extremity function. J Am Geriatr Soc. 2003;51:1602–1609. doi: 10.1046/j.1532-5415.2003.51534.x. [DOI] [PubMed] [Google Scholar]
- 19.Lukasi H, Heymsfield M, et al., editors. Assessing muscle mass Human body composition. Champaign, IL, USA: Human Kinetics; 2005. [Google Scholar]
- 20.Chien MY, Huang TY, Wu YT. Prevalence of sarcopenia estimated using a bioelectrical impedance analysis prediction equation in community-dwelling elderly people in Taiwan. J Am Geriatr Soc. 2008;56:1710–5. doi: 10.1111/j.1532-5415.2008.01854.x. [DOI] [PubMed] [Google Scholar]
- 21.Janssen I, Heymsfield SB, Baumgartner RN, et al. Estimation of skeletal muscle mass by bioelectrical impedance analysis. J Appl Physiol. 2000;89:465–71. doi: 10.1152/jappl.2000.89.2.465. [DOI] [PubMed] [Google Scholar]
- 22.Rolland Y, Czerwinski S, Abellan Van Kan G, et al. Sarcopenia: its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging. 2008;12:433–50. doi: 10.1007/BF02982704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laurentani F, Russo C, Bandinelli S, et al. Age-associated changes in skeletal muscles and their effect on mobility: an operational diagnosis of sarcopenia. J Appl Physiol. 2003;95:1851–60. doi: 10.1152/japplphysiol.00246.2003. [DOI] [PubMed] [Google Scholar]
- 24.Al Snih S, Markides K, Ottenbacher K, et al. Hand grip strength and incident ADL disability in elderly Mexican Americans over a seven-year period. Aging Clin Exp Res. 2004;16:481–6. doi: 10.1007/BF03327406. [DOI] [PubMed] [Google Scholar]
- 25.Bassey EJ, Short AH. A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur J Appl Physiol Occup Physiol. 1990;60:385–90. doi: 10.1007/BF00713504. [DOI] [PubMed] [Google Scholar]
- 26.Hartmann A, Knols R, Murer K, et al. Reproducibility of an isokinetic strength-testing protocol of the knee and ankle in older adults. Gerontology. 2009;55:259–68. doi: 10.1159/000172832. [DOI] [PubMed] [Google Scholar]
- 27.Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–94. doi: 10.1093/geronj/49.2.m85. [DOI] [PubMed] [Google Scholar]
- 28.Working Group on Functional Outcome Measures for Clinical Trials Functional outcomes for clinical trials in frail older persons: time to be moving. J Gerontol A Biol Sci Med Sci. 2008;63:160–4. doi: 10.1093/gerona/63.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cesari M, Kritchevsky SB, Newman AB, et al. Added value of physical performance measures in predicting adverse health-related events: results from the health, aging and body composition study. J Am Geriatr Soc. 2009;57:251–9. doi: 10.1111/j.1532-5415.2008.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raguso CA, Kyle U, Kossovsky MP, et al. A 3-year longitudinal study on body composition changes in the elderly: role of physical exercise. Clin Nutr. 2006;25:573–580. doi: 10.1016/j.clnu.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 31.Nelson M, Rejeski J, Blair S, et al. Physical activity and public health in older adults. Recommendation from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116:1094–1105. doi: 10.1161/CIRCULATIONAHA.107.185650. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell D, Haan MN, Steinberg FM, Visser M. Body composition in the elderly: the influence of nutritional factors and physical activity. J Nutr Health Aging. 2003;7(3):130–139. [PubMed] [Google Scholar]
- 33.Kent-Braun JA, Ng AV. Skeletal muscle oxidative capacity in young and older women and men. J Appl Physiol. 2000;89(3):1072–1078. doi: 10.1152/jappl.2000.89.3.1072. [DOI] [PubMed] [Google Scholar]
- 34.Waters DL, Baumgartner RN, Garry PJ, Vellas B. Advantages of dietary, exercise-related, and therapeutic interventions to prevent and treat sarcopenia in adult patients: an update. Clinical Interventions in Aging. 2010;5:259–270. doi: 10.2147/cia.s6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Johnston AP, De Lisio M, Parise G. Resistance training, sarcopenia, and the mitochondrial theory of aging. Appl Physiol Nutr Metab. 2008;33:191–199. doi: 10.1139/H07-141. [DOI] [PubMed] [Google Scholar]
- 36.Staunton L, O’Connell K, Ohlendieck K. Proteomic profiling of mitochondrial enzymes during skeletal muscle aging. J Aging Res. 2011 doi: 10.4061/2011/908035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–2282. [PubMed] [Google Scholar]
- 38.Hawley JA, Hargreaves M, Zierath JR. Signalling mechanisms in skeletal muscle: role in substrate selection and muscle adaptation. Essays Biochem. 2006;42:1–12. doi: 10.1042/bse0420001. [DOI] [PubMed] [Google Scholar]
- 39.Lira VA, Benton CR, Yan Z, Bonen A. PGC-1alpha regulation by exercise training and its influences on muscle function and insulin sensitivity. Am J Physiol Endocrinol Metab. 2010;299:E145–E161. doi: 10.1152/ajpendo.00755.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcription activation of PGC-1alpha gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forbes SC, Little JP, Candow DG. Exercise and nutritional interventions for improving aging muscle health. Endocrine. 2012;42:29–38. doi: 10.1007/s12020-012-9676-1. [DOI] [PubMed] [Google Scholar]
- 42.Abadi A, Glover EI, Isfort RJ, Raha S, Safdar A, Yasuda N, Kaczor JJ, Melov S, Hubbard A, Qu X, Phillips SM, Tarnopolsky M. Limb immobilization induces a coordinate downregulation of mitochondrial and other metabolic pathways in men and women. PLoS ONE. 2009;4:e6518. doi: 10.1371/journal.pone.0006518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc. Natl. Acad. Sci. USA. 2009;106:20405–20410. doi: 10.1073/pnas.0911570106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Sandri M, Lin J, Handschin C, Yang W, Arany ZP, Lecker SH, Goldberg AL, Spiegelman BM. PGC-1alpha protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc Natl Acad Sci USA. 2006;103:16260–16265. doi: 10.1073/pnas.0607795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hawley JA. Exercise as a therapeutic intervention for the prevention and treatment of insulin resistance. Diabetes Metab Res Rev. 2004;20:383–393. doi: 10.1002/dmrr.505. [DOI] [PubMed] [Google Scholar]
- 46.Holloszy JO. Exercise-induced increase in muscle insulin sensitivity. J Appl Physiol. 2005;99:338–343. doi: 10.1152/japplphysiol.00123.2005. [DOI] [PubMed] [Google Scholar]
- 47.Hildrum B, Mykletun A, Hole T, Midthjell K, Dahl AA. Age-specific prevalence of the metabolic syndrome defined by the International Diabetes Federation and the National Cholesterol Education Program: the Norwegian HUNT 2 study. B.M.C. Public Health. 2007;7:220. doi: 10.1186/1471-2458-7-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenhaff PL, Karagounis LG, Peirce N, Simpson EJ, Hazell M, Layfield R, Wackerhage H, Smith K, Atherton P, Selby A, Rennie MJ. Disassociation between the effects of amino acids and insulin on signaling, ubiquitin ligases, and protein turnover in human muscle. Am J Physiol Endocrinol Metab. 2008;295:E595–E604. doi: 10.1152/ajpendo.90411.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fiatarone MA, O’Neill EF, Ryan ND, Clements KM, Solares GR, Nelson ME, Roberts SB, Kehayias JJ, Lipsitz LA, Evans WJ. Exercise training and nutritional supplementation for physical frailty in very elderly people. N Engl J Med. 1994;330:1769–1775. doi: 10.1056/NEJM199406233302501. [DOI] [PubMed] [Google Scholar]
- 50.Schulte JN, Yarasheski KE. Effects of resistance training on the rate of muscle protein synthesis in frail elderly people. Int J Sport Nutr Exerc Metab. 2001;11:S111–S118. doi: 10.1123/ijsnem.11.s1.s111. [DOI] [PubMed] [Google Scholar]
- 51.Verdijk LB, Gleeson BG, Jonkers RA, Meijer K, Savelberg HH, Dendale P, van Loon LJ. Skeletal muscle hypertrophy following resistance training is accompanied by a fibre type-specific increase in satellite cell content in elderly men. J Gerontol A Biol Sci Med Sci. 2009;64:332–339. doi: 10.1093/gerona/gln050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Smilios I, Pilianidis T, Karamouzis M, Parlavantzas A, Tokmakidis SP. Hormonal responses after a strength endurance resistance exercise protocol in young and elderly males. Int J Sports Med. 2007;28:401–406. doi: 10.1055/s-2006-924366. [DOI] [PubMed] [Google Scholar]
- 53.Mero AA, Hulmi JJ, Salmijärvi H, Katajavuori M, Haverinen M, Holviala J, Ridanpää T, Häkkinen K, Kovanen V, Ahtiainen JP, Selänne H. Resistance training induced increase in muscle fiber size in young and older men. Eur J Appl Physiol. 2013 Mar;113(3):641–50. doi: 10.1007/s00421-012-2466-x. [DOI] [PubMed] [Google Scholar]
- 54.Candow DG, Chilibeck PD, Abeysekara S, Zello GA. Short term heavy resistance training eliminates age-related deficits in muscle mass and strength in healthy older males. J Strength Cond Res. 2011;25:326–333. doi: 10.1519/JSC.0b013e3181bf43c8. [DOI] [PubMed] [Google Scholar]
- 55.Peterson MD, Sen A, Gordon PM. Influence of Resistance Exercise on Lean Body Mass in Aging Adults: A Meta-Analysis. Med Sci Sports Exerc. 2011 Feb;43(2):249–258. doi: 10.1249/MSS.0b013e3181eb6265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Latham NK, Bennett DA, Stretton CM, Anderson CS. Systematic review of progressive resistance strength training in older adults. J Gerontol A Biol Sci Med Sci. 2004;59(1):48–61. doi: 10.1093/gerona/59.1.m48. [DOI] [PubMed] [Google Scholar]
- 57.Liu CJ, Latham NK. Progressive resistance strength training for improving physical function in older adults. Cochrane Database Syst Rev. 2009;(3):CD002759. doi: 10.1002/14651858.CD002759.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raue U, Trappe TA, Estrem ST, Qian HR, Helvering LM, Smith RC, Trappe S. Transcriptome signature of resistance exercise adaptations: mixed muscle and fiber type specific profiles in young and old adults. J Appl Physiol. 2012;112:1625–1636. doi: 10.1152/japplphysiol.00435.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Metter EJ, Conwit R, Tobin J, Fozard JL. Age-associated loss of power and strength in the upper extremities in women and men. J Gerontol A Biol Sci Med Sci. 1997;52:B267–B276. doi: 10.1093/gerona/52a.5.b267. [DOI] [PubMed] [Google Scholar]
- 60.Puthoff ML, Nielsen DH. Relationships among impairments in lower extremity strength and power, functional limitations, and disability in older adults. Phys Ther. 2007 Oct;87(10):1334–1347. doi: 10.2522/ptj.20060176. [DOI] [PubMed] [Google Scholar]
- 61.Sayers SP. High-speed power training: a novel approach to resistance training in older men and women. A brief review and pilot study. J Strength Cond Res. 2007;21:518–526. doi: 10.1519/R-20546.1. [DOI] [PubMed] [Google Scholar]
- 62.Fielding RA, LeBrasseur NK, Cuoco A, Bean J, Mizer K, Fiatarone Singh MA. High-velocity resistance training increases skeletal muscle peak power in older women. J Am Geriatr Soc. 2002;50(4):655–662. doi: 10.1046/j.1532-5415.2002.50159.x. [DOI] [PubMed] [Google Scholar]
- 63.Bottaro M, Machado SN, Nogueira W, Scales R, Veloso J. Effect of high versus low-velocity resistance training on muscular fitness and functional performance in older men. Eur J Appl Physiol. 2007;99(3):257–264. doi: 10.1007/s00421-006-0343-1. [DOI] [PubMed] [Google Scholar]
- 64.Porter MM. Power training for older adults. Appl Physiol Nutr Metab. 2006;31:87–94. doi: 10.1139/h05-034. [DOI] [PubMed] [Google Scholar]
- 65.Henwood TR, Riek S, Taaffe DR. Strength versus muscle power specific resistance training in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2008;63(1):83–91. doi: 10.1093/gerona/63.1.83. [DOI] [PubMed] [Google Scholar]
- 66.Henwood TR, Taaffe DR. Improved physical performance in older adults undertaking a short-term programme of high-velocity resistance training. Gerontology. 2005;51(2):108–115. doi: 10.1159/000082195. [DOI] [PubMed] [Google Scholar]
- 67.Reid KF, Callahan DM, Carabello RJ, Phillips EM, Frontera WR, Fielding RA. Lower extremity power training in elderly subjects with mobility limitations: a randomized controlled trial. Aging Clin Exp Res. 2008;20(4):337–343. doi: 10.1007/bf03324865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bean JF, Leveille SG, Kiely DK, Bandinelli S, Guralnik JM, Ferrucci LJ. A comparison of leg power and leg strength within the InCHIANTI study: which influences mobility more? Gerontol A Biol Sci Med Sci. 2003;58(8):728–733. doi: 10.1093/gerona/58.8.m728. [DOI] [PubMed] [Google Scholar]
- 69.American College of Sports Medicine. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2009 Mar;41(3):687–708. doi: 10.1249/MSS.0b013e3181915670. [DOI] [PubMed] [Google Scholar]
- 70.Global Recommendations on Physical Activity for Health. World Health Organization; 2011. [PubMed] [Google Scholar]
- 71.Physical Activity Guidelines for Americans. US Department of Health and Human Services; 2008. [Google Scholar]