Abstract
Background
Bacterial keratitis is a serious ocular infectious disease that can lead to severe visual disability. Risk factors for bacterial corneal infection include contact lens wear, ocular surface disease, corneal trauma, and previous ocular or eyelid surgery. Topical antibiotics constitute the mainstay of treatment in cases of bacterial keratitis, whereas the use of topical corticosteroids as an adjunctive therapy to antibiotics remains controversial. Topical corticosteroids are usually used to control inflammation using the smallest amount of the drug. Their use requires optimal timing, concomitant antibiotics, and careful follow-up.
Objectives
The objective of the review was to assess the effectiveness and safety of corticosteroids as adjunctive therapy for bacterial keratitis. Secondary objectives included evaluation of health economic outcomes and quality of life outcomes.
Search methods
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2014, Issue 6), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to July 2014), EMBASE (January 1980 to July 2014), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to July 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 14 July 2014. We also searched the Science Citation Index to identify additional studies that had cited the only trial included in the original version of this review, reference lists of included trials, earlier reviews, and the American Academy of Ophthalmology guidelines. We also contacted experts to identify any unpublished and ongoing randomized trials.
Selection criteria
We included randomized controlled trials (RCTs) that had evaluated adjunctive therapy with topical corticosteroids in people with bacterial keratitis who were being treated with antibiotics.
Data collection and analysis
We used the standard methodological procedures expected by The Cochrane Collaboration.
Main results
We found four RCTs that met the inclusion criteria of this review. The total number of included participants was 611 (612 eyes), ranging from 30 to 500 participants per trial. One trial was included in the previous version of the review, and we identified three additional trials through the updated searches in July 2014. One of the three smaller trials was a pilot study of the largest study: the Steroids for Corneal Ulcers Trial (SCUT). All trials compared the treatment of bacterial keratitis with topical corticosteroid and without topical corticosteroid and had follow-up periods ranging from two months to one year. These trials were conducted in the USA, Canada, India, and South Africa.
All trials reported data on visual acuity ranging from three weeks to one year, and none of them found any important difference between the corticosteroid group and the control group. The pilot study of the SCUT reported that time to re-epithelialization in the steroid group was 53% slower than the placebo group after adjusting for baseline epithelial defect size (hazard ratio (HR) 0.47; 95% confidence interval (CI) 0.23 to 0.94). However, the SCUT did not find any important difference in time to re-epithelialization (HR 0.92; 95% CI 0.76 to 1.11). For adverse events, none of the three small trials found any important difference between the two treatment groups. The investigators of the largest trial reported that more patients in the control group developed intraocular pressure (IOP) elevation (risk ratio (RR) 0.20; 95% CI 0.04 to 0.90). One trial reported quality of life and concluded that there was no difference between the two groups (data not available). We did not find any reports regarding economic outcomes.
Although the four trials were generally of good methodological design, all trials had considerable losses to follow-up (10% or more) in the final analyses. Further, three of the four trials were underpowered to detect treatment effect differences between groups and inconsistency in outcome measurements precluded meta-analyses for most outcomes relevant to this review.
Authors’ conclusions
There is inadequate evidence as to the effectiveness and safety of adjunctive topical corticosteroids compared with no topical corticosteroids in improving visual acuity, infiltrate/scar size, or adverse events among participants with bacterial keratitis. Current evidence does not support a strong effect of corticosteroid, but may be due to insufficient power to detect a treatment effect.
INDEX TERMS Medical Subject Headings (MeSH): Adrenal Cortex Hormones [*therapeutic use]; Chemotherapy, Adjuvant [methods]; Eye Infections, Bacterial [*drug therapy]; Keratitis [*drug therapy; microbiology]
MeSH check words: Humans
PLAIN LANGUAGE SUMMARY
Corticosteroid eye drops used in addition to standard antibiotic therapy in the treatment of bacterial keratitis
Review question
We reviewed the available information regarding the added effect of corticosteroid eye drops in people with bacterial keratitis (corneal ulcers) who were also being treated with antibiotics.
Background
Bacterial keratitis, or corneal inflammation due to bacterial infection, is a sight-threatening condition. Contact lens wear, ocular surface disease, corneal trauma, and previous ocular or eyelid surgery have been linked to bacterial keratitis. Antibiotic eye drops are the standard treatment for eyes with bacterial keratitis. Corticosteroid eye drops also may be used to control the inflammation from infection. Eye doctors disagree about whether corticosteroid eye drops should be used with antibiotics to treat this condition due to potential side effects of using steroids in the eye.
Study characteristics
We found four studies in which antibiotics alone had been compared with antibiotics plus corticosteroids for the treatment of bacterial keratitis. These studies were conducted in the USA, Canada, India, and South Africa, and included a total of 612 eyes of 611 participants. The largest study included 500 participants followed for one year. The three smaller studies followed participants for two to three months. The evidence is current to July 2014.
Key results
None of the four studies reported an important difference between topical corticosteroid therapy and placebo or control treatment for reduction in ulcer size, change in visual acuity, adverse events, or quality of life. One study reported that healing or cure time in the steroid group was slower than the placebo group (for every 100 people cured in the control group, only 47 were cured in the steroid group during the same time period), but the largest study did not report any difference (for every 100 people cured in the control group, 92 were cured in the steroid group during the same time interval). For adverse events, none of the studies found a difference between the two groups, except that one study reported that more eyes in the control group developed intraocular pressure (IOP) elevation. We did not find any information on economic outcomes.
Quality of the evidence
Generally, the quality of the evidence based on the four studies we identified was moderate due to the proportions of participants who were not included in the final study analyses and the inconsistency of outcomes assessed across the four studies. In addition, three studies enrolled too few participants (30 to 42) to reach scientifically valid conclusions.
BACKGROUND
Description of the condition
Introduction
Keratitis, also known as corneal inflammation or corneal ulcers, is a potentially sight-threatening condition that may have infectious or non-infectious etiology. Keratitis presents as a diagnostic challenge due to the large number of possible causes that may lead to this condition. Bacterial keratitis is a serious ocular infectious disease that can lead to severe visual loss. Risk factors for bacterial corneal infection include contact lens wear, ocular surface disease, corneal trauma, and previous ocular or eyelid surgery. Certain ocular diseases and systemic conditions that depress the immune system also increase the possibility of bacterial keratitis.
Epidemiology
Approximately 30,000 cases of microbial keratitis, which includes bacterial, fungal, and parasitic causes, are diagnosed annually in the United States (Pepose 1992). The proportion of people who develop corneal blindness secondary to bacterial keratitis is high in developing countries (Chirambo 1986; Feng 1990). The spectrum of bacterial keratitis also may be influenced by geographic and climate factors. Many differences in keratitis profile have been noted between populations living in rural or urban areas, in western or in developing countries (Baker 1996; Bennett 1998; Burton 2011; Kaliamurthy 2013; Schaefer 2001; Vajpayee 2000).
Certain bacteria that make up the normal ocular flora are usually implicated in cases of infectious keratitis. Due to the proximity of these organisms to the cornea, they are easily inoculated into damaged or abnormal corneal tissues. Host defenses are usually sufficient to prevent infection but once these are violated, for example, in trauma or debilitating diseases, florid bacterial contamination of ocular tissue may occur. Common causative organisms include staphylococci and streptococci, inherent residents of the ocular milieu (Miño de Kaspar 2005; Moeller 2005). In the past several years there has been an increase in the number of contact lens wearers (Poggio 1989). The incidence of bacterial keratitis secondary to use of extended-wear contact lenses is about 8000 cases per year. Multiple organisms have been isolated from cases of microbial keratitis in association with contact lens wear (Lee 2014; Poggio 1989). A higher prevalence of gram-negative rods, such as pseudomonas, was reported in contact lens wearers compared to patients who do not use contact lenses (Dart 1991; Schein 1989). Conditions that disrupt ocular homeostasis may set the stage for bacterial contamination of the cornea. Agricultural workers, contact lens wearers, and patients who have received any form of ocular surgery or trauma all have increased risk of bacterial kerati-tis. Ocular surface disorders such as recurrent erosions and tear film abnormalities, lid abnormalities, and use and abuse of topical medications all predispose to infections. Debilitating diseases, immunocompromised states, and chronic use of immunosuppressive drugs also contribute to this disease condition (Bourcier 2003; Killingsworth 1993; Musch 1983).
Presentation and diagnosis
Patients with bacterial keratitis present with unilateral and, in rare instances, bilateral, pain and abnormal sensitivity to light (photophobia). Corneal epithelial breaks that are associated with ulcers expose corneal nerve endings and contribute to the pain and discomfort that are associated with this condition.
Typically, the anterior segment of the eye is inflamed and congested. Intense and diffuse conjunctival vessel injection is frequently observed. The discharge may be thick and profuse and is often mucoid to purulent in nature. The eyelids may be edematous and swollen and the underlying palpebral conjunctivae inflamed.
The infected portion of the cornea usually contains a focal area of stromal infiltrate with an overlying area of epithelial excavation. The infiltrate is often, but not always, well circumscribed with distinct borders. The cornea is edematous and the visual acuity is reduced. The severity of visual loss is dependent on the extent and location of the lesion.
Severe cases of bacterial keratitis lead to profound anterior chamber reaction and hypopyon (an accumulation of pus cells in the anterior chamber). Ciliary body inflammation sometimes causes hypotony (lower intraocular pressure); on the other hand, the presence of inflammatory cells in the aqueous may also clog the trabecular meshwork and increase the intraocular pressure.
Determining the etiology of the keratitis by taking corneal scrapes and appropriate cultures is an essential step before the use of any topical antibiotic. However, empirical therapy need not be withheld until culture results become available. Unfortunately, there are no clear-cut signs that point to a bacterial cause of the keratitis. Patient history and on examination the status of the epithelium, the size and time to diagnosis of the corneal lesions, the degree of the stromal inflammation, the quality and quantity of discharge, and other associated findings all have to be considered to arrive at a presumptive diagnosis.
The yield from microbiological investigation may be low despite direct inoculation of a sample on the culture media. However, the positive culture will guide the ophthalmologist’s choice of appropriate antibiotic therapy.
Description of the intervention
Topical antibiotics constitute the mainstay of treatment in cases of bacterial keratitis, whereas the use of topical corticosteroids remains controversial. The role of topical corticosteroids as an adjunctive therapy for bacterial keratitis has been discussed in many studies. Topical steroids are usually given to control inflammation using the smallest possible amount of the drug. Their use requires optimal timing, concomitant antibiotics, and careful follow-up. The effect of treatment on the viability of bacteria in the cornea, corneal wound healing, corneal scarring, increase in intraocular pressure, clinical outcomes, and adverse events warrants comparison between antibiotics alone and antibiotics plus corticosteroids. Topical fluoroquinolone antibiotics are popular choices for initial broad-spectrum therapy (Gangopadhyay 2000; Hyndiuk 1996; O’Brien 1995; OSG 1997; Parks 1993). However, resistance to these drugs may occur with some organisms (Schaefer 2001). The new generation of fluoroquinolones, such as moxifloxacin and gatifloxacin, are currently gaining in popularity and these drugs show promise in the treatment of infectious corneal ulcers (Hyon 2004; Kowalski 2003).
Dosing of the antibiotic is often dependent on the size of the ulcer and severity of keratitis. In severe cases, subconjunctival, subtenon, or intravenous antibiotics are instituted. Cycloplegic eyedrops may be given to reduce pain and inflammation.
However, the role of topical corticosteroids as adjunctive therapy for bacterial keratitis is less clear.
How the intervention might work
Topical corticosteroids have consistently been avoided by many practitioners for fear of causing immunosuppression and, consequently, potentiating bacterial replication. Judicious use of steroids with adequate antibiotic therapy may, however, be beneficial for the patient. Once the micro-organisms have been eliminated and adequate sterilization has been achieved, lessening the inflammatory response through corticosteroids may reduce corneal neovascularization and scarring.
Why it is important to do this review
Topical steroids are used to control damage from inflammation in bacterial keratitis. Although corticosteroids as an adjunctive therapy for bacterial keratitis have been discussed in many published reports, recommendations regarding the type and concentration of corticosteroid, frequency of dosing, optimal timing with respect to the introduction of topical antibiotics, and stage of healing are not consistent. A systematic review of available studies is needed to further understanding of the use of corticosteroids in treating bacterial keratitis and better define their role as an adjunctive treatment modality for bacterial corneal ulcers.
This review is an update of a Cochrane review first published in 2007 (Suwan-apichon 2007), where there was only one trial published for the use of topical steroid for keratitis (Carmichael 1990). Over recent years, several additional trials have been conducted and investigated the effectiveness and safety of keratitis (Blair 2011; SCUT 2012; Srinivasan 2009), and we included these trials in the data synthesis for this review. The protocol for the review was published in 2005 (Suwan-apichon 2005).
OBJECTIVES
The objective of the review was to assess the effectiveness and safety of corticosteroids as adjunctive therapy for bacterial keratitis. Secondary objectives included evaluation of health economic outcomes and quality of life outcomes.
METHODS
Criteria for considering studies for this review
Types of studies
We included all relevant randomized controlled trials (RCTs) in this review.
Types of participants
We included studies in which participants were diagnosed to have bacterial keratitis either clinically or microbiologically. We excluded studies that included participants who had mixed infections and corneal perforations that warranted surgical intervention.
Types of interventions
We included studies using topical corticosteroids as an adjunct to antibiotics in the management of bacterial keratitis. Eligible studies included placebo-controlled trials and trials that compared different steroids against each other as adjunctive agents.
Types of outcome measures
Primary outcomes
The primary outcomes for comparison of the treatments were:
clinical improvement, defined as lessening of the ocular inflammation, congestion, pain, photophobia, and overall ocular discomfort; improvement in corneal clarity and visual acuity (i.e. best corrected visual acuity, BCVA), decrease in size of infiltrate and epithelial defect;
clinical cure, defined as complete healing (re-epithelialization) of the epithelium with scarring, disappearance of any sign of inflammation such as congestion and anterior chamber cellular reaction.
We reported the primary outcome at different times of follow-up, as described in the included studies.
Secondary outcomes
The secondary outcomes for comparison of the treatments were:
microbiologic cure, defined as sterilization of the cornea and absence of bacterial viability as shown by corneal smears and cultures;
time to clinical or microbiologic cure.
We planned to report the outcomes at different times of follow-up, as described in the included studies.
Adverse effects
Adverse effects of interest included:
persistence and progression of the corneal infection, defined as increasing infiltrate size and/or bacterial colony count in smear or culture-positive isolates;
corneal melting, descemetocele formation, and perforation;
endophthalmitis;
increased intraocular pressure, steroid-induced or inflammatory glaucoma;
ocular surface complications and allergic reactions attributable to the steroid application alone or to the combination of medications used;
recurrence of the corneal ulcer. This can happen at any time after the first ulcer, sometimes years later, so we recorded what was reported in the included studies.
We summarized adverse effects related to topical corticosteroid therapy reported in all included studies. However, we recognize the difficulty of differentiating between adverse effects of corticosteroid therapy and adverse outcomes of the progressive infections being treated with antibiotics.
Quality of life measures
We have described the quality of life findings reported in included studies.
Economic data
We will report the cost of adding steroids to the therapeutic regimen as assessed in any future trial(s) in updates of this review. No such data had been reported in the included studies.
Follow-up
To take into account the possibility of recurrence, we only included studies with at least one-month follow-up for analysis.
Search methods for identification of studies
Electronic searches
We searched CENTRAL (which contains the Cochrane Eyes and Vision Group Trials Register) (2014, Issue 6), Ovid MEDLINE, Ovid MEDLINE In-Process and Other Non-Indexed Citations, Ovid MEDLINE Daily, Ovid OLDMEDLINE (January 1946 to July 2014), EMBASE (January 1980 to July 2014), Latin American and Caribbean Health Sciences Literature Database (LILACS) (January 1982 to July 2014), the metaRegister of Controlled Trials (mRCT) (www.controlled-trials.com), ClinicalTrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (IC-TRP) (www.who.int/ictrp/search/en). We did not use any date or language restrictions in the electronic searches for trials. We last searched the electronic databases on 14 July 2014.
See: Appendices for details of search strategies for CENTRAL (Appendix 1), MEDLINE (Appendix 2), EMBASE (Appendix 3), LILACS (Appendix 3), mRCT (Appendix 5), ClinicalTrials.gov (Appendix 6) and the ICTRP (Appendix 7).
Searching other resources
We identified and screened through all reports that had cited the only trial included in the original version of this review using the Science Citation Index database. We screened the reference lists of newly included studies and earlier reviews, including Wilhelmus 2002, and abstracts from the American Academy of Ophthalmology meeting in 2003 (AAO 2003), to identify additional relevant studies. We did not handsearch any journals or conference proceedings specifically for this review as they are searched routinely by the Cochrane Eyes and Vision Group and added to the CENTRAL database.
Data collection and analysis
Selection of studies
Two review authors independently reviewed the titles and abstracts resulting from the literature searches, according to the inclusion criteria. We classified the titles and abstracts as ‘definitely relevant’, ‘possibly relevant’, or ‘definitely irrelevant’, and resolved disagreement by discussions. After reaching a consensus, we retrieved the full text for articles in the ‘definitely relevant’ or ‘possibly relevant’ categories and re-assessed the eligibility. We then labeled articles as ‘include’ or ‘exclude’, and resolved disagreements through discussion. For any unclear information, we requested additional information from the study investigators and allowed two weeks for them to respond. When they did not respond within two weeks, we used the information as available. We excluded full-text articles labeled as ‘exclude’, and documented the studies, along with reasons for exclusion, in the ‘Characteristics of excluded studies’ table.
Data extraction and management
Two review authors independently extracted data on study characteristics and outcomes listed in the ‘Criteria for considering studies for this review’ from the published reports of included studies using data extraction forms developed for this purpose. We resolved discrepancies through discussion. We contacted primary investigators of the studies for missing or unreported data. When they did not respond within two weeks, we used the information available. One review author entered the data into Review Manager 5.3 (RevMan 2014), and a second review author verified the data entry.
Assessment of risk of bias in included studies
Two review authors independently assessed the included studies for risk of bias according to guidelines set out in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We assessed each domain as ‘low risk’, ‘high risk’, or ‘unclear’ risk of bias. We considered methods employed to address the following risks of bias to determine the methodological quality of included studies:
Selection bias: we assessed random sequence generation and allocation concealment before randomization. Any method of allocation concealment, such as centralized randomization or use of sequential, opaque envelopes, which provided reasonable confidence that the allocation sequence was concealed from participating physicians and patients was to be considered ‘low risk’. We assessed trial reports without such explicit mention of a method of allocation concealment for convincing information on adequacy of allocation concealment. Whenever the adequacy of allocation concealment was unclear from the trial report, we contacted the primary investigators for clarification. If they did not respond within a two-week time period, we classified the studies based on available information and will update our classifications when more information becomes available.
Performance bias: we assessed masking of participants and care providers with regard to treatment allocation.
Detection bias: we assessed masking of outcome assessors with regard to treatment allocation.
Attrition bias: we assessed whether rates of follow-up and reasons for loss to follow-up for intervention and control arms were similar and whether all participants were analyzed in the group to which they were randomized. We also examined whether both participants for whom no outcome was collected, and those who received only some or none of their allotted treatment, were included in the analysis. We interpreted the analysis as intention-to-treat only when both the above criteria were fulfilled. We assessed studies following an intention-to-treat analysis as having ‘low risk’ of attrition bias.
Reporting bias: we considered studies that had reported all outcomes as specified in a protocol, clinical trial registry, or in the methods section of the published report as having ‘low risk’ of reporting bias.
We resolved disagreements through discussion. We contacted the authors of included studies for additional information on issues that we categorized as ‘unclear’ from information available in the trial reports. Whenever they did not respond within a two-week time period, we assessed the studies based on available information and will update our assessments when more information becomes available.
Measures of treatment effect
We reported a summary risk ratio (RR) for dichotomous outcomes (adverse events) when data were available. For continuous data (BCVA), we calculated the mean difference and 95% confidence interval between two intervention groups when sufficient data were provided. We reported time-to-event data (time to re-epithelialization) as a summary log hazard ratio using methods described in Parmar 1998 to extract information on observed and log-rank expected events from the included studies.
Unit of analysis issues
The unit of analysis for this review was the eye or the person, because all four studies included one eye per participant. For future studies including both eyes, where one eye is allocated to one intervention group and the other eye is allocated to the other intervention group, we will consider intra-person correlation when conducting the analysis, and refer to the principles outlined in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b).
Dealing with missing data
We contacted study investigators whenever there was missing or unclear information. When they did not respond within two weeks, we proceeded with available data.
Assessment of heterogeneity
We evaluated clinical and methodological heterogeneity in terms of study characteristics, participant inclusion/exclusion criteria, and primary and secondary outcomes. We assessed statistical heterogeneity using summary test statistics (I2 statistic). When the I2 statistic was greater than or equal to 50%, we also examined the Chi2 statistic for heterogeneity, the degree of overlap in confidence intervals, and the directions of treatment effect of included studies. Poor overlap suggests the presence of heterogeneity.
Assessment of reporting biases
For selective outcome reporting, we assessed the bias by comparing the protocols of the study and the published final report(s). We also compared the outcomes specified in the Methods section and reported in the Results section to identify potential selective outcome reporting. For future updates of the review, when there are at least 10 studies included in a meta-analysis, we will examine the symmetry of the funnel plot for the meta-analysis in order to assess the potential for publication bias.
Data synthesis
We conducted data analysis according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 9 (Deeks 2011). In future updates of the review, we plan to assess the inconsistency of effect estimates across studies using the I2 statistic when sufficient data are available. Our a priori decision was not to combine the outcomes from multiple studies in a meta-analysis in the presence of substantial heterogeneity, i.e. an I 2 statistic greater than 50%. In the absence of substantial statistical and clinical heterogeneity, we combined the results of included studies using a random-effects model. We plan to use a fixed-effect model whenever fewer than three studies contribute data to a meta-analysis.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analysis based on the causative organism. As there was only one article with such information, reporting the Nocardia species, we provided descriptive summaries of those findings.
Sensitivity analysis
We planned to conduct sensitivity analyses to assess how robust the review results are to key decisions and assumptions made during the review. We will conduct sensitivity analyses of data in updates of this review with the following adjustments when a sufficient number of studies can be included:
exclusion of studies with high risk of bias, specifically for loss of follow-up and selective outcome reporting;
exclusion of unpublished studies.
RESULTS
Description of studies
Results of the search
The initial electronic searches returned 336 titles and abstracts as of 15 January 2007. We screened the titles and abstracts of these records and judged 16 to be potentially relevant. On review of full-text reports, we included one trial in the original review and excluded the remaining 15 studies.
Updated searches conducted as of 14 July 2014 yielded a total of 562 titles and abstracts and six records from clinicaltrials.gov. We screened the records and judged 19 titles and abstracts and two records from clinicaltrials.gov to be potentially relevant. Upon retrieving full-text reports and assessing the eligibility for each study, we found three studies that met our inclusion criteria, among which one trial was a pilot study (Srinivasan 2009) of another large trial (SCUT 2012); 16 reports emanated from the same large trial (SCUT 2012) and two reports from another trial (Blair 2011). For the two records from clinicaltrials.gov, one trial already had been included in our review (SCUT 2012); we recorded the characteristics of the other trial in the ‘Characteristics of ongoing studies’ table. The search and selection flow diagram is shown in Figure 1.
Figure 1. Results from searching for studies for inclusion in the review.
Included studies
We included four studies, summarized below. Further details are recorded in the ‘Characteristics of included studies’ table.
Types of participants
The four studies included a total of 612 eyes of 611 participants. The largest trial had 500 eligible participants and eyes (SCUT 2012). The other three studies enrolled 30 to 58 participants. All participants in the included studies were aged 12 years or older at time of presentation with corneal ulcers. One study was conducted in South Africa and randomized 40 eyes of 39 participants (Carmichael 1990). Srinivasan 2009 was a pilot study of SCUT 2012 (the Steroids for Corneal Ulcers Trial, SCUT). The pilot study investigators enrolled 42 participants from 4 January 2005 to 20 August 2005 at a single center in India. SCUT 2012 enrolled 500 participants from 1 September 2006 to 22 February 2010 from multiple centers across India and the United States. The fourth study was conducted in Canada and the investigators enrolled 30 eyes of 30 participants (Blair 2011).
Types of interventions
All four studies included topical corticosteroid as an adjunctive therapy to antibiotics in one arm and compared it with antibiotics only in the other arm. Each of the studies used slightly different drugs and concentrations as described below:
Carmichael 1990 compared corticosteroid (0.1% dexamethasone) plus antibiotics (cefazolin, fortified, 32 g/l, and gentamicin, fortified, 14 g/l) or placebo (0.9% sodium chloride) plus antibiotics. Both the pilot study (Srinivasan 2009) and SCUT (SCUT 2012) compared 1% topical prednisolone phosphate or placebo (0.9% sodium chloride) in adjunct to antibiotics. All participants received a minimum of 48 hours of topical moxifloxacin treatment before randomization and continued to receive the drug every two hours until re-epithelialization and then four times a day until three weeks after enrollment.
Blair 2011 compared an antibiotic-only group (gatifloxacin and placebo) with an antibiotic-steroid group (gatifloxacin and 0.1% dexamethasone).
Types of outcomes
All four studies reported visual acuity, healing rate, ulcer size, or time to re-epithelialization. However, each of them reported data at different time points, or used different methods for data analysis. A comparison across studies is listed in Table 1.
Table 1. Clinical outcomes reported in the included studies.
| Outcomes | Blair 2011 | Carmichael 1990 | SCUT 2012 | Srinivasan 2009 | |
|---|---|---|---|---|---|
| Visual acuity | Effect measure | Mean change in log-MAR visual acuity from baseline, measured by ETDRS chart | Mean BCVA from Snellen chart and then converted to a numerical equivalent number; linear regression model | Mean difference in logMAR BCVA between groups from multiple linear regression model, using a protocol adapted from AREDS using a tumbling “E” chart | Mean logMAR BCVA adapted from AREDS using a tumbling “E” chart; mean difference in logMAR BCVA between groups from multiple linear regression model |
| Time point(s) measured | 10 weeks | 2 months | 3 weeks, 3 months, 12 months | 3 weeks and 3 months | |
| Estimate |
|
|
|
|
|
| Interpretation | No statistically significant difference between groups was found | No statistically significant difference between groups was found | No statistically significant difference between groups was found | No statistically significant difference between groups was found | |
| Healing rate/ulcer size /time to re-epithelialization | Effect measure | Mean change in ulcer area from baseline (mm2), measured by photographic measurement and by clinician estimate (slit lamp) | Mean size of ulcer (mm2) healed per day, measured at the slit lamp | Mean difference in diameter of ulcer area from linear regression models, measured by a Haag-Streit 900 slit lamp biomicroscope; median time to re-epithelialization (days) | Mean difference in diameter of ulcer area from linear regression models, measured by a Haag-Streit 900 slit lamp biomicroscope |
| Time point(s) measured | 10 weeks | 21 days | 3 weeks, 3 months, 12 months | 3 weeks and 3 months | |
| Estimate |
|
|
|
|
|
| Interpretation | No statistically significant difference between groups from photographic measurement; statistically significant difference between groups from clinician estimate | No statistically significant difference between groups was found | No statistically significant difference between groups was found | No statistically significant difference between groups was found | |
AREDS: Age Related Eye Disease Study
BCVA: best corrected visual acuity
CI: confidence interval
ETDRS: Early Treatment Diabetic Retinopathy Study
SD: standard deviation
The study investigators of Carmichael 1990 measured healing rates and visual acuity in the two groups, with follow-up time at a minimum of four weeks.
The primary outcome for both the pilot study (Srinivasan 2009) and the SCUT (SCUT 2012) was BCVA at three months from enrollment. BCVA was measured using a tumbling E chart. Secondary outcomes included BCVA at three weeks, infiltrate/scar size at three weeks and three months measured by slit lamp examination, adverse events, and time to re-epithelialization. The SCUT investigators followed participants for 12 months. At 12 months, 399 out of the initial 500 patients enrolled returned for a follow-up examination (SCUT 2012). At this time point, the outcomes examined were BCVA and scar size.
Blair 2011 reported measurements included ulcer size, visual acuity, time to healing, and quality of life at 10 weeks follow-up.
Funding sources
Funding sources for Carmichael 1990 were not reported.
The SCUT (SCUT 2012) was funded by the National Eye Institute and the pilot study (Srinivasan 2009) was funded by That Man May See, the South Asia Research Fund, and the National Eye Institute.
Blair 2011 was funded by the Physicians’ Services Incorporation Foundation.
Excluded studies
Excluded studies along with reasons for exclusions are listed in the ‘Characteristics of excluded studies’ table.
Risk of bias in included studies
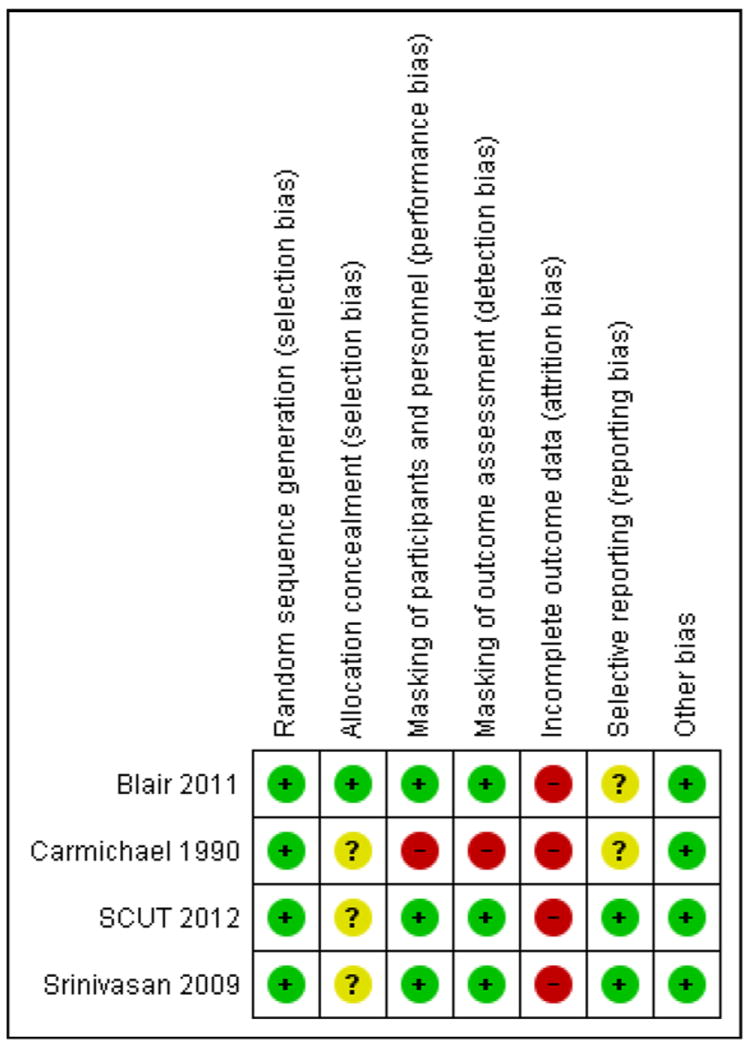
Individually, the studies included in this review were at low risk of bias, except for Carmichael 1990, which was at high or unclear risk of bias for five of the seven domains. Overall, all studies were high risk of attrition bias. The results of the ‘Risk of bias’ assessments are given in the ‘Characteristics of included studies’ table and Figure 2. A descriptive summary of studies included in this review is given below.
Figure 2. ’Risk of bias’ summary: review authors’ judgements about each risk of bias item for each included study.
Allocation
The method for randomization was adequately addressed in all four included studies. Carmichael 1990 used a random numbers table. Srinivasan 2009 used block randomization with block of 10 generated by RAND command in Excel. Blair 2011 used stratification and block randomization with blocks of six. Randomization was stratified by ulcer size in this study, as ulcer size is believed to be an important confounder. SCUT 2012 used permuted blocks within study centers. Random block sizes were four, six, and eight. Allocation was concealed before randomization only in Blair 2011; the allocation schedule was generated at a central office using a computer algorithm from the uniform distribution (STATA Corp, College Station, Tex.). The generator of the random allocation did not participate in executing the intervention and the executors did not participate in generating the schedule.
Masking (performance bias and detection bias)
For Carmichael 1990, neither the participants nor the physicians caring for them were masked to the treatment allocation. Outcomes were assessed independently by two physicians, but neither was masked to the treatment allocation. Masking of participants and investigators was achieved in the other three studies.
Incomplete outcome data
Losses to follow-up were common in all studies. Carmichael 1990 reported healing rates for only 26/40 (65%) eyes and visual acuity for only 28/40 (70%) eyes in the final analysis. Participants were excluded due to adverse events such as persistent epithelial defects and corneal thinning. Blair 2011 reported 26/30 (87%) eyes in the final analysis, where there was one participant (6.7%) missing for the antibiotic-only group and three participants (20%) missing for the antibiotic-steroid group. Srinivasan 2009 reported 33/42 (79%) eyes and SCUT 2012 reported 442/500 (88%) eyes in the final analysis. The reasons for loss to follow-up were not reported for any of the latter three studies. Therefore, we judged all four studies to have high risk of attrition bias.
Selective reporting
The risk of reporting bias for Carmichael 1990 and Blair 2011 was unclear because protocols were not available for these studies. The SCUT trial had reported all outcomes pre-specified in the protocol (SCUT 2012). The protocol for Srinivasan 2009 was not available, but because this is a pilot trial of the SCUT, we compared the two studies as well as the outcomes specified in the methods section and reported in the results sections for Srinivasan 2009, and we found the outcomes reported in these two studies to be the same. Srinivasan 2009 had reported all outcomes specified in the methods section, so we considered Srinivasan 2009 to be at low risk of outcome reporting bias.
Other potential sources of bias
We did not identify any other potential source of bias among the included studies.
Effects of interventions
Although different corticosteroids and different antibiotics were used in these four studies, all studies compared antibiotic treatment with versus without corticosteroids. Three studies performed a sample size calculation (Blair 2011; SCUT 2012; Srinivasan 2009). Blair 2011 estimated that 54 participants (27 participants per arm) would provide 80% power to detect a 4 mm2 difference in ulcer size between groups, with a standard deviation (SD) of 3.3 mm. However, the required sample size was not achieved. Srinivasan 2009 reported that 42 participants (21 participants per arm) would provide 80% power to detect a 0.4 logMAR (four Snellen lines) difference between the two study arms, assuming a SD of 0.4 in the three-month best corrected visual acuity (BCVA); however, due to losses to follow-up, the power was not maintained through the three-month follow-up examination. SCUT 2012 investigators estimated that a sample size of 500 participants (250 per arm) would provide 80% power to detect a 0.20 logMAR (two lines of visual acuity) difference between groups in BCVA three months after enrollment, assuming a SD of 0.65 logMAR for three-month BCVA. The calculation assumed a 20% dropout rate by three months. Only the Steroids for Corneal Ulcers Trial (SCUT) trial had a sample size large enough to detect or rule out an important between-group difference (SCUT 2012). However, even SCUT was not powered to detect or rule out a difference between management approaches within causative organism-specific subgroups.
A summary table is shown in Table 1. We also describe the details below:
Primary outcomes (clinical improvement and clinical cure)
For primary outcomes of clinical improvement and clinical cure, the four studies reported data from a total of 529 eyes. Time to re-epithelialization was reported by two studies, but all other outcomes were reported in different ways that precluded quantitative synthesis of the data.
Visual acuity
The four studies reported visual acuity at the last follow-up (ranged from 2 months to 12 months) for 486 eyes. The four studies reported visual acuity measured in different ways and at different time points. In one study, best corrected visual acuity (BCVA) was measured on a Snellen chart at different times, then converted to author-defined integer numbers ranging from 2 to 13, and fitted to a line for each participant using a linear regression model (Carmichael 1990). In another study, baseline, three-week, and three-month logMAR best corrected visual acuity (BCVA) was reported (Srinivasan 2009). In the third study, the investigators reported change of logMAR visual acuity (VA) from baseline to 10 weeks (Blair 2011). The investigators of the largest trial also reported visual acuity change using a linear regression model (SCUT 2012). Therefore, we were unable to combine the visual acuity outcome data. However, none of the studies reported a significant difference in visual acuity between the treatment groups. A descriptive summary is below:
The original data for Carmichael 1990 could not be located by the primary investigator. Carmichael 1990 reported 28 eyes for best corrected visual acuity at two months, and that there was no significant difference between the two groups from the regression model.
Srinivasan 2009 reported logMAR BCVA in 33 eyes of 33 participants at three weeks and three months. In the placebo group, the mean BCVA at enrollment was 1.15 logMAR (SD 0.63); it improved to 0.75 logMAR (SD 0.75) at three weeks and 0.59 logMAR (SD 0.75) at three months. In the steroid-treated group, the mean enrollment visual acuity was 1.28 logMAR (SD 0.54); it improved to 0.66 logMAR (SD 0.68) at three weeks and 0.71 logMAR (SD 0.72) at three months. The baseline BCVA between the two groups was judged to be comparable by the authors of the study report (mean difference (MD) -0.13; 95% confidence interval (CI) -0.48 to 0.22; P value = 0.48). They concluded that, compared to placebo treatment, steroid treatment resulted in 0.19 lower (better) logMAR visual acuity (1.9 lines) at three weeks (95% CI 20.52 to 0.15, P value = 0.26) and 0.09 lower logMAR visual acuity (0.9 line) at three months (95% CI 20.41 to 0.24, P value = 0.60).
Blair 2011 reported that the mean change of logMAR visual acuity in 26 eyes of 26 participants at 10 weeks was less in the antibiotic alone group than that in the steroid plus antibiotic group (MD 0.42; 95% CI not available; P value = 0.52).
SCUT 2012 reported results from 442 eyes of 442 participants at three months, and 399 eyes of 399 participants at 12 months. Based on a multiple linear regression analysis, the authors concluded that corticosteroids offered no significant improvement compared with placebo with respect to three-month and 12-month BCVA after controlling for enrollment BCVA: change from baseline to three-month follow-up (MD -0.009 logMAR; 95% CI -0.085 to 0.068; P value = 0.82); change from baseline to 12-month follow-up (MD -0.04 logMAR; 95% CI -0.12 to 0.05; P value = 0.39).
Healing rate/ulcer size/time to re-epithelialization
Clinical cure of corneal ulcers was assessed either by quantification of the size of the ulcer or the scar (by the clinician at the slit lamp or by photography) and by evaluating the size of the epithelial defect in order to determine closure or healing of the corneal epithelium. Two studies reported healing rates in 52 eyes (Blair 2011; Carmichael 1990). Carmichael 1990 reported similar healing rates: 0.36 mm2 per day in the corticosteroid group and 0.30 mm2 per day in the control group; the difference was neither clinically nor statistically significant. The Kaplan-Meier analysis of time to healing used in Blair 2011 showed no difference in healing rates between the two groups: median healing time was eight weeks in the antibiotic-only group versus six weeks in the antibiotic-steroid group (data not available).
Three studies reported ulcer size at the last follow-up examination in a total of 458 eyes (Blair 2011; SCUT 2012; Srinivasan 2009). Blair 2011 measured ulcer size from photographs; the mean difference in change from baseline in the antibiotic group was less than in the steroid group (-1.919 mm2 in the antibiotic-only group versus -4.388 mm2 in the antibiotic-steroid group; P value = 0.56) at 10 weeks. However, mean residual ulcer size at 10 weeks estimated by the clinician at the slit lamp differed between the two groups (-0.789 mm2 for the antibiotic-only group and -4.206 mm2 for the antibiotic-steroid group; P value = 0.05).
SCUT 2012 reported that corticosteroid use was not associated with a difference in infiltrate/scar size at three weeks (MD 0.05 mm2; 95% CI -0.09 to 0.15, P value = 0.60), three months (MD 0.06 mm2; 95% CI -0.07 to 0.17, P value = 0.40), or one year (MD 0.03 mm2; 95% CI -0.12 to 0.18; P value = 0.69). Srinivasan 2009 reported that steroid treatment was associated with somewhat smaller infiltrate/scar diameter compared with the placebo group at three weeks and three months (differences not available; P value = 0.23 and P value = 0.53 for three weeks and three months respectively).
In two studies, clinical cure (“healing time” or “cure rate”) was defined by re-epithelialization at the last follow-up examination; 432 eyes contributed to analysis of this outcome (SCUT 2012; Srinivasan 2009). SCUT 2012 reported that the median time to re-epithelialization was 7.0 days (95% CI 5.5 to 8.5 days) in the placebo arm and 7.5 days (95% CI 5.5 to 8.5 days; P value = 0.25) in the corticosteroid arm (hazard ratio 0.92; 95% CI 0.76 to 1.12; P value = 0.44). Srinivasan 2009 reported that the average time to re-epithelialization was 6.3 (SD 3.1) days in the placebo group and 8.6 (SD 4.7) days in the steroid-treated patients; after adjusting for baseline epithelial defect size, the hazard ratio was 0.47 (95% CI 0.23 to 0.94, P value = 0.03). The heterogeneity was high (I2 > 50% and there was poor overlap of confidence intervals on individual estimates), therefore we present the data from the two studies in the forest plot without a pooled analysis (Analysis 1.1). Given that these two studies have the same inclusion/exclusion criteria for participants, such a high heterogeneity might be due to the differences in baseline characteristics of the participants, such as ulcer size/location/depth (lacking these data for the pilot study), organisms distribution, and population origins (India versus India and the USA).
Secondary outcomes (microbiologic cure and time to clinical or microbiologic cure)
No studies reported microbiologic cure and time to clinical or microbiologic cure outcomes as listed in the methods section of the review.
Adverse effects
Four studies, including 612 eyes of 611 participants, contributed data for adverse effects (Table 2).
Table 2. Adverse events reported in the included studies.
| Adverse event | Blair 2011 | Carmichael 1990 | SCUT 2012 | Srinivasan 2009 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Steroid | Control | RR (95% CI) | Steroid | Control | RR (95% CI) | Steroid | Placebo | RR (95% CI) | Steroid | Placebo | RR (95% CI) | |
| Recurrence of the ulcer | 1/14 | 0/12 | 2.60 (0.12 to 58.48) | 1/21 | 2/19 | 0.45 (0.04 to 4.60) | / | / | / | / | / | / |
| Second ulcer | 0/14 | 1/12 | 0.29 (0.01 to 6.50) | / | / | / | / | / | / | / | / | / |
| Corneal thinning | / | / | / | 1/21 | 1/19 | 0.90 (0.06 to 13.48) | 0/250 | 2/250 | 0.20 (0.01 to 4.14) | / | / | / |
| Corneal perforation | / | / | / | 1/21 | 1/19 | 0.90 (0.06 to 13.48) | 7/250 | 8/250 | 0.88 (0.32 to 2.38) | 0/20 | 2/22 | 0.20 (0.01 to 3.94) |
| Uncontrolled infection | / | / | / | 0/21 | 2/19 | 0.18 (0.01 to 3.56) | / | / | / | / | / | / |
| Persistent epithelial defect | / | / | / | 4/21 | 3/19 | 1.21 (0.31 to 4.71) | 44/250 | 27/250 | 1.63 (1.04 to 2.54) | / | / | / |
| Epithelial break-down | / | / | / | 1/21 | 1/19 | 0.90 (0.06 to 13.48) | / | / | / | / | / | / |
| Death | / | / | / | / | / | / | 7/250 | 5/250 | 1.40 (0.45 to 4.35) | / | / | / |
| Systemic infection | / | / | / | / | / | / | 1/250 | 0/250 | 3.00 (0.12 to 73.29) | / | / | / |
| Increase in hypopyon | / | / | / | / | / | / | 4/250 | 4/250 | 1.00 (0.25 to 3.95) | / | / | / |
| IOP elevation | / | / | / | / | / | / | 2/250 | 10/250 | 0.20 (0.04 to 0.90) | 0/20 | 1/22 | 0.37 (0.02 to 8.48) |
| Worsening infiltrate | / | / | / | / | / | / | 9/250 | 4/250 | 2.25 (0.70 to 7.21) | 0/20 | 1/22 | 0.37 (0.02 to 8.48) |
| Other | / | / | / | / | / | / | 9/250 | 13/250 | 0.69 (0.30 to 1.59) | / | / | / |
CI: confidence interval
IOP: intraocular pressure
RR: risk ratio
Carmichael 1990 reported that one eye in each group experienced perforation, corneal thinning, and epithelial breakdown (breakdown of epithelium after initial healing). Two eyes in the control group had uncontrolled infection. Recurrence of infection (recurrence of hypopyon within one week of discharge after adequate treatment of infection) occurred in one eye in the corticosteroid group and two eyes in the control group. Persistent epithelial defect was reported in four eyes in the corticosteroid group and three eyes in the control group. Though the overall number of complications was more in the control group (10/19) compared to that in the corticosteroid group (n = 8/21) (risk ratio (RR) 1.38; 95% CI 0.69 to 2.76), the small number of participants in each group and the high proportion lost to follow-up preclude reliable estimates of complication rates or differences in rates between treatment arms. Blair 2011 reported adverse events in two patients. One patient in the antibiotic-steroid group experienced a possible recurrence of the ulcer at week 10 (five weeks after stopping all medications), but repeat culture was negative. One patient in the control group experienced a second corneal ulcer that was confirmed by culture. Srinivasan 2009 found no systemic adverse events. There were four ocular adverse events and all occurred in the control group. SCUT 2012 reported that there was no significant difference in the total number of adverse events (Table 2). The study also found no cases of other serious adverse event, such as endophthalmitis, intraocular pressure (IOP) > 35 mm Hg, and myocardial infarction or stroke. The overall RR was 1.15 (95% CI 0.56 to 2.37) for the serious adverse events and 1.18 (95% CI 0.77 to 1.79) for the non-serious adverse events.
Quality of life measures
One study of 26 eyes of 26 participants collected data regarding quality of life using the VF-14 (Blair 2011). No difference in mean change in quality of life was reported (6.2 for the antibiotic-only group and 9.7 for the antibiotic-steroid group; standard deviations not available; P value = 0.42).
Economic data
No included study reported economic data.
Subgroup analyses
Outcomes in the only causative organism-specific subgroup, Nocardia species, were reported in two articles based on the SCUT data (SCUT 2012). Nocardia species were the third most commonly isolated organism. Lalitha et al reported that there was no difference in visual acuity, time to re-epithelialization, or perforation rates between the corticosteroid and placebo-treated groups. However, in this organism-specific subgroup, the use of corticosteroids was associated with significantly larger infiltrates or scar sizes at three months when compared to keratitis caused by other organisms, suggesting that the use of corticosteroids is not beneficial and may result in worse outcomes regarding corneal infiltrate and scarring when bacterial keratitis is caused by Nocardia.
In the one-year report of the SCUT trial, a regression analysis that used a model that included a Nocardia-treatment arm interaction term found corticosteroid use associated with a mean one-line improvement in BCVA at 12 months among patients with non-Nocardia ulcers (MD -0.10 logMAR; 95% CI -0.19 to -0.02; P value = 0.02). No significant difference was observed in 12-month BCVA for Nocardia ulcers (MD 0.18 logMAR; 95% CI -0.04 to 0.41; P value = 0.16). In the same article, the authors also reported that corticosteroids were associated with larger mean scar size at 12 months among Nocardia ulcers (MD 0.47 mm; 95% CI 0.06 to 0.88; P value = 0.02), while no difference was identified for the non-Nocardia ulcers.
DISCUSSION
Summary of main results
Microbial keratitis is a potentially sight-threatening condition and an important cause of corneal inflammation. It has been estimated that 500,000 persons develop ulcerative keratitis annually around the world (Wilhelmus 2002).
We identified four randomized controlled trials for inclusion in this review. Based on review of the data from 612 eyes of 611 participants enrolled in those trials, the evidence regarding use of corticosteroids as adjuncts to antibiotics is inconclusive due to the small numbers of participants enrolled in three studies and the large number of enrollees with incomplete outcome data due to losses to follow-up in all four studies.
The investigators of the earliest trial reported no adverse effect when using dexamethasone 0.1% four times a day in addition to topical antimicrobial therapy (Carmichael 1990). The authors suggested that steroid treatment should be initiated 24 hours after antimicrobial therapy. Other helpful suggestions for clinicians have been proposed by Wilhelmus 2002.
The Steroids for Corneal Ulcers Trial (SCUT) was a large randomized, placebo-controlled, double-masked, multicenter clinical trial that compared prednisolone sodium phosphate (1.0%) to placebo as adjunctive therapy for the treatment of bacterial corneal ulcers (SCUT 2012). The pilot study for this trial included 42 participants with culture-confirmed bacterial keratitis that were randomized to the same treatments used in SCUT (all participants received topical moxifloxacin 0.5%) (Srinivasan 2009). Although corticosteroid treatment resulted in a statistically significant delay in re-epithelialization, no difference in best corrected visual acuity (BCVA), infiltrate/scar size, or adverse events at three months was observed.
Although SCUT randomized 500 participants, only 442 participants (88.4%) were evaluated three months later and 399 participants 12 months later (SCUT 2012). No clinically or statistically significant differences between treatment arms were observed for any outcome in this review. However, more participants in the placebo arm developed mildly increased intraocular pressure (IOP) (> 25 but < 35 mmHg) when compared to the corticos-teroid-treated group (P value = 0.04). Notably, subgroup analysis showed that corticosteroid treatment was associated with a benefit in visual acuity compared with the placebo group in participants with the worst visual acuity and central ulcer location at baseline. In the SCUT, 56 participants (11%) presented with ulcers caused by Nocardia species. When this subgroup of patients was analyzed separately (Lalitha et al), the use of corticosteroids was associated with a larger infiltrate or scar size at three months and 12 months when compared to placebo.
In SCUT, most patients experienced the majority of BCVA improvement during the first three months after treatment was initiated, although smaller but still significant improvements were observed up to 12 months after starting treatment.
Blair 2011 found no significant difference between the corticosteroid and placebo groups with respect to residual ulcer size at 10 weeks compared with the baseline size, healing rate, or final BCVA. Only the clinician estimates of ulcer size at the slit lamp provided evidence of benefit of adjunctive corticosteroid.
In summary, the available evidence does not support a benefit of corticosteroid use as adjunctive therapy for bacterial keratitis. Three studies were small in sample size and possibly underpowered to detect differences. The SCUT trial was designed to detect a difference between groups but the results may have limited generalizability (SCUT 2012). (See ‘Agreements and disagreements with other studies or reviews’ section). The available data regarding safety of topical corticosteroid is inconclusive. None of the three small studies found significant differences between groups, but the SCUT trial reported more risk of IOP elevation in the control group. Although three studies were relatively well-designed and had large enough sample sizes to satisfy the power of detection, loss to follow-up was an issue for all included studies. In addition, considering subgroup analyses and the generalizability of findings, one or more well-designed and properly conducted randomized controlled trials are still desirable.
Overall completeness and applicability of evidence
As the mainstay of treatment for bacterial keratitis, topical antibiotics are used primarily to eliminate the causative organisms. However, host corneal inflammatory response may in some cases cause more damage than the infection itself. The use of topical corticosteroids in addition to antimicrobial therapy in the treatment of bacterial keratitis has been controversial for over 50 years. Their anti-inflammatory activity may help control the host response, and reduce corneal neovascularization and scarring, thus favoring the clinical outcome. On the other hand, the immunosuppressive effect of corticosteroids may actually promote bacterial replication and slow recovery of the patient. Based on our review, the evidence regarding the effectiveness and safety of adjunctive corticosteroid use in bacterial keratitis is inconclusive.
Quality of the evidence
Based on our assessment of trial quality per pre-specified criteria, we judged the overall quality of evidence as moderate. Random sequence generation was adequately performed in all four studies. Masking of participants, personnel, and outcome assessors was achieved in three studies, with the exception of Carmichael 1990. Compared with the protocol, the SCUT trial and its pilot study were consistent in reporting all pre-specified outcomes. We did not find publicly available protocols for the other two studies. Incomplete outcome data was a major issue for all these four studies in that more than 10% of the participants were lost to follow-up and not included in the final analyses, possibly biasing the findings from all four studies and, thus, of this review. The analysis of SCUT data regarding outcomes for Nocardia species organisms was an ancillary analysis and not based on randomization to treatment arm within that subgroup of patients.
Potential biases in the review process
We are unaware of any potential biases in the review process. We searched multiple databases to identify randomized controlled trials (RCTs) relevant to this review. As part of our search, we screened nearly 500 citations to identify the four RCTs included. Data extracted from reports focused on clinical and functional outcomes and were confirmed by at least two authors. Thus, our conclusion, i.e. that current evidence does not suggest a difference in the effectiveness of topical corticosteroid use adjunctive to antibacterial therapy in bacterial keratitis, is based on established, reproducible methods.
Agreements and disagreements with other studies or reviews
Steroids have been implicated as a risk factor for the development of corneal ulcers; however, the use of topical steroids adjunctive to antibiotics is still deliberated today.
Several suggestions have been proposed by authors, such as to delay the use of steroids for at least 24 hours to exclude rapidly deteriorating infections and fungal ulcers (Carmichael 1990). Other useful suggestions were published by Wilhelmus 2002, which included certain recommendations such as to minimize corticosteroid use if inflammation is not near the visual axis and the corneal wound is healing adequately; to avoid the use of topical corticosteroid if the causative micro-organisms are unknown and effective antibacterial therapy cannot be provided; and to continue a topical corticosteroid, usually at a lower frequency or concentration, for patients already justifiably using a topical corticosteroid for another serious ocular condition or inflammatory disease. Despite the fact that all of these recommendations seem prudent and may be useful clinically, they are not supported by any evidence in this review.
In general, authors agree with this review, stating that there is no benefit or harm in using steroids adjunctive to antibiotics in the treatment of bacterial keratitis. One publication pointed out that the SCUT may not be applicable to the US population due to several key differences, such as the reduced number of contact lens wearers and the high incidence of trauma in the SCUT patient population, compared to a higher incidence of keratitis due to contact lenses and low incidence of trauma-induced keratitis in western countries (Tuli 2013).
The American Academy of Ophthalmology (AAO) published a Preferred Practice Pattern (PPP) guideline on the management of bacterial keratitis on 21 September 2013 (AAO Bacterial Keratitis PPP 2013). No conclusive evidence was provided as to the treatment effect of corticosteroids on clinical outcomes. However, the guideline recommended using only the minimum dose of corticosteroid required to alleviate inflammation and to avoid use of corticosteroids when the ulcer is associated with Acanthamoeba, Nocardia, fungus, or HSV (AAO Bacterial Keratitis PPP 2013).
AUTHORS’ CONCLUSIONS
Implications for practice
The effectiveness of corticosteroids as an adjunctive treatment for bacterial keratitis remains unknown at present. The completed randomized controlled trials (RCTs) are either inconclusive or have limitations when applied to other populations (SCUT 2012). Two studies reported no benefit of topical steroids in regards to visual acuity, ulcer healing, and re-epithelialization (Blair 2011; SCUT 2012). None of the studies reported harmful effects of adding topical steroids to antibiotics in the treatment of bacterial corneal ulcers. The Steroids for Corneal Ulcers Trial (SCUT) investigators reported that steroids may be beneficial in some cases, such as central ulcers with severely decreased vision, based on a post hoc subgroup analysis. They also suggested that steroids may be contraindicated in the subgroup of patients that present with Nocardia keratitis.
Although many currently recommend waiting at least 24 hours before instituting corticosteroid therapy, there is no evidence available that argues for or against immediate institution concurrent with antibiotic therapy. Identification of the bacterial pathogen is crucial for appropriate selection of antibiotics. Close observation for complications and wound healing is essential.
Implications for research
The studies included in this review have shown neither harm nor benefit from the use of adjunctive steroids together with antibiotics for the treatment of bacterial ulcers. Further, results have not been clear when dealing with severe bacterial keratitis where corneal scarring is of great concern. In addition, a majority of studies (3/4) were inadequately powered to detect a treatment effect. To demonstrate benefits and harms of adjunctive corticosteroid use larger studies, with severe corneal ulcer subgroups, are needed. With modern clinical imaging techniques, the assessment of ulcer severity as well as treatment results should be more reliable. Furthermore, all studies began the use of topical steroids after a minimum of 24 to 48 hours of exclusive antibiotic treatment. Therefore, the early treatment of ulcers with adjunctive steroids has yet to be studied.
Any future trial should be designed with a target sample size calculated based on realistic assumptions regarding the effect size that could be expected based on currently available estimates of outcomes and likely follow-up rates. In addition, strategies for assuring complete or nearly complete follow-up of all randomized participants should be designed and implemented. The feasibility of such a trial requires careful evaluation. In addition, a future trial should also collect quality of life data and economic outcome data.
Characteristics of included studies [ordered by study ID].
|
Blair 2011
| ||
| Methods | Study design: RCT | |
| Number randomized: | ||
| Total: 30 | ||
| Per group: 15 | ||
| Exclusions after randomization and reasons for exclusion: none | ||
| Number analyzed: | ||
| Total: 26 | ||
| Per group: 14 in the antibiotic only group, 12 in the antibiotic + steroid group | ||
| Unit of analysis: 1 eye per individual | ||
| Losses to follow-up and reasons for loss to follow-up: | ||
| Total: 4 | ||
| Per group: 1 in the antibiotic-only group, 3 in the antibiotic + steroid group | ||
| Reasons not reported | ||
| How were missing data handled?: excluded from analysis | ||
| Reported power calculation: yes: “In order to detect a difference of 4 mm² between groups with a standard deviation of 3.3 mm, a type I error of 0.05, and a power of 0.08, a crude sample size (2N) of 54 was calculated.” | ||
| Any issues with study design?: none | ||
|
| ||
| Participants | Country: Canada | |
| Age (mean ± SD): 40.7 ± 21.12 years in the antibiotic-only group, 48.7 ± 19.88 years in the antibiotic-steroid group | ||
| Gender (male:female): 6:9 in the antibiotic-only group, 4:11 in the antibiotic-steroid group | ||
Inclusion criteria:
| ||
Exclusion criteria:
| ||
Equivalence of baseline
characteristics: no
| ||
|
| ||
| Interventions | Antibiotic-only group: gatifloxacin (Zymar, Allergan Inc, Irvine, Calif.) and a masked placebo | |
| Antibiotic-steroid group: gatifloxacin and masked dexamethasone 0.1% (Maxidex, Alcon Inc, Fort Worth, Tex.) | ||
| Note: “If the treating physician felt fortified antibiotics were necessary, the option of topical cefazolin 50 mg/ml and tobramycin 14 mg/ml, in place of gatifloxacin, was allowed.” | ||
| Length of follow-up: | ||
| Planned: 10 weeks | ||
| Actual: 10 weeks | ||
|
| ||
| Outcomes | Primary outcome, as defined in the study: reduction in ulcer size at 10 weeks compared with the baseline size | |
| Measurement of primary outcome in the study: digital photographic measurement | ||
Secondary outcomes and measurements, as
defined in the study:
| ||
| Adverse events reported: yes | ||
| Intervals at which outcome were assessed: all outcomes were reported at 10 weeks | ||
|
| ||
| Notes | Type of study: published | |
| Study period: not reported | ||
| Source of funding: the Physicians’ Services Incorporation Foundation, North York, Ontario | ||
| Declaration of interest: “The authors have no proprietary or commercial interest in any materials discussed in this article” | ||
| Reported subgroup analyses: none | ||
|
| ||
|
Risk of
bias
| ||
| Bias | Authors’ judgement | Support for judgement |
|
| ||
| Random sequence generation (selection bias) | Low risk | “Stratified block randomization in blocks of 6 was used to force a reasonably equal number of eyes in the 2 groups. Randomization was stratified by ulcer size (<2 mm greatest diameter, 2-4 mm greatest diameter, or >4 mm greatest diameter) as ulcer size is an important confounder in this study.” |
|
| ||
| Allocation concealment (selection bias) | Low risk | “The allocation schedule was generated by a central office using a computer algorithm from the uniform distribution (STATA Corp, College Station, Tex.). The generator of the random allocation did not participate in executing the intervention and the executors did not participate in generating the schedule.” |
|
| ||
| Masking of participants and personnel (performance bias) | Low risk | “Both the investigators and the patients were blinded to the treatment allocation.” |
|
| ||
| Masking of outcome assessment (detection bias) | Low risk | “Two independent and blinded observers used validated software to precisely map ulcer areas. Theoretically, photographic measurement should be quite accurate.” |
|
| ||
| Incomplete outcome data (attrition bias) All outcomes | High risk | “Primary analysis was performed based on an intent-to-treat scenario regardless of compliance or protocol deviations.” |
| Patients who were lost to follow-up were not included for the analysis: 1 patient (6. 7%) in the antibiotic-only group and 3 patients (20%) in the antibiotic-steroid group were lost to follow-up. The reasons for loss to follow-up were not reported | ||
|
| ||
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available |
|
| ||
| Other bias | Low risk | No other bias identified |
|
| ||
|
Carmichael 1990
| ||
| Methods | Study design: RCT | |
| Number randomized: | ||
| Total: 40 eyes of 39 participants | ||
| Per group: 19 eyes to non-steroid group, 21 eyes to steroid group | ||
| Exclusions after randomization and reasons for exclusion: 1 participant (1 eye) in the steroid group did not receive treatment due to descemetocele formation the morning after admission; another participant (1 eye) had corneal thinning with early descemetocele formation and steroids were discontinued after 12 days | ||
| Number analyzed: | ||
| Total analyzed for healing rates: 26 eyes | ||
| Per group: “Healing rates were calculated with data available only for 15 eyes in steroid group and 11 eyes in non-steroid group”. Participants were excluded from analysis if they had persistent epithelial defects (more than 21 days), required therapy other than that in the protocol, such as pressure padding for perforations or corneal thinning and if they had uncontrolled infection that did not heal | ||
| Total analyzed for VA at 2 months: 28 eyes | ||
| Per group: 13 eyes in non-steroid group and 15 eyes in steroid group | ||
| Unit of analysis: eye | ||
| Losses to follow-up: 14 eyes for healing rates and 12 eyes for VA | ||
| How were missing data handled?: excluded from analysis | ||
| Reported power calculation: no | ||
| Any issues with study design?: none | ||
|
| ||
| Participants | Country: South Africa | |
| Age: range 19 to 81 years; mean age was 51.4 years in non-steroid group and 51.6 years in steroid group | ||
| Gender (male:female): 19:2 in the non-steroid group, 14:5 in the steroid group | ||
| Inclusion criteria: central or paracentral corneal ulcers severe enough to warrant admission to the hospital | ||
| Exclusion criteria: identification of fungal isolates, perforated ulcers, or descemetoceles, underlying viral corneal conditions, atopic ulcers; no light perception on admission; less than 13 years of age | ||
| Equivalence of baseline characteristics: no, there were fewer females in the steroid group; greater number of eyes in steroid group had paracentral ulcers (n = 14) compared with non-steroid group (n = 10); greater number of eyes in steroid group (n = 16) had hypopyon at admission compared with non-steroid group (n = 12) | ||
|
| ||
| Interventions | Antibiotic-only group: general therapy on the day following admission with no additional corticosteroid therapy | |
| Antibiotic-steroid group: 0.1% dexamethasone eye drops 4 times a day were added to general therapy on the day following admission if the condition of the ulcer was adjudged to be the same or improved | ||
| Length of follow-up: | ||
| Planned: 18 months | ||
| Actual: VA outcome was only reported at 2 months | ||
|
| ||
| Outcomes | Primary outcome: healing rate of ulcer | |
| Measurement of primary outcome in the study: each ulcer was drawn to scale onto a 1 mm ruled graph paper and the number of squares was counted to calculate the area for each ulcer at admission and to calculate the area of ulcer healed per day | ||
| Secondary outcomes: VA | ||
| Measurement of secondary outcomes in the study: measured with Snellen charts at 2 months by 2 physicians independently (VA was categorized using an arbitrary scale to compare the improvement in the 2 treatment arms) | ||
| Adverse events reported: yes | ||
| Intervals at which outcome were assessed: VA was measured at 2 months; time points measured for healing rates were not specified | ||
|
| ||
| Notes | Type of study: published | |
| Study period: not reported | ||
| Source of funding: not reported | ||
| Declaration of interest: not reported | ||
| Reported subgroup analyses: none | ||
|
| ||
|
Risk of
bias
| ||
| Bias | Authors’ judgement | Support for judgement |
|
| ||
| Random sequence generation (selection bias) | Low risk | Randomization was done using a random numbers table. The first case number was randomly selected, with odd numbers being allocated to one treatment group and even numbers to the other |
|
| ||
| Allocation concealment (selection bias) | Unclear risk | Not reported |
|
| ||
| Masking of participants and personnel (performance bias) | High risk | Study participants and personnel were not masked |
|
| ||
| Masking of outcome assessment (detection bias) | High risk | All assessments were conducted independently by 2 unmasked physicians |
|
| ||
| Incomplete outcome data (attrition bias) | High risk | Total number analyzed was 26 out of 40 eyes (65%). Healing rates were calculated with data available only for 15 eyes in the steroid group and 11 eyes in the non-steroid group. Analysis of visual acuity at 2 months included only 15 eyes in the steroid group and 13 eyes in the non-steroid group |
| All outcomes | ||
|
| ||
| Selective reporting (reporting bias) | Unclear risk | Protocol was not available |
|
| ||
| Other bias | Low risk | No other bias identified |
|
| ||
|
SCUT 2012
| ||
| Methods | Study design: RCT | |
| Number randomized: | ||
| Total: 500 | ||
| Per group: 250 | ||
| Exclusions after randomization and reasons for exclusion: | ||
| Total: none | ||
| Per group: none | ||
| Number analyzed: | ||
| Total: 442 | ||
| Per group: 220 in the placebo group; 222 in the corticosteroid group | ||
| Unit of analysis: 1 eye per individual | ||
| Losses to follow-up and reasons for loss to follow-up: | ||
| Total: 58 | ||
| Per group: 30 patients (12.0%) in the placebo group, 28 patients (11.2%) in the corticosteroid group | ||
| How were missing data handled?: excluded from analysis | ||
| Reported power calculation: yes: a sample size of 500 participants (250 per arm) can provide 80% power to detect a 0.20 logMAR (2 lines of visual acuity) difference between groups in BCVA 3 months after enrollment, assuming a SD of 0.65 logMAR for 3-month BCVA | ||
| Any issues with study design?: none | ||
|
| ||
| Participants | Country: India, USA | |
| Age, median (25th to 75th percentile): 54.5 (40.0 to 61.0) in the placebo group, 52.0 (40.0 to 62.0) in the corticosteroid group, 53.0 (40.0 to 61.0) in total | ||
| Gender (male:female): 147:103 in the placebo group, 126:124 in the corticosteroid group | ||
Inclusion criteria:
| ||
Exclusion criteria:
| ||
| Equivalence of baseline characteristics: yes | ||
|
| ||
| Interventions | Antibiotic-only group: placebo, after a cornea culture that tested positive for bacteria and after they had received 48 hours of topical moxifloxacin | |
| Antibiotic-steroid group: topical prednisolone sodium phosphate 1.0% after a cornea culture that tested positive for bacteria and after they had received 48 hours of topical moxifloxacin | ||
| Length of follow-up: | ||
| Planned: 12 months | ||
| Actual: 12 months (follow-up ongoing) | ||
|
| ||
| Outcomes | Primary outcome, as defined in the study: best corrected visual acuity (BCVA) at 3 months from enrollment | |
| Measurement of primary outcome in the study: tumbling E chart | ||
Secondary outcomes and measurements, as
defined in the study:
| ||
| Adverse events reported: yes | ||
| Intervals at which outcome were assessed: baseline, every 3 days ± 1 day until reepithelialization, at 3 weeks, and at 3 months | ||
|
| ||
| Notes | Type of study: published | |
| Study period: 1 September 2006 to 22 February 2010 | ||
| Source of funding: “The trial was funded by National Eye Institute grant U10 EY015114 (Dr Lietman). Dr Acharya is supported by National Eye Institute grant K23 EY017897 and a Research to Prevent Blindness Award. Alcon/Novartis AG provided moxifloxacin(Vigamox) for the trial. The Department of Ophthalmology at the University of California, San Francisco, is supported by core grant EY02162 from the National Eye Institute.” | ||
| Declaration of interest: “None of the authors have any financial disclosures to report” | ||
| Reported subgroup analyses: “Prespecified subgroups included baseline BCVA (<20/40, 20/40 to 20/800, and counting fingers or worse), geometric mean of baseline infiltrate/scar size (0-1.90, 1.91-2.70, 2.71-4.06, and 4.07-8.90 mm), infiltrate depth (>0%-33%, >33%-67%, and >67%-100%), and ulcer location (completely filling the 4-mm central artificial pupil, partially filling the 4-mm central pupil, and entirely in the periphery)” | ||
|
| ||
|
Risk of
bias
| ||
| Bias | Authors’ judgement | Support for judgement |
|
| ||
| Random sequence generation (selection bias) | Low risk | “Participants were randomized in a 1:1 ratio either to placebo drops or prednisolone phosphate drops using permuted blocks within study centers. Block sizes were randomized in sizes of 4, 6, and 8.” |
|
| ||
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment was not reported |
|
| ||
| Masking of participants and personnel (performance bias) | Low risk | “Double-masking was achieved because the prednisolone phosphate solution was identical to placebo. Only the study biostatisticians were unmasked.” |
|
| ||
| Masking of outcome assessment (detection bias) | Low risk | “Double-masking was achieved because the prednisolone phosphate solution was identical to placebo. Only the study biostatisticians were unmasked.” |
|
| ||
| Incomplete outcome data (attrition bias) All outcomes | High risk | 30 (12.0%) in the placebo group and 28 (11.2%) in the corticosteroid group were not included in the analysis |
|
| ||
| Selective reporting (reporting bias) | Low risk | All pre-specified outcomes in the Clinical-Trials.gov record were reported in the full-length publications |
|
| ||
| Other bias | Low risk | No other bias identified |
|
| ||
|
Srinivasan 2009
| ||
| Methods | Study design: RCT | |
| Number randomized: | ||
| Total: 42 participants | ||
| Per group: 22 in the placebo group and 20 in the steroid group | ||
| Exclusions after randomization and reasons for exclusion: none | ||
| Number analyzed: | ||
| Total: 33 participants | ||
| Per group: 17 in the placebo group and 16 in the steroid group for analysis of best spectacle-corrected visual acuity at 3 months | ||
| Unit of analysis: 1 eye per individual | ||
| Losses to follow-up and reasons for loss to follow-up: at week 3, 5 participants in the placebo group and 1 participant in the steroid group discontinued intervention; at month 3, an additional 5 participants in the placebo group and 4 participants in the steroid group discontinued intervention; reasons for discontinuation were not specified | ||
| How were missing data handled?: excluded from analysis | ||
| Reported power calculation: yes: 42 participants (21 participants per arm) can provide 80% power to detect a 0.4 logMAR (4 Snellen lines) difference between the 2 study arms, assuming a SD of 0.4 in the 3-month BCVA | ||
| Any issues with study design?: none | ||
|
| ||
| Participants | Country: India | |
| Age: greater than 16 years; mean age was 44.1 years in the placebo group and 49.9 years in the steroid group | ||
| Gender (male:female): 50:50 in the placebo group and 55:45 in the steroid group | ||
| Inclusion criteria: “presence of a corneal ulcer at presentation (defined by an epithelial defect and signs of stromal inflammation); cornea culture on blood or chocolate agar indicates the presence of bacteria; antibiotic given for more than 48 hours; the patient able to verbalise a basic understanding of the study after it was explained to the patient, as determined by a physician examiner (this understanding included a commitment to return for follow-up visits); appropriate consent” | ||
| Exclusion criteria: “overlying epithelial defect, 0.75 mm at its greatest width at presentation; impending perforation; evidence of fungus on KOH or Giemsa stain at time of presentation; evidence of acanthamoeba by stain; evidence of herpetic keratitis by history or exam; history of corneal scar in the affected eye; use of a topical steroid in the affected eye during the course of the present ulcer, including use after the symptoms of the ulcer started but before presentation; use of systemic steroids during the course of the present ulcer; age less than 16 years; bilateral ulcers; previous penetrating keratoplasty; pregnancy (by history or urine test); outside 200 km radius of Aravind Eye Hospital; evidence of fungus on culture at time of enrollment; best spectacle-corrected vision worse than 6/60 (20/200) in the fellow eye; corneal perforation or descemetocoele; known allergy to study medications (steroid or preservative); no light perception in the affected eye; not willing to participate or to return for follow-up visits” | ||
| Equivalence of baseline characteristics: yes | ||
|
| ||
| Interventions | Antibiotic-only group: adjunctive placebo, 0.9% sodium chloride, administered topically to the cornea 4 times a day for 1 week, followed by 2 times a day for 1 week, then once a day for 1 week and then stopped | |
| Antibiotic-steroid group: adjunctive topical corticosteroids, topical prednisolone phosphate 1% administered topically to the cornea 4 times a day for 1 week, followed by 2 times a day for 1 week, then once a day for 1 week and then stopped | ||
| General therapy: “treatment for all participants in the two groups included: administration of study drops (steroid or placebo) to the cornea four times a day for 1 week, followed by two times a day for 1 week, then once a day for 1 week and then stopped; all patients continued to receive topical moxifloxacin every 2 hours while awake until re-epithelialisation, and then four times a day until 3 weeks after enrolment; antibiotics were then discontinued unless the treating physician thought that longer treatment was warranted” | ||
| Length of follow-up: | ||
| Planned: 3 weeks and 3 months after enrollment | ||
| Actual: 3 weeks and 3 months after enrollment | ||
|
| ||
| Outcomes | Primary outcome, as defined in the study: best corrected visual acuity (BCVA) at 3 months, adjusting for enrolment BCVA and group | |
| Measurement of primary outcome in the study: BCVA at 3 months in the study eye, using a linear regression model with 3-month logMAR BCVA as the outcome variable and treatment arm (placebo versus steroid) and enrolment logMAR BCVA as covariates | ||
| Secondary outcomes, as defined in the study: re-epithelialization time, infiltrate/scar size, and adverse events | ||
| Measurement of secondary outcomes in the study: BCVA at 3 weeks, adjusting for enrolment BCVA, and infiltrate/scar size at 3 weeks and 3 months, adjusting for enrolment infiltrate/scar size. For analysis, infiltrate/scar size was characterized by the geometric mean of the longest dimension and the longest perpendicular. The association between enrolment and 3-month BCVA was assessed using Pearson’s correlation coefficient | ||
| Adverse events reported: yes | ||
| Intervals at which outcome were assessed: 3 weeks and 3 months after the enrollment | ||
|
| ||
| Notes | Type of study: published | |
| Study period: 4 January 2005 to 20 August 2005 | ||
| Source of funding: That Man May See and the South Asia Research Fund. The Department of Ophthalmology at UCSF is supported by a core grant from the National Eye Institute, EY02162. N Acharya is supported by a National Eye Institute K23EY017897 grant and a Research to Prevent Blindness Career Development Award. TM Lietman is supported by a National Eye Institute grant U10-EY015114 and a Research to Prevent Blindness award. M Zegans is supported by a K08 EY13977-01 NEI grant | ||
| Declaration of interest: “None declared. Alcon donated moxifloxacin for the study. The sponsors had no role in the design or conduct of the study, data analysis or manuscript preparation. None of the authors have any financial disclosures related to this manuscript.” | ||
| Reported subgroup analyses: none | ||
|
| ||
|
Risk of
bias
| ||
| Bias | Authors’ judgement | Support for judgement |
|
| ||
| Random sequence generation (selection bias) | Low risk | Randomization was done using “block randomisation in groups of ten generated by RAND command in Excel by TML; implementation including enrolment and assignment of participants by RM.” |
|
| ||
| Allocation concealment (selection bias) | Unclear risk | Did not describe explicitly; stated only that after a minimum of 48 hours of moxifloxacin treatment, patients were randomized (block randomization in groups of 10 generated by RAND command in Excel by TML; implementation including enrolment and assignment of participants by RM) to receive topical prednisolone phosphate 1% or placebo drops |
|
| ||
| Masking of participants and personnel (performance bias) | Low risk | “Double-masking of treatment assignment was achieved since the prednisolone phosphate solution could not be differentiated from the placebo. All study-site personnel and patients were masked to treatment assignment. Only the biostatisticians responsible for the randomisation coding and the study pharmacist were unmasked.” |
|
| ||
| Masking of outcome assessment (detection bias) | Low risk | “Double-masking of treatment assignment was achieved since the prednisolone phosphate solution could not be differentiated from the placebo. All study-site personnel and patients were masked to treatment assignment. Only the biostatisticians responsible for the randomisation coding and the study pharmacist were unmasked.” |
|
| ||
| Incomplete outcome data (attrition bias) All outcomes | High risk | “Efficacy endpoints were analysed on an intent-to-treat basis for all randomised patients enrolled in the study. The primary analysis included patients with both enrolment and 3-month data. A sensitivity analysis was also performed in which 3-week values were carried forward to 3 months if the 3-month visit was missed.” |
| “Forty-two patients with culture-proven BK at Aravind Eye Hospital were enrolled: 22 in the placebo arm and 20 in the steroid arm. Thirty-three patients (79%) were followed-up at 3 months, and an additional three patients were followed-up at their 3-weeks but missed their 3-month visit.” | ||
|
| ||
| Selective reporting (reporting bias) | Low risk | We did not have access to the reported outcomes with the original study protocol. We assessed the selective outcome reporting by comparing the reported outcomes in the methods and results sections. Also, because this is the pilot trial for the SCUT trial, and the SCUT trial has a protocol, we compared these 2 studies and they have similarly reported all expected outcomes |
|
| ||
| Other bias | Low risk | No other bias identified |
BCVA: best corrected visual acuity
ETDRS: Early Treatment Diabetic Retinopathy Study
g/l: grams per liter
KOH: potassium hydroxide
mg: milligrams
mm: millimeters
RCT: randomized controlled trial
SD: standard deviation
VA: visual acuity
Characteristics of excluded studies [ordered by study ID].
| Study | Reason for exclusion |
|---|---|
| Barequet 2001 | Not relevant to bacterial keratitis |
| Buhren 2001 | Non-comparative case report |
| Callegan 1994 | Review article; no new randomized trial described |
| Cosar 2004 | Not RCT and not relevant to bacterial keratitis |
| Dighiero 2005 | Not RCT; amniotic membrane transplantation is not one of the interventions in the inclusion criteria |
| Goldberg 2002 | Case report |
| Gris 2004 | Case report |
| Hanada 2001 | Not RCT; no clear mention of inclusion of bacterial keratitis |
| Hoffman 2003 | Cohort study |
| Morlet 1999 | Cross-sectional study |
| Nakajima 2001 | Retrospective study |
| Shulman 1996 | Inclusion criteria do not include bacterial keratitis |
| Vajpayee 1998 | Retrospective study |
| Wilhelmus 2002 | Review article; no new randomized trial described |
| Zegans 2006 | Abstract only; no full length publication available |
RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID].
|
NCT01721694
| |
| Trial name or title | Antibiotic Steroid Combination Compared With Individual Administration in the Treatment of Ocular Inflammation and Infection |
|
| |
| Methods | Study design: interventional, randomized, parallel-group design, double-blinded (participant, investigator) trial |
| Expected number to be randomized: | |
| Total: 60 participants | |
| Per group: 30 participants | |
| Unit of analysis: 1 eye per individual | |
| Reported power calculation: not reported | |
|
| |
| Participants | Country: Brazil |
| Age: eligibility is 18 years and older | |
| Gender (male:female): the study has not yet recruited participants, but plan to recruit both genders | |
| Inclusion criteria: central or paracentral corneal ulcers severe enough to warrant admission to the hospital | |
| Exclusion criteria: identification of fungal isolates, perforated ulcers or descemetoceles, underlying viral corneal conditions, atopic ulcers; no light perception on admission; less than 13 years of age | |
|
| |
| Interventions | Intervention 1: fixed combination of azithromycin 1.5%/loteprednol 0.5% eye drops + placebo eye drops |
| Intervention 2: azithromycin 1.5% + loteprednol 0.5% eye drops (separately) | |
|
| |
| Outcomes | Primary outcome measures: “clinical cure achieved when the score of the cardinal ocular signs (hyperemia in the bulbar conjunctiva, palpebral, exudate / conjunctival discharge, eyelid erythema and flaking / crust eyelid) is zero at the time of conclusion of the study” on day 8 |
| Secondary outcome measures: eradication of pathogens defined as “success obtained with microbiological irradication of pathogens present at baseline” on day 8 | |
| Other outcome measures: “decrease of visual acuity, corneal/anterior chamber changes, IOP increase and adverse event reporting” on day 4 and day 8 | |
|
| |
| Starting date | December 2012 |
|
| |
| Contact information | Cristina Muccioli, MD: 5511-99748809; crissmucci@gmail.com |
| Luci Silva, MBA: 5511-71571967; luci.pesquisa@gmail.com | |
| Locations: Brazil | |
| Department of Ophthalmology of Hospital São Paulo | |
| São Paulo, Brazil, 04023-062 | |
| Principal Investigator: Rubens Belfort, MD | |
| Sub-Investigator: Cristina Muccioli, MD | |
|
| |
| Notes | Estimated study completion date: May 2013 |
| The study had not yet recruited participants as of 5 November 2012 | |
| The study is sponsored by Adapt Produtos Oftalmológicos Ltda | |
IOP: intraocular pressure
Acknowledgments
We wish to acknowledge Iris Gordon and Lori Rosman, Trials Search Co-ordinators for CEVG, for devising and running the search strategies. We wish to acknowledge Drs. Marie Diener-West, Lawrence Pe and Richard L. Nepomuceno, for their helpful comments on this review. We thank Sueko M. Ng (SMN) for her help in the data extraction when updating the review. We also wish to thank the CEVG editorial base for their support and suggestions in preparing and revising this review.
SOURCES OF SUPPORT
Internal sources
• Brown University, USA.
• Johns Hopkins University, USA.
External sources
• Contract N01-EY-2-1003, National Eye Institute, National Institutes of Health, USA.
• National Eye Institute, National Institutes of Health, USA.
Xue Wang is supported by the Cochrane Eyes and Vision - US Project through the National Eye Institute Grant 1 U01 EY020522
• National Institute for Health Research (NIHR), UK.
• Richard Wormald, Co-ordinating Editor for the Cochrane Eyes and Vision Group (CEVG) acknowledges financial support for his CEVG research sessions from the Department of Health through the award made by the National Institute for Health Research to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
• The NIHR also funds the CEVG Editorial Base in London.
The views expressed in this publication are those of the authors and not necessarily those of the NIHR, NHS, or the Department of Health.
APPENDICES
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Keratitis] explode all trees
#2 MeSH descriptor: [Corneal Ulcer] explode all trees
#3 MeSH descriptor: [Eye Infections, Bacterial] explode all trees
#4 cornea* near/3 ulcer*
#5 keratitis near/3 bacteria*
#6 bacteri* near/3 infec* near/5 ocular
#7 bacteria* near/3 infec* near/5 eye*
#8 #1 or #2 or #3 or #4 or #5 or #6 or #7
#9 MeSH descriptor: [Adrenal Cortex Hormones] explode all trees
#10 corticosteroid* or steroid*
#11 MeSH descriptor: [Prednisolone] this term only
#12 MeSH descriptor: [Dexamethasone] this term only
#13 MeSH descriptor: [Fluorometholone] this term only
#14 (prednisolone or pred forte or dexamethasone or fluorometholone or rimexolone)
#15 #9 or #10 or #11 or #12 or #13 or #14
#16 #8 and #15
Appendix 2. MEDLINE (OvidSP) search strategy
randomized controlled trial.pt.
(randomized or randomised).ab,ti.
placebo.ab,ti.
dt.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1-7
exp animals/
exp humans/
9 not (9 and 10)
8 not 11
exp keratitis/
exp eye infections bacterial/
(cornea$ adj3 ulcer$).tw.
(keratitis adj3 bacteria$).tw.
(bacteri$ adj3 (infec$ adj5 ocular)).tw.
(bacteri$ adj3 (infec$ adj5 eye$)).tw.
or/13-18
exp Adrenal Cortex Hormones/
(corticosteroid$ or steroid$).tw.
exp prednisolone/
exp Dexamethasone/
Fluorometholone/
(prednisolone or pred forte or dexamethasone or fluorometholone or rimexolone).tw.
or/20-25
19 and 26
12 and 27
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville (Glanville 2006).
Appendix 3. EMBASE (OvidSP) search strategy
exp randomized controlled trial/
exp randomization/
exp double blind procedure/
exp single blind procedure/
random$.tw.
or/1-5
(animal or animal experiment).sh.
human.sh.
7 and 8
7 not 9
6 not 10
exp clinical trial/
(clin$ adj3 trial$).tw.
((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
exp placebo/
placebo$.tw.
random$.tw.
exp experimental design/
exp crossover procedure/
exp control group/
exp latin square design/
or/12-21
22 not 10
23 not 11
exp comparative study/
exp evaluation/
exp prospective study/
(control$ or prospectiv$ or volunteer$).tw.
or/25-28
29 not 10
30 not (11 or 23)
11 or 24 or 31
exp keratitis/
bacterial eye infection/
(cornea$ adj3 ulcer$).tw.
(keratitis adj3 bacteria$).tw.
(bacteri$ adj3 (infec$ adj5 ocular)).tw.
(bacteri$ adj3 (infec$ adj5 eye$)).tw.
or/33-38
corticosteroid/
(corticosteroid$ or steroid$).tw.
prednisolone/
dexamethasone/
fluorometholone/
(prednisolone or pred forte or dexamethasone or fluorometholone or rimexolone).tw.
or/40-45
39 and 46
32 and 47
Appendix 4. LILACS search strategy
bacterial keratitis and corticosteroid or steroid or prednisolone or pred forte or dexamethasone or fluorometholone or dexamethasone or fluorometholone or rimexolone
Appendix 5. metaRegister of Controlled Trials search strategy
bacterial keratitis AND (corticosteroid OR steroid OR prednisolone OR pred forte OR dexamethasone OR fluorometholone OR dexamethasone OR fluorometholone OR rimexolone)
Appendix 6. ClinicalTrials.gov search strategy
bacterial keratitis AND (corticosteroid OR steroid OR prednisolone OR pred forte OR dexamethasone OR fluorometholone OR dexamethasone OR fluorometholone OR rimexolone)
Appendix 7. ICTRP search strategy
bacterial keratitis = Condition AND corticosteroid OR steroid OR prednisolone OR pred forte OR dexamethasone OR fluorometholone OR dexamethasone OR fluorometholone OR rimexolone = Intervention
DATA AND ANALYSES
Comparison 1. Steroid versus placebo for the treatment of corneal ulcers
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Time to re-epithelialization | 2 | Hazard Ratio (Fixed, 95% CI) | Totals not selected |
Analysis 1.1.
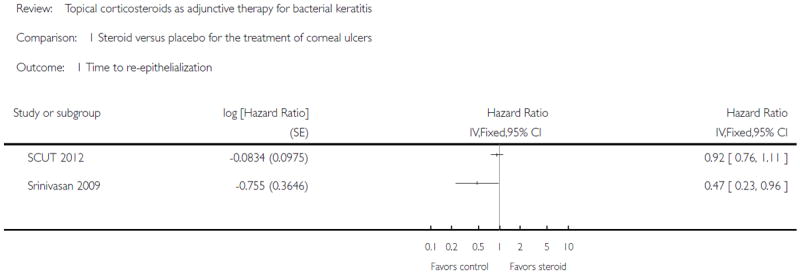
Comparison 1 Steroid versus placebo for the treatment of corneal ulcers, Outcome 1 Time to re-epithelialization.
WHAT’S NEW
| Date | Event | Description |
|---|---|---|
| 15 October 2014 | New search has been performed | Issues 10, 2014: Searches updated. |
| 15 October 2014 | New citation required but conclusions have not changed | Issue 10, 2014: Three additional studies included (Blair 2011; SCUT 2012; Srinivasan 2009). |
Last assessed as up-to-date: 14 July 2014
HISTORY
| Date | Event | Description |
|---|---|---|
| 22 March 2009 | New search has been performed | Updated search. |
| 11 October 2008 | Amended | Converted to new review format. |
| 19 August 2007 | New citation required and conclusions have changed | Substantive amendment. |
Protocol first published: Issue 3, 2005
Review first published: Issue 4, 2007
Footnotes
CONTRIBUTIONS OF AUTHORS
For the original review (please refer to Suwan-apichon 2007):
Conceiving the review: OS, RSC
Designing the review: OS, JMGR
Co-ordinating the review: OS, JMGR, SSV
Data collection for the review
- Designing electronic search strategies: Cochrane Eyes and Vision Group editorial base
- Undertaking manual searches: OS, JMGR, SH
- Screening search results: OS, RSC, SH
- Organizing retrieval of papers: OS, SH
- Screening retrieved papers against inclusion criteria: OS, JMGR, SH, SSV
- Appraising quality of papers: OS, RSC, SSV
- Extracting data from papers: OS, JMGR, SSV
- Writing to authors of papers for additional information: OS, SSV
- Obtaining and screening data on unpublished studies: OS
Data management for the review
- Entering data into RevMan: OS, JMGR
- Analysis of data: OS, JMGR, SSV
- Interpretation of data: OS, RSC, SSV
Writing the review: OS, JMGR, RSC, SSV
For the updated review: Data collection for the review
- Designing electronic search strategies: Cochrane Eyes and Vision Group editorial base
- Screening search results: XW, SMN, SSV
- Organizing retrieval of papers: XW
- Screening retrieved papers against inclusion criteria: XW, SMN
- Appraising quality of papers: XW, SMN
- Extracting data from papers: XW, SMN
- Writing to authors of papers for additional information: XW
Data management for the review
- Entering data into RevMan: XW, SH
- Analysis of data: XW, SH, RSC
- Interpretation of data: XW, SH, RSC
Writing the review: SH, XW, RSC
Guarantor of the review: SH
DECLARATIONS OF INTEREST
Xue Wang: none known
Johann MG Reyes: none known
References to studies included in this review
- *Blair 2011 {published data only}.Blair J, Hodge W, Al-Ghamdi S, Balabanian R, Lowcock B, Pan YI, et al. Comparison of antibiotic-only and antibiotic-steroid combination treatment in corneal ulcer patients: double-blinded randomized clinical trial. Canada Journal of Ophthalmology. 2011;46(1):40–5. doi: 10.3129/i10-054. [DOI] [PubMed] [Google Scholar]
- Hodge WG, Blair J, Al-Ghambdi S, Balabanian R, Lowcock BC, Pan YI, et al. Comparison of antibiotic-only and antibiotic-steroid combination treatment in corneal ulcer patients: a double-blinded, randomized clinical trial - interim report. Investigative Ophthalmology and Visual Science. 2007;48 doi: 10.3129/i10-054. ARVO E-Abstract 4275. [DOI] [PubMed] [Google Scholar]
- Carmichael 1990 {published data only}.Carmichael TR, Gelfand Y, Welsh NH. Topical steroids in the treatment of central and paracentral corneal ulcers. British Journal of Ophthalmology. 1990;74(9):528–31. doi: 10.1136/bjo.74.9.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *SCUT 2012 {published and unpublished data}.Acharya N, Srinivasan M, Mahalakshmi R, Jayashree D, Prajna L, House J, et al. Steroids for Corneal Ulcers Treatment - SCUT pilot study results. Investigative Ophthalmology and Visual Science. 2006;47 ARVO Eabstract 4752. [Google Scholar]
- Chen A, Prajna L, Srinivasan M, Acharya N, Lietman T. Does in vitro susceptibility testing predict clinical outcomes in bacterial keratitis? Results from the Steroids for Corneal Ulcers Trial (SCUT) pilot study. Investigative Ophthalmology and Visual Science. 2007;48 ARVO E-Abstract 4277. [Google Scholar]
- Dulku S. Subgroup analysis in the steroids for corneal ulcers trial. Archives of Ophthalmology. 2012;130(6):807–8. doi: 10.1001/archophthalmol.2011.1919. [DOI] [PubMed] [Google Scholar]
- Garg P, Vazirani J. Can we apply the results of the Steroid Corneal Ulcer Trial to Nocardia infections of the cornea? Expert Review of Ophthalmology. 2013;8(1):41–4. [Google Scholar]
- Lalitha P, Srinivasan M, Manikandan P, Bharathi MJ, Rajaraman R, Ravindran M, et al. Relationship of in vitro susceptibility to moxifloxacin and in vivo clinical outcome in bacterial keratitis. Clinical Infectious Diseases. 2012;54(10):1381–7. doi: 10.1093/cid/cis189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalitha P, Srinivasan M, Rajaraman R, Ravindran M, Mascarenhas J, Priya JL, et al. Nocardia keratitis: clinical course and effect of corticosteroids. American Journal of Ophthalmology. 2012;154(6):934–9. doi: 10.1016/j.ajo.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClintic SM, Prajna NV, Srinivasan M, Mascarenhas J, Lalitha P, Rajaraman R, et al. Visual outcomes in treated bacterial keratitis: four years of prospective follow-up. Investigative Ophthalmology and Visual Science. 2014;55(5):2935–40. doi: 10.1167/iovs.14-13980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldenburg CE, Lalitha P, Srinivasan M, Manikandan P, Bharathi MJ, Rajaraman R, et al. Moxifloxacin susceptibility mediates the relationship between causative organism and clinical outcome in bacterial keratitis. Investigative Ophthalmology and Visual Science. 2013;54(2):1522–6. doi: 10.1167/iovs.12-11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray KJ, Prajna L, Srinivasan M, Geetha M, Karpagam R, Glidden D, et al. Fluoroquinolone treatment and susceptibility of isolates from bacterial keratitis. JAMA Ophthalmology. 2013;131(3):310–3. doi: 10.1001/jamaophthalmol.2013.1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray KJ, Srinivasan M, Mascarenhas J, Rajaraman R, Ravindran M, Glidden DV, et al. Early addition of topical corticosteroids in the treatment of bacterial keratitis. JAMA Ophthalmology. 2014;132(6):737–41. doi: 10.1001/jamaophthalmol.2014.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- See CW, Srinivasan M, Saravanan S, Oldenburg CE, Esterberg EJ, Ray KJ, et al. Prior elicitation and Bayesian analysis of the Steroids for Corneal Ulcers Trial. Ophthalmic Epidemiology. 2012;19(6):407–13. doi: 10.3109/09286586.2012.735332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M, Mascarenhas J, Rajaraman R, Ravindran M, Lalitha P, Glidden DV, et al. Corticosteroids for bacterial keratitis - The Steroids for Corneal Ulcers Trial (SCUT) Archives of Ophthalmology. 2012;130(2):143–150. doi: 10.1001/archophthalmol.2011.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M, Mascarenhas J, Rajaraman R, Ravindran M, Lalitha P, Glidden DV, et al. The Steroids for Corneal Ulcers Trial: study design and baseline characteristics. Archives of Ophthalmology. 2012;130(2):151–7. doi: 10.1001/archophthalmol.2011.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M, Mascarenhas J, Rajaraman R, Ravindran M, Lalitha P, O’Brien KS, et al. The Steroids for Corneal Ulcers Trial (SCUT). secondary 12-month clinical outcomes of a randomized controlled trial. American Journal of Ophthalmology. 2014;157(2):327–33. doi: 10.1016/j.ajo.2013.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan M, Mascarenhas J, Rajaraman R, Ravindran M, Lalitha P, Ray KJ, et al. Visual recovery in treated bacterial keratitis. Ophthalmology. 2014;121(6):1310–1. doi: 10.1016/j.ophtha.2013.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy A, Srinivasan M, Mascarenhas J, Lalitha P, Rajaraman R, Ravindran M, et al. Pseudomonas aeruginosa keratitis: outcomes and response to corticosteroid treatment. Investigative Ophthalmology and Visual Science. 2012;53(1):267–72. doi: 10.1167/iovs.11-7840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan 2009 {published data only}.Srinivasan M, Lalitha P, Mahalakshmi R, Prajna NV, Mascarenhas J, Chidambaram JD, et al. Corticosteroids for bacterial corneal ulcers. British Journal of Ophthalmology. 2009;93(2):198–202. doi: 10.1136/bjo.2008.147298. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Barequet 2001 {published data only}.Barequet IS, Jabbur NS, Barron Y, Osterhout GJ, O’Brien TP. Perioperative microbiologic profile of the conjunctiva in photorefractive keratectomy. Journal of Refractive Surgery. 2001;17(1):55–62. doi: 10.3928/1081-597X-20010101-07. [DOI] [PubMed] [Google Scholar]
- Buhren 2001 {published data only}.Buhren J, Baumeister M, Kohnen T. Diffuse lamellar keratitis after laser in situ keratomileusis imaged by confocal microscopy. Ophthalmology. 2001;108(6):1075–81. doi: 10.1016/s0161-6420(01)00567-x. [DOI] [PubMed] [Google Scholar]
- Callegan 1994 {published data only}.Callegan MC, O’Callaghan RJ, Hill JM. Pharmacokinetic considerations in the treatment of bacterial keratitis. Clinical Pharmacokinetics. 1994;27(2):129–49. doi: 10.2165/00003088-199427020-00005. [DOI] [PubMed] [Google Scholar]
- Cosar 2004 {published data only}.Cosar CB, Sener AB, Sen N, Coskunseven E. The efficacy of hourly prophylactic steroids in diffuse lamellar keratitis epidemic. Ophthalmologica. 2004;218(5):318–22. doi: 10.1159/000079473. [DOI] [PubMed] [Google Scholar]
- Dighiero 2005 {published data only}.Dighiero PL, Merciã M, Gicquel J. Early use of amniotic membrane transplantation combined with topical steroids in severe bacterial keratitis. Investigative Ophthalmology and Visual Science. 2005;45 ARVO E-abstract 2767. [Google Scholar]
- Goldberg 2002 {published data only}.Goldberg RA, Li TG. Postoperative infection with group A beta-hemolytic Streptococcus after blepharoplasty. American Journal of Ophthalmology. 2002;134(6):908–10. doi: 10.1016/s0002-9394(02)01848-2. [DOI] [PubMed] [Google Scholar]
- Gris 2004 {published data only}.Gris O, Guell JL, Wolley-Dod C, Adan A. Diffuse lamellar keratitis and corneal edema associated with viral keratoconjunctivitis 2 years after laser in situ keratomileusis. Journal of Cataract and Refractive Surgery. 2004;30(6):1366–70. doi: 10.1016/j.jcrs.2003.11.033. [DOI] [PubMed] [Google Scholar]
- Hanada 2001 {published data only}.Hanada K, Shimazaki J, Shimmura S, Tsubota K. Multilayered amniotic membrane transplantation for severe ulceration of the cornea and sclera. American Journal of Ophthalmology. 2001;131(3):324–31. doi: 10.1016/s0002-9394(00)00825-4. [DOI] [PubMed] [Google Scholar]
- Hoffman 2003 {published data only}.Hoffman RS, Fine IH, Packer M. Incidence and outcomes of LASIK with diffuse lamellar keratitis treated with topical and oral corticosteroids. Journal of Cataract and Refractive Surgery. 2003;29(3):451–6. doi: 10.1016/s0886-3350(02)01835-7. [DOI] [PubMed] [Google Scholar]
- Morlet 1999 {published data only.Morlet N, Minassian D, Butcher J. Risk factors for treatment outcome of suspected microbial keratitis. Ofloxacin Study Group British Journal of Ophthalmology. 1999;83(9):1027–31. doi: 10.1136/bjo.83.9.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima 2001 {published data only}.Nakajima H, Yamada M, Mashima Y. Review of infectious keratitis following keratoplasty. Japanese Journal of Clinical Ophthalmology. 2001;55(5):1001–6. [Google Scholar]
- Shulman 1996 {published data only}.Shulman DG, Sargent JB, Stewart RH, Mester U. Comparative evaluation of the short-term bactericidal potential of a steroid-antibiotic combination versus steroid in the treatment of chronic bacterial blepharitis and conjunctivitis. European Journal of Ophthalmology. 1996;6(4):361–7. doi: 10.1177/112067219600600403. [DOI] [PubMed] [Google Scholar]
- Vajpayee 1998 {published data only}.Vajpayee RB, Sharma N, Chand M, Tabin GC, Vajpayee M, Anand JR. Corneal superinfection in acute hemorrhagic conjunctivitis. Cornea. 1998;17(6):614–7. doi: 10.1097/00003226-199811000-00009. [DOI] [PubMed] [Google Scholar]
- Wilhelmus 2002 {published data only}.Wilhelmus KR. Indecision about corticosteroids for bacterial keratitis: an evidence-based update. Ophthalmology. 2002;109(5):835–42. doi: 10.1016/s0161-6420(02)00963-6. [DOI] [PubMed] [Google Scholar]
- Zegans 2006 {published data only}.Zegans ME, Toutain C, Saine P, Srinivasan M, Jayashree D, Mahalakshmi R, et al. Analysis of digital images of corneal ulcers in the pilot study for the Steroid Corneal Ulcer Trial (SCUT) Investigative Ophthalmology and Visual Science. 2006;47 ARVO E-abstract 1885. [Google Scholar]
References to ongoing studies
- NCT01721694 {unpublished data only}. NCT01721694. Association of azithromycin 1,5% loteprednol 0,5%eye drops versus individual administration of azithromycin 1,5%and loteprednol 0,5%in the treatment of ocular inflammation and infection. [3 December 2013]; clinicaltrials.gov/show/NCT01721694.
Additional references
- AAO 2003.American Academy of Ophthalmology. American Academy of Ophthalmology Final Program (conference abstracts), Chicago. San Francisco, USA: American Academy of Ophthalmology; 2003. [Google Scholar]
- AAO Bacterial Keratitis PPP 2013.Bacterial keratitis Preferred Practice Pattern (PPP) guideline. American Academy of Ophthalmology. 2013 [Google Scholar]
- Baker 1996.Baker RS, Flowers CW, Jr, Casey R, Fong DS, Wilson MR. Efficacy of ofloxacin vs cefazolin and tobramycin in the therapy for bacterial keratitis. Archives of Ophthalmology. 1996;114(5):632–3. doi: 10.1001/archopht.1996.01100130624031. [DOI] [PubMed] [Google Scholar]
- Bennett 1998.Bennett HG, Hay J, Kirkness CM, Seal DV, Devonshire P. Antimicrobial management of presumed microbial keratitis: guidelines for the treatment of central and peripheral ulcers. British Journal of Ophthalmology. 1998;82(2):137–45. doi: 10.1136/bjo.82.2.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourcier 2003.Bourcier T, Thomas F, Borderie V, Chaumeil C, Laroche L. Bacterial keratitis: predisposing factors, clinical and microbiological review of 300 cases. British Journal of Ophthalmology. 2003;87(7):834–8. doi: 10.1136/bjo.87.7.834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton 2011.Burton MJ, Pithuwa J, Okello E, Afwamba I, Onyango JJ, Oates F, et al. Microbial keratitis in East Africa: why are the outcomes so poor? Ophthalmic Epidemiology. 2011;18(4):158–63. doi: 10.3109/09286586.2011.595041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirambo 1986.Chirambo MC, Tielsch JM, West KP, Jr, Katz J, Tizazu T, Schwab L, et al. Blindness and visual impairment in Southern Malawi. Bulletin of the World Health Organization. 1986;64(4):567–72. [PMC free article] [PubMed] [Google Scholar]
- Dart 1991.Dart JK, Stapleton F, Minassian D. Contact lenses and other risk factors in microbial keratitis. Lancet. 1991;338(8768):650–3. doi: 10.1016/0140-6736(91)91231-i. [DOI] [PubMed] [Google Scholar]
- Deeks 2011.Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta-analyses. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; [March 2011]. 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- Feng 1990.Feng CM. The causes of blindness by corneal diseases in 3499 cases. Chinese Journal of Eye Science [Zhonghua Yan Ke Za Zhi] 1990;26(3):151–3. [PubMed] [Google Scholar]
- Gangopadhyay 2000.Gangopadhyay N, Daniell M, Weih L, Taylor HR. Fluoroquinolone and fortified antibiotics for treating bacterial corneal ulcers. British Journal of Ophthalmology. 2000;84(4):378–84. doi: 10.1136/bjo.84.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville 2006.Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials inMEDLINE: ten years on. Journal of theMedical Library Association. 2006;94(2):130–6. [PMC free article] [PubMed] [Google Scholar]
- Higgins 2011a.Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration; [March 2011]. 2011. Available from www.cochrane-handbook.org. [Google Scholar]
- Higgins 2011b.Higgins JPT, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) The Cochrane Collaboration. 2011 Available from www.cochrane-handbook.org.
- Hyndiuk 1996.Hyndiuk RA, Eiferman RA, Caldwell DR, Rosenwasser GO, Santos CI, Katz HR, et al. Comparison of ciprofloxacin ophthalmic solution 0.3% to fortified tobramycin-cefazolin in treating bacterial corneal ulcers. Ciprofloxacin Bacterial Keratitis Study Group. Ophthalmology. 1996;103(11):1854–62. doi: 10.1016/s0161-6420(96)30416-8. [DOI] [PubMed] [Google Scholar]
- Hyon 2004.Hyon JY, Joo MJ, Hose S, Sinha D, Dick JD, O’Brien TP. Comparative efficacy of topical gatifloxacin with ciprofloxacin, amikacin, and clarithromycin in the treatment of experimental Mycobacterium chelonae keratitis. Archives of Ophthalmology. 2004;122(8):1166–9. doi: 10.1001/archopht.122.8.1166. [DOI] [PubMed] [Google Scholar]
- Kaliamurthy 2013.Kaliamurthy J, Kalavathy CM, Parmar P, Nelson Jesudasan CA, Thomas PA. Spectrum of bacterial keratitis at a tertiary eye care centre in India. BioMed Research International. 2013 doi: 10.1155/2013/181564. Article ID 181564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killingsworth 1993.Killingsworth DW, Stern GA, Driebe WT, Knapp A, Dragon DM. Results of therapeutic penetrating keratoplasty. Ophthalmology. 1993;100(4):534–41. doi: 10.1016/s0161-6420(13)31631-5. [DOI] [PubMed] [Google Scholar]
- Kowalski 2003.Kowalski RP, Dhaliwal DK, Karenchak LM, Romanowski EG, Mah FS, Ritterband DC, et al. Gatifloxacin and moxifloxacin: an in vitro susceptibility comparison to levofloxacin, ciprofloxacin and ofloxacin using bacterial keratitis isolates. American Journal of Ophthalmology. 2003;136(3):500–5. doi: 10.1016/s0002-9394(03)00294-0. [DOI] [PubMed] [Google Scholar]
- Lee 2014.Lee YS, Tan HY, Yeh LK, Lin HC, Ma DH, Chen HC, et al. Pediatric microbial keratitis in Taiwan: clinical and microbiological profiles, 1998-2002 versus 2008-2012. American Journal of Ophthalmology. 2014;157(5):1090–6. doi: 10.1016/j.ajo.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Miño de Kaspar 2005.Mino de Kaspar H, Koss MJ, He L, Blumenkranz MS, Ta CN. Antibiotic susceptibility of preoperative normal conjunctival bacteria. American Journal of Ophthalmology. 2005;139(4):730–3. doi: 10.1016/j.ajo.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Moeller 2005.Moeller CT, Branco BC, Yu MC, Farah ME, Santos MA, Hofling-Lima AL. Evaluation of normal ocular bacterial flora with two different culture media. Canadian Journal of Ophthalmology. 2005;40(4):448–53. doi: 10.1139/i05-014. [DOI] [PubMed] [Google Scholar]
- Musch 1983.Musch DC, Sugar A, Meyer RF. Demographic and predisposing factors in corneal ulceration. Archives of Ophthalmology. 1983;101(10):1545–8. doi: 10.1001/archopht.1983.01040020547007. [DOI] [PubMed] [Google Scholar]
- O’Brien 1995.O’Brien TP, Maguire MG, Fink NE, Alfonso E, McDonnell P. Efficacy of ofloxacin vs cefazolin and tobramycin in the therapy for bacterial keratitis. Report from the Bacterial Keratitis Study Research Group Archives of Ophthalmology. 1995;113(10):1257–65. doi: 10.1001/archopht.1995.01100100045026. [DOI] [PubMed] [Google Scholar]
- OSG 1997.The Ofloxacin Study Group. Ofloxacin monotherapy for the primary treatment of microbial keratitis: a doublemasked, randomized, controlled trial with conventional dual therapy. Ophthalmology. 1997;104(11):1902–9. [PubMed] [Google Scholar]
- Parks 1993.Parks DJ, Abrams DA, Sarfarazi FA, Katz HR. Comparison of topical ciprofloxacin to conventional antibiotic therapy in the treatment of ulcerative keratitis. American Journal of Ophthalmology. 1993;115(4):471–7. doi: 10.1016/s0002-9394(14)74449-6. [DOI] [PubMed] [Google Scholar]
- Parmar 1998.Parmar MKB, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Statistics in Medicine. 1998;17(24):2815–34. doi: 10.1002/(sici)1097-0258(19981230)17:24<2815::aid-sim110>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Pepose 1992.Pepose JS, Wilhelmus KR. Divergent approaches to the management of corneal ulcers. American Journal of Ophthalmology. 1992;114(5):630–2. doi: 10.1016/s0002-9394(14)74496-4. [DOI] [PubMed] [Google Scholar]
- Poggio 1989.Poggio EC, Glynn RJ, Schein OD, Seddon JM, Shannon MJ, Scardino VA, et al. The incidence of ulcerative keratitis among users of daily-wear and extended-wear soft contact lenses. New England Journal of Medicine. 1989;321(12):779–83. doi: 10.1056/NEJM198909213211202. [DOI] [PubMed] [Google Scholar]
- RevMan 2014.The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration; 2014. [Google Scholar]
- Schaefer 2001.Schaefer F, Bruttin O, Zografos L, Guex-Crosier Y. Bacterial keratitis: a prospective clinical and microbiological study. British Journal of Ophthalmology. 2001;85(7):842–7. doi: 10.1136/bjo.85.7.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schein 1989.Schein OD, Ormerod LD, Barraquer E, Alfonso E, Egan KM, Paton BG, et al. Microbiology of contact lens-related keratitis. Cornea. 1989;8(4):281–5. [PubMed] [Google Scholar]
- Tuli 2013.Tuli SS. Topical corticosteroids in the management of bacterial keratitis. Current Ophthalmology Reports. 2013;1(4):190–3. doi: 10.1007/s40135-013-0026-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vajpayee 2000.Vajpayee RB, Dada T, Saxena R, Vajpayee M, Taylor HR, Venkatesh P, et al. Study of the first contact management profile of cases of infectious keratitis: a hospital-based study. Cornea. 2000;19(1):52–6. doi: 10.1097/00003226-200001000-00011. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Suwan-apichon 2005.Suwan-apichon O, Reyes JM, Chuck RS, Herretes S, Vedula SS. Topical corticosteroids as adjunctive therapy for bacterial keratitis. Cochrane Database of Systematic Reviews. 2005;3 doi: 10.1002/14651858.CD005430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- * Suwan-apichon 2007.Suwan-Apichon O, Reyes JM, Herretes S, Vedula SS, Chuck RS. Topical corticosteroids as adjunctive therapy for bacterial keratitis. Cochrane Database of Systematic Reviews. 2007;(4) doi: 10.1002/14651858.CD005430.pub2. Indicates the major publication for the study. [DOI] [PMC free article] [PubMed] [Google Scholar]


