Abstract
Background
Black/white disparities in HIV incidence and prevalence among men who have sex with men (MSM) in the United States remain largely unexplained. We examined the impact on HIV prevalence and incidence of interventions that decrease disparities in HIV care. Deciding which interventions have most impact on reducing disparities is critical.
Methods
Using available US Centers for Disease Control and Prevention (CDC) estimates, we constructed HIV care continua for black and white MSM for 2009-2010. These estimates were used as parameters in a deterministic model to yield estimated race-specific transmissions, transmission rates, incidence rates, and rate-ratios (RR). We examined the impact of changes in the care continuum for black MSM on transmission and incidence rates.
Findings
Marked disparities were found throughout the care continuum: ultimately, 16% of black and 34% of white MSM achieved viral suppression. Based on these care continua, 9,833 and 9,710 new HIV transmissions were estimated annually respectively from HIV-positive black and white MSM (transmission RR=1·36 and incidence RR=7·92). In a model where black and white MSM have identical care outcomes, transmission RR=1·00 and incidence RR=5·80. Scenarios of 95% diagnosis, 95% retention, and concurrent 95% diagnosis and 95% retention respectively yield transmission RR=1·00, 1·02, 0·56, and incidence RR=5·81, 5·93, 3·28.
Interpretation
Disparities in HIV transmission rates may be reduced by improving the HIV care continuum outcomes, but existing racial disparities in HIV prevalence will likely continue to drive higher incidence among black MSM for decades to come.
Introduction
In the United States, the incidence of HIV infection is rising among men who have sex with men (MSM). As with numerous other conditions, there are important racial differences in HIV infection.1-3Black MSM have experienced disproportionate incidence and prevalence since the beginning of the epidemic.4 Although the factors that gave rise to black/white disparities in HIV among MSM are incompletely understood, data are emerging to suggest important factors in sustaining those disparities. Meta-analyses have shown that black MSM do not have higher risk behaviors than white MSM,5 possible hypotheses have been enumerated that consider the effects of social network structures and treatment disparities.6 Existing disparities in HIV prevalence and socioeconomic factors might also contribute to ongoing disparities.7-9 For example, because of higher HIV prevalence and lower extent of HIV suppression among black MSM and substantial racial concordance in sexual partnerships, equivalent risk behaviors among black MSM confer a higher probability of exposure to an HIV-transmitting male partner, compared to white MSM.8 In a study of MSM in Atlanta, having black partners statistically accounted for the black/white HIV incidence disparity.10Others have suggested that differences in clinical care outcomes by race among HIV-infected MSM magnify such disparities.11,12
New HIV infections in a populationare a function of behavioral and biological parameters, including the number of serodiscordant sexual partnerships, number of unprotected sex acts, and viral load in infected partners.13 Within a serodiscordant partnership, the transfer of HIV may be seen from the perspective of either the person acquiring or transmitting HIV.
Most studies report racial disparities in HIV prevalence (infection burden) or HIV incidence (new infections).10,11,14,15 Fewer studies have considered disparities in HIV transmission – i.e., the extent to which HIV-infected black MSM are more likely to transmit HIV, relative to HIV-infected white MSM. Behavioral studies have examined differences in HIV transmission risk behavior among MSM by race to help explain disparate infection rates among black MSM.16,17 A recent study using HIV surveillance data furthered these analyses by calculating HIV transmission rates (average transmissions per personliving with HIV) for those diagnosed and undiagnosed, and additionally found that, although there are about one-fifth as many black men, compared to white men, in the United States, there are about the same number of black and white MSM living with HIV and not virally suppressed.18
The HIV care continuum has become an important model for measuring HIV care in populations through nested steps of HIV infection, diagnosis, retention in care, antiretroviral therapy (ART) prescription, and viral suppression.19,20 None of the above studies constructed a full HIV care continuum for MSM by race, nor modeled the degree to which drop-off across the continuum contributes to HIV infection disparities. Using available national data sources, we illustrate how existing racial disparities in HIV prevalence and in the HIV care continuum translate into and explain disparate rates of HIV incidence among MSM.
Methods
HIV care continua for black and white MSM, 2009/2010
Using nationally-representative US Centers for Disease Control and Prevention (CDC) data on people living with HIV in 2009 and 2010 in the United States(Table 1), we estimated the HIV care continuum during this period separately for black and white MSM.1,12,18Where competing estimates were available, we selected ones with greater subpopulation detail.
Table 1. Data sources for estimating the HIV Continuum of Care for Black and White MSM, United States 2009-2010.
| Continuum step | Data source representation | Reference | Estimation notesa |
|---|---|---|---|
| 1. Total living with HIV |
50 US states, DC, Puerto Rico, 5 other dependent areas; 2009 and 2010 |
Hall et al, 2013 18; CDC, 2013 1 |
Black and white MSM total diagnosed (step 2), divided by Black and white MSM proportion diagnosed |
| 2. Diagnosed | 50 US states, DC, Puerto Rico, 5 other dependent areas; 2010 |
CDC, 2013 1 | Black and white MSM totals provided |
| 3. Retained in care b | 50 US states, DC, Puerto Rico; 2009 | Beer et al, 2013 12 | Black and white MSM totals providede |
| 4. Prescribed ART c | 50 US states, DC, Puerto Rico; 2009 | Beer et al, 2013 12 | Black and white MSM total retained in care (step 3), multiplied by proportion on ARTe |
| 5. Virally suppressed d | 50 US states, DC, Puerto Rico; 2009 | Beer et al, 2013 12 | Black and white MSM total retained in care (step 3), multiplied by proportion currently suppressede |
The chosen method for continuum estimation using US CDC National HIV Surveillance System and Medical Monitoring Project data more closely resembles that used to construct national care continua for the United States by multiple subgroups,21 than another method using the same data systems, but partly uses data from a 19 jurisdiction subset and excludes those undiagnosed from estimation, and has been applied to the care continuum for blacks in the United States.30
Had a medical care visit in the first 4 months of 2009
Based on self-report
Most recent viral load documented as undetectable or <200 copies/ml
The estimate for the total retained in care represents all MSM in care, whereas the proportion on ART and proportion currently suppressed were estimated among MSM who were sexually active in the previous year. It may represent a minimal source of error if these care indicators were differential by sexual activity in the previous year
Population sizes along the care continuum were represented in three ways. The first was the typical cumulative prevalence method that monotonically decreases from HIV infection to viral suppression.19,21The second was the percent attaining a given step of care, conditional on attaining the previous step. Finally, by subtraction we obtained the number at three broader, mutually exclusive stages of care; HIV-infected but undiagnosed, diagnosed but not virologically suppressed, and virologically suppressed. This view is most informative for understanding the relative contribution of individuals at each stage to ongoing transmission, and thus targeting prevention efforts.
HIV transmission calculations
We used published annual per-person transmission rates from those living with HIV in the US in 2009 for those with undiagnosed infection (rate=0.108), diagnosed infection but not virally suppressed (rate=0.046), and those virally suppressed (rate=0).18,22Most (83%) of MSM diagnosed but not suppressed are estimated to be out of care.21 Stage-specific transmission rates, and thus transmission risk behaviors, were held constant between black and white MSM, consistent with previous research on comparable sexual behaviors by race, due to the unavailability of race-specific estimates.5,11
Next, we estimated the number of HIV transmissions originating from black and white MSM living with HIV infection at each step of care by multiplying the number of MSM of each race at each care step with that step’s transmission rate. Division by the race-specific number of MSM living with HIV yielded the race-specific transmission rate, the ratio of which estimated the transmission rate-ratio, or the disparity in HIV transmission.
HIV incidence calculations
Total transmissions by race were used to imply incident infections (under differing racial mixing scenarios, described below), and these were compared with CDC back-calculation estimates of 2009 race-specific incident infection counts.2 Although national incidence rates remain unpublished, we estimated these using other population-based sources. The number of MSM living in the United States was computed based on 2008 estimates from a meta-analysis of population-based surveys using behavioral definitions of MSM, accounting for population growth among males ages ≥13 years from 2008 to 2010(Appendix, Table 1).23,24Race-specific totals were determined per the overall US race distribution.23Using the number living with HIV, we next computed race-specific HIV prevalence, the number living without HIV, and thus the incidence rates, and incidence-ratio comparing black vs. white MSM.
Intervention scenarios
We next implemented hypothetical interventions along the continuum of care for black MSM as both a sensitivity analysis and to understand the relative contributions of the steps of care and existing prevalence to transmission and incidence among black MSM.19 Using the observed care continuum as a base case (“Observed continuum”), we examined HIV transmission rates, incidence rates, and rate-ratios under counterfactual scenarios that began with the same number of black MSM HIV-infected, but altered coverage of subsequent steps in the care continuum by modifying the percentages attaining subsequent steps. These four scenarios were equivalent care achievement as white MSM (“Racially-equivalent care”), 95% diagnosis, 95% retention, or concurrent 95% diagnosis and 95% retention.
Race mixing sensitivity analyses
The primary analysis assumedall serodiscordant MSM partnerships were with same-race men (ie: no racial mixing). Previous studies have indicated greater racial mixing among MSM than heterosexuals, but mixing varies regionally and no nationally-representative partnership race-mixing data are available for MSM.25Furthermore, transmission analyses require race-mixing data on the specific subset of HIV serodiscordant partnerships, ideally those in which transmission is likely to occur or has occurred. In sensitivity analyses we reassessed all outcomes under varied racial mixing scenarios. These included hypothetical scenarios, as well as those based on data from 5,978 anal intercourse (AI) partnerships and 432 serodiscordant AI partnerships from three sources: a national online study, and an Atlanta-based cohort, and an Atlanta-based sexual networks study (Appendix Table 2). To account for all MSM transmissions in the population, a third group of Hispanic ethnicity/Other race MSM was included, with a care continuum approximated from the above sources.18,21A spreadsheet demonstrating all results is at http://sgiz.mobi/s3/03589c4b2f09.
Role of the funding source
The funding source had no role in data analysis or interpretation, nor writing of or decision to submit the report.
Results
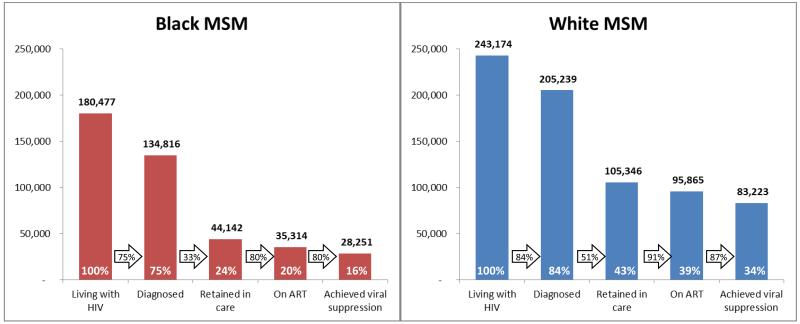
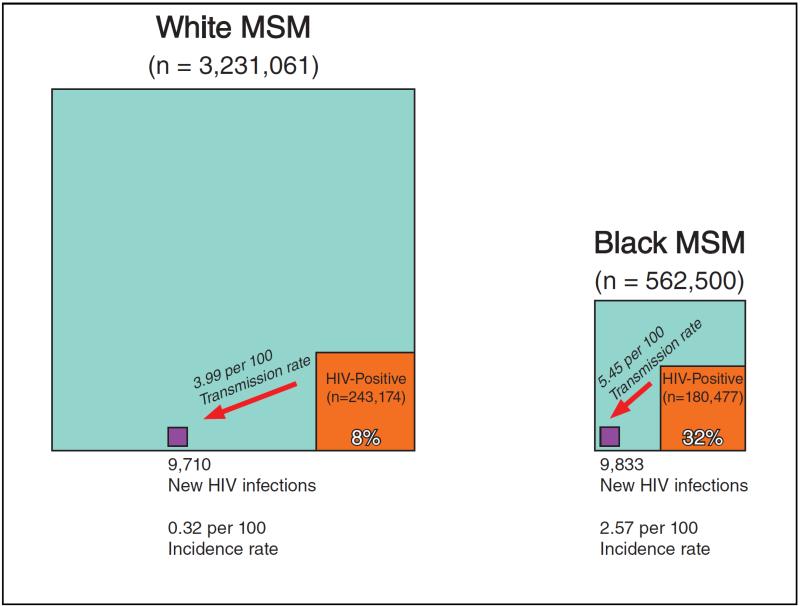
In 2010, approximately 562,500 black and 3,231,061 white adult MSM were living in the United States. Of these, 180,477 black and 243,174 white MSM were estimated living with HIV, respectively yielding HIV prevalences of 32% and 8% (Figures 1-2). Disparities were found at all steps of the HIV care continuum, most notably in retention in care, with 33% of black and 51% of white diagnosed MSM retained. Ultimately 16% of black and 34% of white MSM were estimated to have attained HIV virological suppression.
Figure 1. Estimated HIV care continuum for black and white MSM in the United States, 2009/2010.
Bold numbers above bars represent the estimated total MSM of each race attaining steps of care, white percentages within bars represents the estimated percentage of MSM of each race attaining steps of care, and black percentages within arrows represent the percentage of MSM of each race percent attaining a given step of care, conditional on attaining the previous step.
Figure 2. Illustration of estimated HIV transmission and incidence, using the observed HIV care continuum.
White and black MSM have similar numbers of new HIV infections, but black MSM have a smaller total population and greater proportion of prevalent HIV-positive men, resulting in incidence disparities. All representations of area in the boxes are proportionally scaled to the corresponding group sizes. This illustration assumes a scenario of 100% racial concordance in HIV serodiscordant relationships; 5 alternative scenarios are described in the Appendix, Tables 2-3.
Applying per-person transmission rates to each care continuum, 9,710 transmissions were attributable to white MSM and 9,833 to black MSM (Table 2, “Observed Continuum”), resulting in a transmission rate-ratio of 1·36 (black rate=5·45 vs. white rate=3·99, per 100) and an incidence rate-ratio of 7·92 (black rate=2·57 vs. white rate=0·32, per 100). The higher incidence rate-ratio was due to the smaller total population of HIV-negative black MSM compared with white MSM and the larger proportion of prevalent positives (Figure 2).
Table 2. Estimated HIV transmission and incidence,estimated from the HIV care continuum, for black and white MSM in the United States, 2009/2010.
| HIV Transmission |
HIV Incidence |
|||||
|---|---|---|---|---|---|---|
| New HIV infections |
Transmission rate, per 100 a |
Transmission rate ratio |
Incidence rate, per 100 b |
Incidence rate ratio |
Change in rate ratio from Scenario 1 |
|
| Scenario 1: Observed continuum(for each race) | ||||||
| White MSM | 9,710 | 3·99 | ref. | 0·32 | ref. | -- |
| Black MSM | 9,833 | 5·45 | 1·36 | 2·57 | 7·92 | ref. |
|
| ||||||
| Black MSM, under HIV care continuum interventions c | ||||||
| Scenario 2: Racially-equivalent care | 7,206 | 3·99 | 1·00 | 1·89 | 5·80 | −27% |
| Scenario 3:95% diagnosis | 7,209 | 3·99 | 1·00 | 1·89 | 5·81 | −27% |
| Scenario 4:95% retention | 7,362 | 4·08 | 1·02 | 1·93 | 5·93 | −25% |
| Scenario 5: Concurrent 95% diagnosis and 95% retention | 4,066 | 2·25 | 0·56 | 1·06 | 3·28 | −59% |
|
| ||||||
| Estimated Incident HIV Infections. 2009 - Prejean et al. 2011d | ||||||
| White MSM | 11,400 | -- | -- | 0·35 | ref. | -- |
| Black MSM | 10,800 | -- | -- | 2·55 | 7·21 | -- |
Number of new HIV infections per 100 persons living with an HIV infection
Number of new HIV infections per 100 persons living without an HIV infection
Interventions applied to black MSM only, while holding constant levels of diagnosis and care for white MSM at the levels observed for 2009/2010
Presented to illustrate similarities of estimated incident infections and incidence rate ratios between previously published CDC estimates and our current estimates
Considering the scenario where black MSM assume the care continuum of white MSM, the transmission rate-ratio is definitionally fixed at 1·00. The equalizing of transmission likelihood due to care equality alone results in a 27% decline in the estimated black MSM incidence rate and the rate-ratio (Table 2, “Racially-equivalent Care”). The complement of 73% represents the portion of incidence attributable to current disparities in prevalence.
Holding the care continuum for white MSM constant, the “95%Diagnosis” scenario for all black MSM yields nearly identical changes inestimated transmission and incidence as “Racially-equivalent Care”. Similarly, the “95%Retention” scenario for black MSM results in an estimated transmission rate-ratio of 1·02 and a 25% incidence reduction. Under the “concurrent 95% Diagnosis and 95% Retention” scenario, the estimated transmission rate-ratio declines to 0·56 and incidence by 59%. Under this extreme level of intervention for black MSM, the estimated incidence rate-ratio remains elevated at 3·28, owing to the proportionally larger prevalent HIV-positive population among black MSM – of whom 42% are not virally suppressed. Considering the same interventions at a perfect 100% coverage gave nearly identical results.
In sensitivity analyses under varying racial mixing configurations, “Observed Continuum” incidence rate-ratio estimates for black and white MSM varied from 7.89 to 9.22 (Appendix, Table 3). In all cases, incidence reductions among black MSM achieved through improvements among black MSM in HIV diagnosis and care were attenuated because these changes now partly reduced transmissions to other racial/ethnic groups while those groups were responsible for unmodified continued transmissions to black MSM.
Comparing the “Observed Continuum” scenario estimates to other studies of US surveillance data which used independent methods provided validation of model findings. Predicted infection totals are close to previously published back-calculated incidence estimates of 10,800 for black MSM and 11,400 for white MSM (Table 2).2
Discussion
By synthesizing existing nationally-representative CDC estimates, we illustrate the role of differences in HIV care continua in the perpetuation of black-white disparities in HIV among MSM. Our results extend existing work by presenting separate HIV care continua for black and white MSM, including those living with HIV regardless of their diagnosis status. Our results suggest important lessons that may inform prevention priorities and prospects for mitigating these disparities among MSM in the United States, one of many health-related racial disparities.
In the United States, one in three black MSM is living with HIV, compared to less than one in ten white MSM.23 Among them, the race-specific HIV care continua results depict consistent disparities at each step of the continuum for black compared to white MSM: black MSM are less likely to be diagnosed with HIV, be retained in care, be on ART, and achieve viral suppression. These continua culminate with black MSM achieving less than half of the virologic suppression of white MSM. These disparity estimates are validated by a previously published meta-analysis of studies comparing black and white HIV-infected MSM,11 but our analysis furthers those findings by demonstrating that disparate HIV transmission rates stemming from racial differences in care may help magnify HIV incidence disparities among black MSM.
In our analysis, we present both HIV transmission and incidence rates. According to our model, the disparity in HIV transmission rate is substantially lower than the observed disparity in HIV incidence: the black/white transmission rate-ratio in our model is 1·36, but the HIV incidence rate-ratio is 7·92 due to differences in HIV prevalence, population size and the tendency towards racially concordant relationships.
Our counterfactual scenarios illustrate the challenges with addressing HIV disparities by race because of the existing differences in prevalent positives. Even assuming that black MSM have a comparable continuum to white MSM and reducing the transmission rate-ratio to 1·0, black MSM will still experience an estimated HIV incidence 5·8 times that of white MSM. Under ideal (and challenging to achieve) concurrent 95% diagnosis and retention in care for black MSM, the results include an estimated reduction in the HIV transmission disparity to below 1·0 and a greatly reduced but nonetheless three fold incidence disparity. These findings suggest that, even if transmission-rate disparities are addressed, excess HIV prevalence among black MSM will continue to fuel disparities in HIV incidence for many years to come. Reversing this trend will only be possible by a sustained reduction in the HIV transmission rate to less than 1.0 for a sufficient period of time that will allow the current number of prevalent HIV-positive black MSM to be replaced by HIV-negative cohorts in successive generations.
The results of our analyses underscore the substantial challenges to reducing or eliminating black-white disparities in HIV incidence in the near-term. Our results suggest that we must address all elements of the HIV care continuum to achieve meaningful reductions in HIV incidence disparities. Doing so for all MSM is critical for care and transmission outcomes, given the low levels of viral suppression across racial/ethnic groups. Increasing HIV testing as a sole approach to reducing racial disparities will likely have limited impact. Even public health approaches that substantially address disparities in transmission rates for black MSM will not produce comparable reductions in HIV incidence. Therefore, it is important to bring additional approaches such as pre-exposure prophylaxis (PrEP) to scale as part of combination HIV prevention strategies for black MSM.26Because adequate estimates of the protective effective of PrEP among black MSM have yet to be published, such interventions for HIV-uninfected black MSM are not included in our model of the HIV-infected population. Although PrEP has a great potential in reducing incidence, scale-up may be inhibited by the same social/structural barriers that limit care outcomes among black MSM living with HIV.7
Our model and interpretations have important limitations. First, our input parameters are derived from different data systems from both 2009 and 2010. However, both of our data sources are nationally-representative data systems. Second, our primary model assumes serodiscordant partnerships that are fully racially-assortative. Accordingly, all changes to a racial group’s HIV care and resulting transmissions are attributed to that same group’s incident infections. Thus, the estimated changes in incidence reported reflect best-case scenarios. From sensitivity analyses of racial mixing in serodiscordant partnerships, we expect prioritizing HIV-positive black MSM for intervention may have lesser impact on black MSM incidence than our model estimates, but would benefit other racial groups. Also, our overall estimate of HIV incidence among MSM is lower than that reported in a meta-analysis representing mainly urban US, European, and Australian HIV epidemics.27 It is important to note that previous estimated HIV incidence rates among MSM populations were derived largely from men recruited in bars and other risk venues in urban areas, and represented mostly younger MSM, who tend to have higher HIV incidence than their older counterparts. Further, our estimates of HIV incidence are nearly identical to that derived from combining CDC’s estimates of 2009 incident infections among MSM using independent methodology. Further, our national estimate of HIV incidence rates improves earlier work by using incident infections, rather than diagnoses, in the numerator and adjusting the denominator of MSM “at risk” by subtracting the number of MSM living with HIV.23 Transmission rates used may not fully capture the incompletely understood role of acute infection in the MSM epidemic, possible undocumented behavioral or circulating viral differences between HIV-positive black and white MSM, or differences in host-susceptibility.28 Finally, because some source data reports did not include estimates of random error, we could not include these for our model results.
Our study has clear programmatic and policy implications. Because disparities in the HIV care continuum likely account for most HIV transmission rate disparities between black and white MSM, there is an urgent need to improve our rates of HIV testing, linkage and retention in care and prescription of and adherence to ART for black MSM living with HIV. Efficacious and cost-effective interventions are available to increase HIV testing, care engagement, and adherence to ART, although more research is needed on tailored interventions and resource allocation for this population.29 In addition, important socioeconomic disparities between white and black MSM need to be addressed as these may negatively impact the effectiveness of care continuum interventions. Dynamic models are needed to better evaluate the long-term outcomes of prevention interventions which achieve parity in HIV transmission rates between black and white MSM. Further, the transmission rate-ratio should be used as a more proximate indicator of the success of programs designed to reduce black/white HIV disparities. In terms of policy, our results highlight the importance of the 2013 presidential executive order focusing on the HIV care continuum, as well as the National HIV/AIDS Strategy’s prioritization of reducing HIV-related health disparities.20Lastly, our data illustrate the urgency of research towards an HIV cure and/or a highly effective HIV vaccine. Absent such transformational biomedical advances in HIV prevention, disparities by race in HIV incidence among MSM are likely to be a stubbornly tenacious characteristic of the US HIV epidemic in the foreseeable future.
Research in context
Systematic Review
We updated findings from a meta-analysis we recently published.11 Additional United States care-continuum and transmission results for MSM were located via Pubmed searches for the criteria “HIV care continuum MSM” and “HIV transmission MSM United States”, and by searching the HIV case surveillance report listing on the US Centers for Disease Control and Prevention website.
Interpretation
Large gaps exists in both existing infections and care continuum outcomes between black and white men who have sex with men (MSM), resulting in even larger racial disparities in HIV incidence. Even if extreme care interventions resulted in 2.3 transmissions per 100 HIV+ black MSM, compared to 4.0 transmissions per 100 HIV+ white MSM, HIV incidence disparities will persist because of many more prevalent positives in the black MSM community.
Supplementary Material
Acknowledgments
Sources of Funding
United States National Institutes of Health (NIH) R01MH085600, R21HD075662, R01DA038196, and P30AI050409 (The Emory Center for AIDS Research).
Funding
NIH R01MH085600;R21HD075662;R01DA038196;P30AI050409
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copy editing, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors state that no conflicts of interest exist.
Author contributions
ER conceived of the work, led the analysis and writing, and approved of the final version to be published
GM contributed to the concept development, analysis, writing, and approved of the final version to be published
PS contributed to the concept development, analysis, writing, and approved of the final version to be published
CdR contributed to the concept development, writing, and approved of the final version to be published
JC contributed to the concept development, writing, and approved of the final version to be published
References
- 1.Centers for Disease Control and Prevention . HIV Surveillance Report, 2011. Atlanta, GA: [accessed August 15, 2014]. 2013. Available at: http://www.cdc.gov/hiv/library/reports/surveillance/2011/surveillance_Report_vol_23.html. [Google Scholar]
- 2.Prejean J, Song R, Hernandez A, Ziebell R, Green T, Walker F, et al. Estimated HIV incidence in the United States, 2006-2009. PloS one. 2011;6(8):e17502. doi: 10.1371/journal.pone.0017502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.S. Department of Health and Human Services [accessed October 5, 2014];Office of Disease Prevention and Health Promotion. Healthy People 2020 - Disparities. Available at: http://www.healthypeople.gov/2020/about/foundation-health-measures/Disparities.
- 4.Samuel M, Winkelstein W. Prevalence of HIV in Ethnic Minority Homosexual/Bisexual Men. JAMA. 1987;257:1901–2. [PubMed] [Google Scholar]
- 5.Millett GA, Flores SA, Peterson JL, Bakeman R. Explaining disparities in HIV infection among black and white men who have sex with men: a meta-analysis of HIV risk behaviors. AIDS. 2007;21(15):2083–91. doi: 10.1097/QAD.0b013e3282e9a64b. [DOI] [PubMed] [Google Scholar]
- 6.Millett GA, Peterson JL, Wolitski RJ, Stall R. Greater risk for HIV infection of black men who have sex with men: a critical literature review. American Journal of Public Health. 2006;96(6):1007–19. doi: 10.2105/AJPH.2005.066720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sullivan PS, Peterson J, Rosenberg ES, Kelley CF, Cooper H, Vaughan A, et al. Understanding racial HIV/STI disparities in black and white men who have sex with men: a multilevel approach. PloS one. 2014;9(3):e90514. doi: 10.1371/journal.pone.0090514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kelley CF, Rosenberg ES, O’Hara BM, Frew PM, Sanchez T, Peterson JL, et al. Measuring population transmission risk for HIV: an alternative metric of exposure risk in men who have sex with men (MSM) in the US. PloS one. 2012;7(12):e53284. doi: 10.1371/journal.pone.0053284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Characteristics associated with HIV infection among heterosexuals in urban areas with high AIDS prevalence --- 24 cities, United States, 2006-2007. MMWR Morbidity and mortality weekly report. 2011;60(31):1045–9. [PubMed] [Google Scholar]
- 10.Rosenberg ES, Sullivan PS, Kelley CF, Sanchez TH, Luisi N, Del Rio C, et al. Race and Age Disparities in HIV Incidence and Prevalence Among MSM in Atlanta, GA. 2014 Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2014. [Google Scholar]
- 11.Millett GA, Peterson JL, Flores SA, Hart TA, Jeffries WLt, Wilson PA, et al. Comparisons of disparities and risks of HIV infection in black and other men who have sex with men in Canada, UK, and USA: a meta-analysis. Lancet. 2012;380(9839):341–8. doi: 10.1016/S0140-6736(12)60899-X. [DOI] [PubMed] [Google Scholar]
- 12.Beer L, Oster AM, Mattson CL, Skarbinski J. Disparities in HIV transmission risk among HIV-infected black and white MSM, Medical Monitoring Project, 2009. AIDS. 2014;28(1):105–14. doi: 10.1097/QAD.0000000000000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baggaley RF, White RG, Boily MC. HIV transmission risk through anal intercourse: systematic review, meta-analysis and implications for HIV prevention. International journal of epidemiology. 2010;39(4):1048–63. doi: 10.1093/ije/dyq057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koblin BA, Husnik MJ, Colfax G, Huang Y, Madison M, Mayer K, et al. Risk factors for HIV infection among men who have sex with men. AIDS. 2006;20(5):731–9. doi: 10.1097/01.aids.0000216374.61442.55. [DOI] [PubMed] [Google Scholar]
- 15.Sifakis F, Hylton JB, Flynn C, Solomon L, Mackellar DA, Valleroy LA, et al. Racial disparities in HIV incidence among young men who have sex with men: the Baltimore Young Men’s Survey. J Acquir Immune Defic Syndr. 2007;46(3):343–8. doi: 10.1097/QAI.0b013e31815724cc. [DOI] [PubMed] [Google Scholar]
- 16.Marks G, Millett GA, Bingham T, Bond L, Lauby J, Liau A, et al. Understanding Differences in HIV Sexual Transmission among Latino and Black Men who have Sex with Men: The Brothers y Hermanos Study. AIDS Behav. 2009 Aug;13(4):682–90. doi: 10.1007/s10461-008-9380-6. [DOI] [PubMed] [Google Scholar]
- 17.MacKellar DA, Hou SI, Whalen CC, Samuelsen K, Valleroy LA, Secura GM, et al. HIV/AIDS complacency and HIV infection among young men who have sex with men, and the race-specific influence of underlying HAART beliefs. Sexually transmitted diseases. 2011;38(8):755–63. doi: 10.1097/OLQ.0b013e31820d5a77. [DOI] [PubMed] [Google Scholar]
- 18.Hall HI, Holtgrave DR, Tang T, Rhodes P. HIV transmission in the United States: considerations of viral load, risk behavior, and health disparities. AIDS Behav. 2013;17(5):1632–6. doi: 10.1007/s10461-013-0426-z. [DOI] [PubMed] [Google Scholar]
- 19.Gardner EM, McLees MP, Steiner JF, Del Rio C, Burman WJ. The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clin Infect Dis. 2011;52(6):793–800. doi: 10.1093/cid/ciq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Executive order -- the HIV Care Continuum Initiative House TW 2013. Accessible at: http://www.whitehouse.gov/the-press-office/2013/07/15/executive-order-hiv-care-continuum-initiative.
- 21.Hall HI, Frazier EL, Rhodes P, Holtgrave DR, Furlow-Parmley C, Tang T, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA internal medicine. 2013;173(14):1337–44. doi: 10.1001/jamainternmed.2013.6841. [DOI] [PubMed] [Google Scholar]
- 22.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS. 2006;20(10):1447–50. doi: 10.1097/01.aids.0000233579.79714.8d. [DOI] [PubMed] [Google Scholar]
- 23.Purcell DW, Johnson CH, Lansky A, Prejean J, Stein R, Denning P, et al. Estimating the Population Size of Men Who Have Sex with Men in the United States to Obtain HIV and Syphilis Rates. The Open AIDS Journal. 2012;6:98–107. doi: 10.2174/1874613601206010098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bureau USC National Intercensal Estimates (2000-2010) 2010 Accessible at: http://www.census.gov/popest/data/intercensal/national/nat2010.html.
- 25.Raymond HF, McFarland W. Racial mixing and HIV risk among men who have sex with men. AIDS Behav. 2009;13(4):630–7. doi: 10.1007/s10461-009-9574-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sullivan PS, Carballo-Dieguez A, Coates T, Goodreau SM, McGowan I, Sanders EJ, et al. Successes and challenges of HIV prevention in men who have sex with men. Lancet. 2012;380(9839):388–99. doi: 10.1016/S0140-6736(12)60955-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stall R, Duran L, Wisniewski SR, Friedman MS, Marshal MP, McFarland W, et al. Running in place: implications of HIV incidence estimates among urban men who have sex with men in the United States and other industrialized countries. AIDS Behav. 2009;13(4):615–29. doi: 10.1007/s10461-008-9509-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen MS, Shaw GM, McMichael AJ, Haynes BF. Acute HIV-1 Infection. The New England Journal of Medicine. 2011;364(20):1943–54. doi: 10.1056/NEJMra1011874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Castro FG, Barrera M, Jr., Martinez CR., Jr. The cultural adaptation of prevention interventions: resolving tensions between fidelity and fit. Prevention science. 2004;5(1):41–5. doi: 10.1023/b:prev.0000013980.12412.cd. [DOI] [PubMed] [Google Scholar]
- 30.Whiteside YO, Cohen SM, Bradley H, Skarbinski J, Hall HI, Lansky A. Progress along the continuum of HIV care among blacks with diagnosed HIV-United States, 2010. MMWR Morbidity and Mortality Weekly Report. 2014;63(5):85–9. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.